Note: Descriptions are shown in the official language in which they were submitted.
~2~
The present invention is related to a method and apparatus
for recovering heat from gase containing vaporized, ~olten and
eutectic components by bringing it into contact with the heat
transfer surfaces of a heat exchanger.
The process industry produces great amounts of hot gases. The re-
covery of heat from these gases is rendered difficult by the vaporized or
lten components in the gases contaminating heat transfer surfaces. A
typical example of this are the waste gases of the pyrcmetallurgical
industry. The cleaning of the heat transfer surfaces by means of the
methods available at the mcment is in most cases extremely difficult,
which leads to diminished usability of the plant and therefore also to
considerable costs.
~ xperience has shown that the cleaning problems and most severe in
that temperature range, where part of the solid compounds are in a
eutectic state. As an example in non-ferrous metallurgic
foundry processes small concentrations oF Zn, As and Pb are enough to
cause the eutectic behaviour of the entire dust. Dust in a eutectic state
catches on the heat transfer surfaces and especially when crystallizing
forms a dirt layer, the removal of which by means of the known cleaning
methods (blowing or mechanical sweepers) is in some cases impossible.
Field research has shown ~hat the kest endurance values are obtained in
such steam boilers m which, due to the nature of the process, there has
been natural erosion of the layers. It has also been possible to judge
from the form of the dirt layers that even effective blc~ers or mechanical
2 ~ 7~
sweepers cannot considerably affect the dirt layers. On the other
hand, erosion has kept the heat transfer surfaces parallel to the
flow direction fairly clean.
It is an object of the present invention to further advance
the efficiency of heat recovery from hot gases by further
reducing the rate of contamination of the heat transfer surfaces.
In general terms and in one aspect of the invention, a
method of recovering heat from gas containing moltPn components,
said method comprising the steps of:
(a) directing hot gases having temperature in the range from
about 800 C to about 1500-C from a furnace in a generally
vertical direction;
(b) reducing the temperature of the hot gases to a
temperature below the eutectic range of molten components
contained in said hot gases b~ mixing into said hot gases
recirculated solid particles;
(c) directing the mixture of the solid particles and of the
hot gases having said reduced temperature to a heat exchanger and
in the heat exchanger further reducing the temperature of said
gases;
(d) the velocity of the gases passing through the heat
exchanger being within the range of about 3 m/s (meters per
second) to about 20 m/s and being selected, with respect to the
volume of particles added to the hot gases upstream of the heat
exahanger, such as to assure a generally pneumatic passage of the
particles through the heat exchanger.
The invention also provides apparatus for reco~ering heat
from gas containing molten aomponents, said apparatus
compr.ising:
1~
, . ; ',, '
": ~ '''
2a ~ 2~i~7~
(a) generally vertical conduit means for directing hot
gases having temperature in the range of about 800 C to about
1500-C from a furnace;
(b) said conduit means having an upper end portion
communicating with a downstream end of a pre-cooling chamber
disposed above the conduit means, which pre-cooling chamber
further communicates with a discharge end of a feeding device of
recirculated paxticulate material for admixing the particulate
material into said pre-cooling chamber in amount sufficient to
bring the temperature of the hot gases within the pre-cooling
chamber to a temperature below the eutectic range of molten
components contained in the respective hot gases;
(c) said pre-cooling chamber having a downstream end
portion which merges with a heat exchanger chamber provided with
heat exchange medium passage means whose heat transmitting
surface means are exposed to the flow of a mixture of the pre-
cooled hot gases and the particulate matærial;
(d) gas velocity control means adapted to generate and
maintain the velocity of said gases in the heat exchanger
chamber at a rate within the range o~ 3 m/s to 20 m/s, said rate
being sufficient to assure a generally pneumatic passage of the
particles of the particulate material through the heat exchanger
chamber.
The cross-sectional area of the pre-cooling chamber taken
across the flow of said hot gases may be generally equal to that
of the heat exchanger chamber, whereby the velocity of gas flow
ln both chambers is generally the same.
The term "pneumatic passage" with reference ta the
particles means that the velocity o~ gases is selected, with
1[~
2b
respect to the size and amount of the particles, to be
sufficiently high to assure that the particles are carried along
at generally the same speed as the velocity of the gas flow.
Experiments show that the speed within the mentioned range of 3
to 30 ~eters per second provides satisfactory results in
practical applications.
The term "eutectic`' has the common meaning as set forth, for
instance, in Webster~s New World Dictionary, Second College
Edition, 1970, at page 484: "designating or of a mixture or alloy
with a melting point lower than that of any other combination of
the same components.
The drawing shows an embodiment according to the invention
for heat recovery.
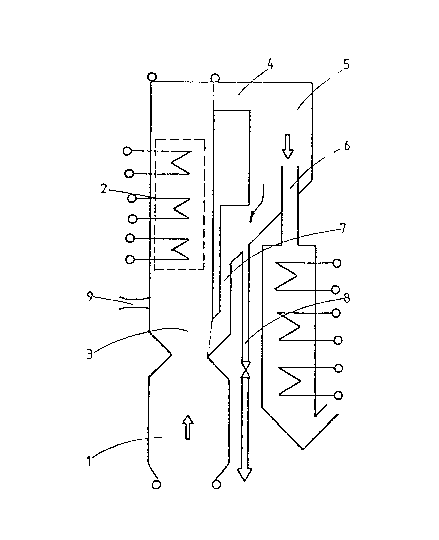
In the apparatus shown in the figure, hot gas containing
vaporized and molten components flow through a channel 1 provided
with radiation surfaces. When approaching a heat exchanger 2, the
temperature of the gas is near the upper limit of the eutectic
range. The temperature downstream of the heat exchanger is chosen
to be sufficiently below the eutectic temperature range so that
the dust contained by the gas in pul~erous by nature and thus
does not catch on the heat transfer ~urfaces. The sweeping
eEfect re~uired for keeping the heat transfer surfaces clean is
acquired when the sweeping dust is concentrated in the heat
exchanger so much that when mixing with the dusty ~as entering
the heat exchanger in point 3, the temperature of the mixture
drops near the limit of the eutectio range.
After the m.ixing and the droppin~ of the temperature that occurred
D
'7~
in point 3, the suspension containing a sufficient amount of abrading
particles flows through the heat exchanger 2 and thus by erosion prevents
the forming of dirt layers on the heat transfer surfa oe s.
After the heat exchanger 2, the suspension has cooled below the
eutectic range and is led tangentially through a channel 4 to a flow-
through cyclone 5 from which the gases containing essentially no dust
are discharged through a central pipe 6 and the
separated solid material is returned through a pipe 7 to the gas flow to
point 3 of the channel, upstream of the heat exchanger. An outlet 8 is
disposed in the return pipe 7 for the circulating solid material. Thus the
circulating solid material flow and the erosion effect can be regulated.
The circulating material is preferably the solid material used m the
process or some other inexpensive material, such as sand, which is added
to t~,e process through a pipe 9.
m e following advantages are obtained by means of the method accord-
ing to the invention:
1. The heat transfer surfaces are kept clean by means of a controlled
erosion effect.
2. By mixing, the temperature can be rapidly dropped.
3. A so called dry washing effect is obtained, as the circulating
solid particles condensate the co~pounds that have come to their
surface in the vapo~ phase.
4. The amount of sulphur emissions can be decreased by arrang mg
e.g. a Ca-based circulating material
5. The radi~tlon and convection heat exchan~e ar~ activa-ted.
. . . : .
~Q~G~784
The operation ranges of the method according to the invention are
the following:
Gas velocity 3 to 20 m/s
Particle content in gas 10 to 500 g/mol
Temperature of incoming gas 800 to lS00 &
Temperature of outgoing gas S00 to 1200 C
Mean diameter of particles 100 to 2000 ~m
Example 1:
The values of the offgases of a Cu-smeltery after the smelting
furnace are:
gas flow mol/s 1740
dust content g/mol 2.7
temperature C 1400
The offgases are cooled by radiatlon cooling in the channel 1 to
about 900 &, whereby a temperature range having difficult prcperties as
regards the contamination of the heat transfer surfaces is arrived at. The
thermal capacity of the dusty offgas is about 1~7 kJ/(Nm3 C) = 38 J(molC)
i.e. the thermal capacity flow is 66.1 kW/C. A preferable te~perature
before the heat transfer surfaces of the heat exchanaer 2 is 700 & and
after the heat transfer surfaces 550 &. Thus the circulating thermal
capacity flow is 88.1 k~^1/C. The specific thermal capacity of the circula-
ting material can be estimated to be about 0.8 kJ/(kgC), which gives a
value of 110 kg/s for the circulating mass flow. Thus after the mixing
the solid m~tter content of the gas is 63 g/mol (= 2.81 kg/Nm3~. In
practise solid n~atter content~ of 900 to 1~00 g/mol have been used in a so
called cir~ulating b~d reactor. A conc~ntrat~, fiand or a mixture of them
i7~
can be used as the circulating material in a smeltery. Furthennore,
particles included in the offgases are concentrated in the cooling
circulationO
Example 2
The black liquor flow of a soda boiler is 5.6 kg/s and its dry
matter content 0.60. A typical dry matter analysis is as follows:
C 35.5 % (of pulp)
Na 20.8 %
S 5.2 %
O3501 %
H 3.4 %
In case the combustion is carried out in a normal soda boiler, ca.
30 % of the sulphur feed and 10 % of the sodium feed follow the flue
gases from the furnace partly as gaseous compounds and partly as small
molten components. In case the combustion is carried out in a separate
cambustion chamber, the flue gases ma~ contain even 50 ~ of the sulphur
and 30 % of the sodium after the co~bustion zone. When the flue gases
get cooler, the inorganic chemicals form mostly sodium sul~ate and sodium
carbonate as well as sulphur dioxide. Depending on the com~osition of the
liquor and on the running circumstances, this may in some cases lead to
the formation of a difficult sodiumpyrosulfate layer on the heat transfer
surfaces.
The offg~s values in the ~ibove ccmbustion chamber are:
gas flow mol/s ~40
N~-~low molts 4.$6
S-fl~wmol/s 2.75
temperature C 900
dust (cond.~ g/mol 0.23 (10.3 g/~m3)
Thermal capacity flcw of the gas 29.4 kl~/ C
Gas tem~eratures:
upstream of the exchanger 870 C
after mixing 700 &
downstream of the exchanger 550 &
Circulating thermal capacity flow 33.0 kW/C
Circulating mass flow (0.8 kJ/kg&~ 41.7 kg/s
~ust content of gas in the exchanger 50.0 glmol
The circulation flcw comprises the Na2003-based dust of the flue
gases and the Na2C03 or Na2$04 added to point 3.
Those skilled tn the art will appreciate that many mcdifications
of the method and apparatus of the invention can be carried out within
the context of the present invention. Accordingly, we wish to secure
by letters patent which may issue on this application all such
embodiments as properly fall within the scope of our contribution to
the art.
:: .