Note: Descriptions are shown in the official language in which they were submitted.
i5~iS
Specification of Inv~ntion Patent:..... ~... ~.... ;
PRO(:~SS FOR DRII.LING UD ADDITIVE ~I~EPARATION AND
ADDITIVE FOR DRILLING MUD
The present invention relates to a new
method for obtaining a high efficiency and low cost
sulfidc sequestering agent, as well as aqueous dril-
ling muds containing the sequestrant.
i More particularly, the present invention
des,-ribes a method for obtaining high surface area
magnetite~'eO.FQ203), with a high capacity for quick
absorption of sulfides~H2S, HS and s2 )~ with the
magnetite bei~g obtained from pyrite roasting tailings~
One of several instances where the presen-
ce of a sulfide sequesteri~g agent is required, is in
j oil or gas well drilling areas, where sulfides are
oEten evolved, which are well known to be toxic,even
in small amounts, and also to cause damage to the
drilling equipment, through the sulfide reacting with
water, to produce a highly corrosive acid.
.
- At present, there are basically two types
of sequestrants which are preferred as additives for
drilling muds or fluids, to react with sulEides such
as to render them inert and harmless to human beings
and equipment: zinc ccmpounds(e.g., water-insoluble
zinc carbonate and water-soluble zinc chelates)lwhicl-
react with sulfides by forming ZnS, and iron compounds
i~5~j~is
solely represented by a synthetic magnetite of high
surace area, Fe3O4.
The effectiveness of an iron compound as
a sequestering agent for sulfides depends on the ex -
posed reactive surface area, the pH value, the mud's
shear conditions, the temperature, reaction time and
post-reaction ageing.
Under optimum reaction conditions, for a
suitable exposed surface area of the magnetite, the
capacity of Fe3O4 for sulfide removal may reach up to
2000 mg/l of sulfides by 2,85 kg/m3(1 lb/bbl) of treat-
ment with Fe3~4.
Effective sequestering action of an a~di-
tive in aqueous sulfide-containing muds is based on
the possibility of achieving an irreversible and com
plete chemical reaction between the sequestrant and
the sulfide.species, with the latter existing, at am-
bient pressure and temperature, as molecular hydrogen
sulfide~H25) within t:he acid range of pH values,while
in the neutral range, up to a pH value of 13(the ran-
ge occurring in muds), the hydrosulfide ion ~HS ) is
the prevailing species, and above a pH of 13, the
dominant species is the divalent sulfide ion, s2
In solution, there is a mutual equilibrium among the
three species, wi~h the irreversible and complete re-
moval of any one of the three species serving to re-
~2~j566S
move all of them. If chemical reaction between thesequestrant and the sulfide species is incomplete,
not all soluble sulfides present will be removed.
Further properties of the ideal seques-
trant are as ~ollows:
1) An ideal sequestrant reacts with sul-
fides in a complete, quick and reliable manner;
2) The sequestrant must act un~er varying
p~l r temperature, and pressure, competitive reaction
and shearing conditions, in the presence o~ all other
reactants contained in the mud;
3 1 MUd performance must not be impaired
by maintainin~ excess sequestrant in the mud, even at
high temperatures;
- 4) The actual amount of sequestering react-
-ant in the mud must be readily measurable in the field;
5~ Both the sequestrant and its reaction
products should not be corrosive to metals and mate-
rials in contact with the mud; and
6) The sequestrant must be widely availa-
ble and economical for the purpose of acceptance by
the industry, and have a low cost and hi~h removal
effectiveness, under any and all conditions of use.
The search for an iron compound meeting
the above requirements has been the objec~ of a number
of studies and patents.
56~i5
-- 4 --
Thus, for instance, Sooiety of Petroleum
Engineers Report No. 7499, 53rd Annual SPE of AIME Fall
Conf. Houston, Oct. 1-3, 1978, "Chemical Scavengers for
Sulfides in Water-Base Drilling Fluids", by R.L. Garrett
et al., di~clo3es an overview of the product~ usea as
sulfide seguestering agent6 iIl aqueous muds, and Society
of Petroleum Engineers Report No. 7498, 53rd Annual SP~
of AIME Fall Conf. Houston, Oct. 1~3, 1978, ~U~e of
Reacti~e Iron Oxide to Remove H~S from Drilling Fluid",
by J.D. Ray et al., describe6 in detail the removal
chemistry of H2S from underground formations with the
help of magnetic sponge iron.
Fox, U.S. Patent No. 4,008,775, i6sued February
22, 1977, disclo~es manufacture of a porous iron oxide
with a surface area much yreater than that of magnetite,
and which can be succe6~fully used as a sulfide
s~questrant in formation~. The oxide is prepared by
oxidizing carbon-containing iron under controlled
oxidation oonditions, so that oxidation doe6 not proceed
up to Fe2O3. ~hs raw material ~mployed for preparing the
porous material has a chemical composition such that the
high surface area porous material is obtained at the end
of the proce6s, suitable as a sulfide sequestering agent
in aqueous muds for underground formations.
On the other hand, Fox, U.S. Patent 4,324,298,
issued February 13, 1982, discloses a high surface area
Fe2O3 iron oxide, of about 3.5 m2/g surface area,
A.~
~5~ 5
- 4a -
u6eful as a 6ulfide ~equestrant in underground
formations. Thi~ product i~ made by high temperature
oxidation of ferrou6 sulfate followed by quick cooling.
~1~,
~s~
-- 5
A further approach is that of patents des-
cri~)ing methods for cbtaining magnetic or non-magnetic
iron oxides, but solely as densifiers for drilling
muds, without any su]fide absorption capacity.Among
those patents, there are: Brazilian Patent Application
No. PI 81 00245, Miden Argentina S.A., published July 6,
1982; British Patent No. 1,554,4:30 of Metallgesellschaft
AG, dated October 24, 1979; and B:ritish Patent No.
1,379,067 of Metallgesellschaft AG, dated January 2, 1975.
In contrast to prior art processes, the
applicant has developed a method for obtaining a high
surface area magnetite, employing, as a raw material,
the residue rrom pyrite roasting, with the product
thus prepared exhibiting a quick and efficient absorpt-
ion of sulfides in basic aqueous drilling muds, with-
out changing the mud's rheological properties, thus
correspor:ding to the ideal sequestrant hereinabove
described.
Thexefor~r it is an object of the instant
invention to provide a method for the production of
high surface area FeO.Fe2O3 magnetite, for effective-
ly and quickly absorbing the sulfides emanating from
oil and gas formation.;, which sulfides are hazardous
to human health and corrosive to drilling equipment.
A further object comprises provi~ing a
simple and economic process for high surface area ma2~
netite preparation, from pyrite roasting residues,
with the magnetite-serving as a sulfide sequestering
~t~
~j56~;5
agent in aqueous drilling muds.
A still further object is to prepare aque-
ous drilling muds containing, in addition to the usual
additives, such as barytei lignine sulfonates, etc.,
the high surface area magnetite according to the pres-
ent invention, with the mud keeping its rheological
properties intact, even on the addition of large quan-
tities of the present invention's iron oxide, with the
danger of sulfide emanations being averted.
The process for preparing the high surfa--
ce area FeO.Fe203 magnetite, according to the present
invention, uses as a starting material, the residue
from the roasti.ng of pyrites, in a fluidized bed, in
the presence of oxygen, at high temperatures.The me-
thod according to the present invention basically comr
prises subjecting the iron oxide residue from pyrite
roasting, said residue having a suitable particle si-
ze, to a magnetic separation process, with a high sur-
face area magnetic fraction, and another non-magnetic
fraction, which is rejected, being obtained.
Pyrites are materials composed of iron
sulfide mixtures: FeS2(pyrite), FeS(pyrrhotite) and
FeAsS(arsenopyrite), with such materials comprising
an average 40-44 weight per cent of iron. Carbonaceous
pyrites are those obtained from physical treatment
of coals, which retain some carbon not xemovable by
i ", ~
5~tis
-- 7
oxidizing trea~ment.
The roasting of l~yrites yields el~ment~l
sulfur or sulfuric acid, in addition to iron oxides
for the iron and steel industry. The reaction proceeds
at 850QC, in air and without the lpresence of a liquid
phase, that is, without melting, with the evolved gas
comprising about 13.5% sulfur dioxide, and a solid
residue, which is the roasted product, comprised o~
a purple-colored iron oxide mixture( "purple ore").
It is this residue, i.e., this blend of oxides, which
is to be subjected to the present invention's ~rocess
whereby a high surface area magnetite ~raction, of a
particle size less than 0.075mm~ 200 mesh) is obtain-
ed, which is useful as an additive for aqueous dril-
ling muds, said additive acting as a sequestrant upon
the sulfides emanating from the f~rmation.
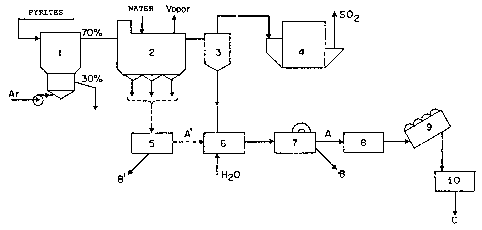
In the drawings appended to this specification:
Figuras 1 and 2 represent two preferred embodi-
ments of the present invention r and Figure 3 represents
the apparatus used to measuxe the consumption of s2 ions
by the invention product, through titration of the S
content in a sample of the same product invention. In
Figure 1, the preferred solids fraction is directed to a
pulp tank, and a less preferred fraction goes to a hydro-
cyclone from where is separated a fraction to be incorpor-
in the pulp tank. In Figure 2, the boiler and cyclone
fractions are routed to a pulp tank to which is optionally
l~S6~i5
- 7a -
coupled a hydrocyclone from where is dlscarded a fraction
of undesirable granulometry.
The process according to the present inv-
ention will now be illustrated and described with ref-
erence to the accompanying Figures 1 and 2.
With reference to Figure 1, a fluidized
bed reactor 1 is fed with pyrites, for example, and
oxidized with air at a rate of about 1,900 Nm3Jh and
by square meter of distributor area. The size parti-
cle distribution of the pyrites is typically: 3~ bet-
E ween 4 and 6 mm, 15~ between 2 and 4 mm, 25~ between
1 and 2 mm, 30~ between 0.5 and 1 mm, and 27% below
5~;~;S
0.5 mm. The pyrite roasting reaction in the presence
of air at 850QC yielcls a sulfurized gaseous stream,
which will eventually produce sulfur or sulfuric acid
and haviny suspended iron oxides entrained therein,
with the solids having varying particle sizes.By means
of successive treatmellt and separating steps, the
product of the present invention will be obtained.
Thus, according to Figure 1, the pyrites
are routed, through a feeding silo(not snown) to a
conventional fluidized bed reactor 1, whereinto air
is blown, and the roasting process is then started,
at 850QC. The roasting process product is typically
the above-mentioned mixture of iron oxides with vary-
ing particle sizes. A portion of this product leaving
the reactor's flu;dized bed is the reactor underflow,
comprising 30% of the total solids and a coarse par-
ticle size(greater than 1 mm~, which is disposed of.
From the top of reactor 1, a gas stream issues, cont-
aining the remaining solids(70%) in suspension and
gaseous SO2. The gaseous stream is directed to a heat
exchanging boiler 2, provided with a coil and barrierS
and having a water inlet and a steam outlet. Boiler 2
retains mainly the fraction with a particle size bet-
ween 1 mm and 0.208 mm( 65 mesh), with the finer sol-
ids passing on to the next stage, and the coarser ones
being disposed of or subjected to sizing, for reclaim
~ ~5t;~S
ing of fines. From the 70~ solids entering boilex 2,
the coarser 40~ are thus separated, there still rem-
aining 30% suspended solids, which are directed to a
cyc]one 3, with a cylindrical upper portion and a con~
ical lower portion, and wherein the next separation
step is conducted, with 27% of the solids being rec-
overed, comprising iron oxide material of a particle
size between .208 mm and .037 mm (65 to ~00 mesh).This
is the preferred fraction for processing in accordan-
ce ~ith the present invention. The remaining suspend-
ed solids, which amount to 3%, of particle size under
.037 mrl~(400 mesh) t are fed to an electrostatic separ-
ator 4, which settles the particles and the sulfuriz-
ed solid-free gases are coilected and processed to
produce sulfuric acid and elemental sulfur.
Optionally, the boiler fraction is direct-
ed to a particle size classifier 5, with an A' fract-
ion of particle size less than .208 mm, and a B' tail-
ing fraction, of particle size greater than .208 mm,
which is rejected, being obtained.
Tne cyclone fraction, to which the A'
fraction may be joinecl, representing 27% of the init-
ial solids subjected to roastin~ is recover~d by a
conveyor belt system( not shown~, and water is added
to said fraction in a pulp tank 6, whereby a pulp is
formed, containing from 20 to 40% solids, preferably
" , .
- 10~;5~i~i5
30% iron oxide sol~ds. This aqueous pulp is then led
to a low strength magnetic separator 7, where magne-
tic separation is effected, with a magnetic A fraction
(50-60% of the total cyclone fraction)- ~he present
invention's product - being collected, and a non-mag-
netic B fraction being discarded. It should be noted
that there is an important relationship among parti-
cle porosity, surface area and sulfide se~uestering
activity efficiency. For the cyclone fraction, surfa-
ce area measurements have been carried out: the .208
mm fraction (-65 mesh) exhibiting, by the BET method,
a surface area of 2~04 m2/g; the .053 mm(-270 mesh)
and 0.037 mm(-400 mesh) fractions with 2~97 and 3.33
m~/g, respectively. ~urther, it was experimentally
found that at comparable surface areas, the iron oxi-
de particle sizes most effective for sulfide sequester-
ing action are the ~ma.lest ones, between .037 and
.074 mm~400 and 200 mesh).
Following magnetic separation, which can
be carried out in a low strength magnetic separator 7
the magnetic concentrate is demagnetized in a demag-
netizer 8, thickened in a thickener 9, to remove most
of the water from the pulp, forming a suspension of
about 70% solids, filtered in filter (10), and dried
in a rotating dryer Inot shown) whereby a final product
C is obtained, which is a high surface area magnetite ooncentrate,
~'
~3,1
iL~656~5
dry and ready for incorporating into drilling muds
with a particle size of less than .074 mm ~200 mesh).
It should be noted that the temperature of thickener
9 should not exceed 220C, in order to avoid reoxidat-
ion of magnetite to hematite.
Referring now to Figure 2, which illustra-
tes another preferred embodiment of the present inv-
ention, a suspension of sulfurized gases containing
magnetite is subjected to the separating action in
heat exchanging boiler 2 and cyclone 3, and the unclas-
sified products are collectecl in a pulp tank 6, where
a pulp of about 30% solids is.formed upon addition of
water. At this point, a hydrocyclone 5 may be inter-
posed, for the purpose of separating the coarser fract-
ions, of greater than .074 mm(200 mesh) particle si-
ze, which will be discarded. The pulp is then fed to
a low strength magnetic separator 7, whereafter, mag-
netic fraction A, in the event of beiny still unclas-
sified, is subjected to sizing in hydrocyclone 5, to
separate a tailing B' fraction, of particle size great-
er than .074 mm(200 mesh3, which is the hydrocyclone
5 underflow, and an A' fraction, the hydrocyclone S
overflow, of a particle size under .074 mm(200 mesh),
suitable as a sulfide sequestrant additive ~or dril-
ling muds. The hydrocyclone 5 overflow A' fraction is
then demagnetized in demagnetizer B, thickened in thi-
..
,
~s~s
- 12
ckener 9, to 70% solids, and the classified and thick-
ened magnetic concentrate is filtered in a fi~ter ~', and
dried in dryer 10, so as to ~)rovide a classified and
dried magnetic concentrate C', of a particle ;ize less
than .074 mm ~200 mesh), suitable as a sulfid~ sequest-
ering ad~}tive for aqueous drilling muds.
Accordingly, the drilling mud aaditive
preparation process o~ the pres~nt invention is char-
acterized in that it comprises the steps of:
(a) sub~ecting the overflow iron oxide residue (70% by
weight of the initial pyrite charge) from a roasting reactor to a
particle size separation in a heat exchanger boiler, thus
obtainin~ a ~raction B' (40% by weight of the initial charge), of
particle size greater than û.074 mm, said fraction being rejected
or classified in a hydrocyclone and joined to the next fraction,
with the fines from said heat exchanger boiler (30% by weight of
the initial charge) being separated in a cyclone, the fraction of
particle size less than 0.074 mm (27% by weight) being collected;
or the boiler and cyclone fractions are joined without
classifying;
(b) forming a pulp in a pulp tank, with the fraction or the
fractions from step (a), under addition of water, said pulp
having a solids content of about 30%, said solids being comprised
of the cyclone fraction or the classified boiler fraction and the
cyclone fraction, or the unclassified boiler and cyclone
fractions;
(c) subjecting the pulp from step ~b), formed with the
cyclone fraction or the cyclone fraction and the boiler fraction,
with or without classifying, to a magnetic separation in a low
strength magnetic separator, or classifyin~ the pulp formed by
;56~;5
- 13
the boiler and cyclone fractions, and thereafter carrying out
magnetic separation in said low strength magnetic separator, thus
obtaining a magnetic fraction A, which comprises the product of
the present invention and a non-magnetic fraction B, which is
rejected;
(d) subjecting magnetic fraction A from step (c) to the
demagneti~ing action nf a demagnetizer, or subjecting said
fraction to c~assifiration in a hydrocyclone, and afterwards
demagnetizing said fraction in said demagnetizer;
(e) thickening the demagnetized pulp from step (d) in a
thickener, to obtain a concentrated, 70% solids pulp;
(f) drying the pulp from step (e) in a rotating dryer, at a
temperature between 90C and 210QC, or filtering the concentrated
pulp in a filter and thereafter, drying the same in said dryer,
to obtain a dried product C or a dried and classified product C',
of less than 0.074 mm particle size, suitable as a sulfide
sequesterin~ additive for drilling muds, of 2.4 to 3.3 m2/g
surface area, allowing a quick and irreversible absorption of
sulfides emanating from underqround formations.
~Z~:iS6~
- 14 -
The pre:~nt invention will now be further
illustrated by the following Examples, wherein:
Examples 1 and 2, of reactivity to S
ions and kinetic testing witl- S ions, evaluate the
prod.uct's nature; Example 3 is an evaluation of s2
ion consumption at pH 8.5, and Example ~ is a H~S con-
sumption test at pH 7, being related to the actual
~ performance of the product.
~ Further, in all of the Examples, sul-fide
ion concentrati.on was determined by potentiometric
.. ,
, titration with a 0.1 ~ sil~er nitrate, AgN03,s~lution
~ The autornated titration system includes a"Metrohm He-
:~. risau~odel E 535 potentiograph and an automatic"Me-
~ trohm Herisau"~odel E 535 burette. Potential was mea-
'1 . *
sured through a "~etrohm Heri.sau Model EA 245 silver
.electrode, coated with a silver sulfide film an~ a
etrohm"Model EA 109 glass electrode.
All reasents used were analytical grade.
~ EXAMPLE 1
- 1.40 grams Na2S.9 H20 were dissolved in
47.5 ml water, and NallC03 was added to pH 8 5. The
sulfide ion content A was d~termined in a 5 ml aliquct.
To the remaining solution there was added 2 5 grams
* Tradema-k
** Tradcma-k
l ~
~s~is
- 15
test product and reaction was allowed to proceed for
one hour at room temperature. A 5 ml aliquot was with-
drawn, which was received in 4ml of Na2CO3 solution
at a pH of about 12. '~aCl( 2 ~) was added, the mixture
was centrifuged and the s2 ion content B was deter-
mined in the supernatant liquid.
The reactivity index, A -B/A for each test
product was calculated, with the results being set
forth in Table I.
In this table, the meaning of the term~
is as follows;
- COAT 1131*designates a commerclal blend
of salts containing chromium and zinc.
- ~yclone stands for cyclone residue.
- boiler stands for boiler residue.
- tlle numeral 270 after "cyclone" or "boil-
er" refers to the fraction of particle size between
53 and 74 mlcrons~270 and 200 mesh).
TABLE I
Product Reactivity Index, %
-
COAT 1131* 100
Ironite Sponge - 100
Overall magn. Cyclone 100
Magn. Cyclone 27~ 93.5
* Trademark
., ,.. . ~ . . .
iS
- 16
TABLE I(cont'd)
Product Reactivity Index, %
Overall magn. Boiler 91
Magn. Boiler 27082
Overall non-magn.Cyclone 68
Non-magn. Cyclone 270 90
Overall non-magn. Boiler 63
Non-magn. Boiler 270 67
EXA~PLE 2
The procedure of Example 1 was repeated,
except that, instead of withdrawing only one aliquot,
four ali~uo~s were taken at 15 min., 30 min., 45 min.
and 60 min. reaction time. Treatment was the,same for
all aliquots.
- The non-consumed fraction B/A was calcu-
lated for each aliquot, the results being summarized
in Table II. The terms used have the same meaning as
in Table I.
TABLE II
Product - % non-consumed s2 ions after
t (min)
t= 0 t=15 t=30 t=45 t-60
. . . _ _ . . _ . . . _ . _ . _ _
COAT 1131 100 3 2 1 0
1;~656~;S
- 17
TABLE II(cont'd)
. . _ _
Product~ non-consumed s2 ions after
t(min)
t=0 t=15 t=30t=45 t=60
... _ .. . . _ . . _ _
Ironite Sponge 100 33 12 4 0
Overall magn.Cyclone 100 34 13 4
Magn. Cyclone 270 100 34 14 4 .5
Overall magn.Boiler 100 71 67 50 37
Magn. ~oiler 270 100 72 64 53 33
Overall Non-magn. 100 69 53 45 32
Cyclone
Non-magn.Cyclone 270 100 70 44 23 10
From Table II, it can be seen that de s2
ion sequestering efficiency o~ the various iron oxide
fractions is a function of the particles' magnetism,
and their origin, iOe., boiler or cyclone, with the
greatest s2 ion absorption being found for the over-
all magnetic c~clone residue, as was expected.
EXAMPLE 3
1.40 grams Na2S.9H2O was dissolved in wa-
ter, diluted to 55 ml, and NaHCO3 was added to pH 8.5
A 5 ml aliquot was withdrawn and its sulfide ion cont-
ent A was determined. To the remaining solution test
product was addecl, in a limited amount P -0O100 g ~
1~i5~;~i5
- 18
and the mixture was allowed to react for one hour at
room temperature. A 5 ml allquot was then taken and
received in 4 ml o~ Na2CO3 s~lut:ion at a pH value of
about 12. NaCl( 2 g) was added, the mixture was cen-
trifuged and the s2 ion content B determined in the
supernatant liquid.
The s2 ion ~onsumption per gram of test
product was calculated as follows:
S B = g S- /g product, where A and B
P x 20,000
are given in mg/l and P is in grams.
Results are set forth in Table III.
TABLE III
Products2 Ion consumption (g s2 /g prod-
uct~
.. . .. . .
COAT 1131 0~120
Ironite Sponge 0.085
Overall magn.Cyclone 0.090
Magn. Cyclone 270 0.105
. . ....
EXAMPLE 4
This Example illustrates the H2S consumpt~
ion at pH 7.0 for one hour, ~or the product of the
invention.
~ An apparatus as illustrated in Figure 3
;6iS
-- 19
was set up, where A is a round-bottomed flask of 250
ml capacity, provided with a burette containing an
aqueous 4:1 HCl solution, a nitrogen or argon inlet
and a connecting tube to washing bottle B.
28 grams Na2S.9H~O were initially disso]v-
ed in 500 ml water, with a 1000 mg s2 ion/l stock so-
lution being obtained. The s2 ion content was deter-
mined in a 5 ml aliquot, according to the abo~e-des-
cribed procedure. 100 ml of s2 stock solution were
transferred with a pipette to f]as~ ~. 0.1 grams of
test product were then weighed and transferred to the
washing bottle B, and 100 ml 1% Na~CO3 solution were
added to the bottle. 50 ml 0.1 N NaOH solution were
placed in tub~ C. The burette fitted to flask A con-
tains 1:4 HCl solution, which is added dropwise, at
the rate of l ml/minute, flas~ A having been previous-
ly purged with nitrogen or argon for 15 minu'es, and
with HCl addition also being made under a nitrogen or
argon atmosphere. After adding 1:4 HCl solution for
one hour, the s2 ion content, in milligrams, present
in tube C, was determined.
H2S consumption per gram of product is
calculated by:
H2S Consumption = (0.1X Y? = g H2S/g o~ pr~auct
where:
X = initial s2 ion content in solution
~iS6~;5
Y = amount of s2 ions present in tube C
P = weight of test product, in mg
The results for H2S consumption at pH 7.0
during one hour, for the product of the instant inven-
tion and comparative commercial products are listed
in Table IV.
TABLE IV
Test ProductConsumption g H2S/g product
. . . ~
COAT 1131 0.13
Ironite Sponge 0.91
Overall magn. Cyclone 1.05
Magn. Cyclone 270 1.12
.. ..
In Table IV the excellence of the present
invention's products can be seen, as compared to com-
mercial products, at a neutral pH.
Examples 1 to 4 therefore show the excel-
lent p~rformance of the present invention's produc~
in the basic(8.5) and neutral (7.0) pH ranges, which
are the pH conditions normally found in aqueous dril-
ling muds.
The Eollowing Examples illustrate the ex-
cellent rheological properties of the present invent-
ion's product, as used in drilling muds. That is, the
additive developed by the applicant is not only an
1.2~;56~;5
excellent sequestrant for s2 ions, but also leaves
unchanged the rheological properties of aqueous dril-
ling muds, which is a necessary condition for its use
in those muds.
Several typical aqueous drilling muds
were prepared, for use in underground formations with
sulfide emanation, and their rheological and filter-
ing properties were measured, both initially and af-
ter aging at 65.5eC(150F), for 16 hours in a rotat-
ing oven.
EXAMPLE 5
In this example, the influence of the 2re-
sent invention's product on the rheological and filt-
ering propert;es of a polymer treated sea-water based
mud is studied. Results are presented in Table V.
TABLE V
.
a) MUD COMPOSITION
. _ . . .. _
ADDITIVE CONCENTRATION/UNIT
Pre-hydrated Bentonite 1 volume (333.3 ml)
Sea Water 2 volumes (666.6 ml)
NaOH 2.85 kg/m3( 1~0 lb/bbl)
Pre-gelled starch 22.80 kg/m3(8.0 lb/bbl)
Bactericide 1,71 kg/m3(0.6 lb/bbl)
Carboxymethyl-cell~lose 1.42 kg/m3(0.5 lb/bbl)
~56~5
- 22
TABLE V(cont'd)
ADDITIVE CONCENTRATION/UNIT
Case A- Product of the - -
Invention
Case B-Product of the 57 kg/m3 (20 lb/bbl)
Invention
Case C-Product of the 114 k~/m3 (40 lb/bbl)
Invent.ion
. _ _ _ ... .. . . .
b) RHEOLOGICAL AND FILTERING PROPERTIES OF MUDS
. . . . _ ~
Case A Case B Case C
Initial Aged Init l Aged Initial Ag~d
Apparent Viscosity 20 21 19.5 21 20 18
~cP)
Plastic Viscosity 10 10 8.0 8.0 11 8
(cP)
Yield Strength(kg/ 98 107.8 112.7 107.8 88.2 98.0
100 m2)(lb/100 ft2) 20 22 23 22 18 20
Initial Gel(kg/100m2) 102.978.9 98.0 6B.6 88.2 93.1
(lb/100 ft2) 21 16 20 14 18 19
Final Gel(kg/100m2) 186.2 166.6 191.1 142.1 16t.7 166.6
(lb/100ft2) 38 39 39 29 33 34
API Filtrate(ml) - 4.4 - 4.2 - 4.0
i6~
- 23
By observing the results i~ Table V, it
is apparent that presence of the drilling mud additi--
ve of the present invention does not change the mud's
rheological and filtering properties.
EXAMPLE 6
In this Example, the influence of the mag-
netite sponge product of the present invention on the
rheological and filtering properties of a potassium
chloride based drilling mud is studied. Results are
listed in Table VI.
TABLE VI
a) MUD COMPOSITION
.
A~ITIVE CONCENTRATION/~IT
Pre-hydrated bentonite 1 volume (225 ml)
.
Sea Water 3 volumes (675 ml)
NaOH 1.27 kg/m3 (1.5 lb/bbl)
Hydrated Lime 1.42 kg/m3 (0.5 lb/bbl)
Pre-gelled Starch 42.75 kg/m3 (15.0 lb/bbl)
Carboxymethyl-cellulose 2,85 kg/m3 (1~0 lb~bbl)
Bactericide 1.71 kg/m (0.6 lb/bbl)
Potassium Chloride 114 kg/m3 (40 lb/bbl)
Baryte 270.8 kg/m3(95 lb/bbl)
Case A-Product o~ the
Invention
6~is
- 24
T?,BLE VI(cont'd)
ADDITIVE CO~POSITION/UNIT
. _ .
Case B-Product of the57 kg/m3(20 lb/bbl)
Invention
Case C-Product of the114 kg/m3(40 lb/bbl)
Invention
.
b) RHEOLO~ICAL AND PILTERING PROPERTIES OF MUD
.. ...
Case A Case B Case C
Initial Aged Initial A~ed Initial Aqed
_
Apparent Viscosity 26 26 27.5 30 28.5 29.5
(cP)
Plastic Viscosi.~y ~7 18 17 20 18 19
~cP) , . .
Yield Strength(kg/ 88.2 78.4 102.9 98.0 tO2.9 102.?
100m2)(lb/100ft2) 18 16 21 20 21 21
Initial Gel(kg/lOOm ) 73.5 73.5 68.6 83.3 93.1 83.3
(lb/100ft2) lS 15 14 17 19 17
Final Gel(kg/10Gm2~ 191.1 171.5 196.0 225.4 210.7 186.2
(lb/100 ft ) 39 35 90 46 43 38
~PI Filtrate(ml) - 3.9 - 3~7 - 3.6
~5~tjs
- 25
EXAMPLE 7
. _
In this Example, the influence of the prod-
uct of the present invention on the rheoloyical ana
filtering properties on a fresh water based, lignine-
sulfonate-containing mud is studied. Results are sUTn-
marized in Table VII.
TABLE VII
a) MUD COMPOSITION
. _
ADDITIVE CONCENTRATION/UNIT
. . _ . _ _ . . . _ . .
Industrial Water 1 liter
. _
Activated Clay42.75 kg~m3(15 lb/bbl)
Fe and Cr Lignine-Sulfonate 2.85 kg/m3( 1.0 lb/bbl)
NaOH 1.42 kg/m3(0.5 lb/bbl)
Pre-gelled Starch34.2 kg/m3t12 lb/bbl)
Bactericide2.0 kg/m3(0.7 lb/bbl)
Case A-Product of the-. -
Invention
Case B-Product of the57.0kg/m3(20 lb/bbl~
Invention
Case C-Product of the114 kg/m3 (40 lb/bbl)
Invention
. ~ .
~X~56~5
- 26
b) RHEOLOGICAL AND FILTERING PROPERTIES OF ~UD
-
Case A Case B _a!;e C
Init~al ~ged Initial Aged Initial
A~ent Viscosity 24 27 24.8 28 2.6 31
(cP)
Plastic Visoosity 17 18 17 19 ~9 ~0
~cP)
Yield Strength(kg/ 68.6 14.7 73.5 38.2 68.6 107.
100m2)(lb/100ft2) 14 1~ 15 18 14 22
Initial Gel(kg/lOOm2) 14.7 1~.7 9.8 14.7 14.7 19.6
tlb/100 ft ~ 3 2 3 3 3 4
Final Gel(kg/100m2) 44.1 93.1 39.2 73.5 39.~ 78.4
(lb/100 ft ) 9 19 8 15 ~ ~
API Filtrate(ml - 3.~ - 3.0- 3.0
~ It is thus seen that the product accordin~
3 : to the present invention~ when added to other common-
,~ , .
ly used drilling mud additives, such as baLyte, bento-
nite and lignine-sulfonates, does not change the rheo-
logical and filterin~ properties of the muds, although
the amount of additive reaches quite high levels,such
as 114 kg/m (40 lb/bbl).
Accordingly, the sulfide sequestering ad-
ditive prepared in accordance wi~h the present invent-
ion
.'
~LX~;5~;S
- 27
is an efficient and quick absorbent of the sulfides
present in underground formations, without changing
the rheological and filtering properties of the mud
to which it is added. Its simple and low cost preparat-
ion renders it ideal for the proposed applications.