Note: Descriptions are shown in the official language in which they were submitted.
7 ~
METHOD OF RECOVERIMG COPPER AND ZrNC CONCENTRATES
FRO~ COMPLEX SULFIDE ORES BY DIFFERENTIAL FLOTATION
The present invention relates to a method of
recovering copper and zinc concentrates separately from
complex sulfide ores containing sulfides of copper, zinc,
iron and other minerals by differential flotation. More
particularly, the invention relates to an improved
differential flotation technique wherein copper sulfide
minerals are caused to float while selectively depress-
in~ ~inc sulfide mineeals and iron sulfide minerals.
BRIEF DESCRI~'TION OF THE DRAWINGS
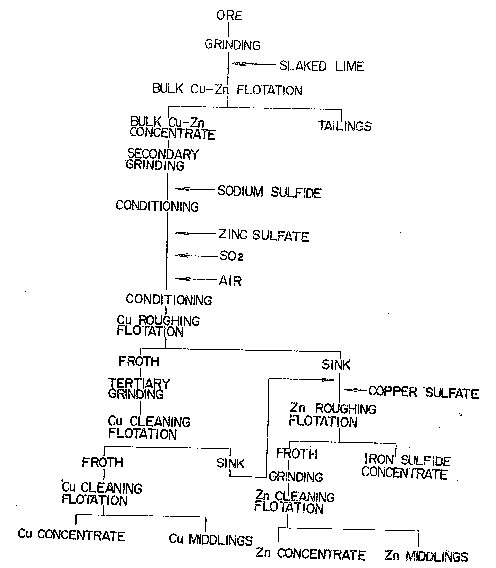
Fig. 1 is a flowsheet for the process of benefici-
ation of complex sulfide ores by flotation in accordance
with the prior art technique;
Fig. 2 is a flowsheet for the beneficiation of
complex sulfide ores by flotation in accordance with one
embodiment of the method of the present invention; and
Fig. 3 is a flowsheet for the beneficiation of
complex sulfide ores by flotation in accordance with
another embodiment of the method of the present invention.
BACKGROUND OF THE INVENTION
It is known to use the differential flotation
method for recovering copper and zinc concentrates
separately from complex sulfide ores containing sulfides
of copper, zinc, iron and other minerals~ In the
differential flotation method, copper sul~ide minerals
in the complex sulfide ores are caused to float whereas
'.~ - 1 -
~ 7~
zinc sul~ide and iron sulfide minerals are selectively
depressed. Two techniques are conventionally used to
realize the di~erential flotation method; in one techni-
que, the minerals pulp is conditioned with lime or other
oH conditioners and subsequently treated with sodium
cyanide and zinc sul~ate, and the other technique which
is shown in Japanese Patent Publication No. 15310/1962 is
characterized by using sodium sulEide and sulfur dioxide
in combination.
The first technique using sodium cyanide and zinc
sulfate does not achieve high efficiency in the selective
depression of zinc sulfide and iron sulfide minerals, and
is practically inefEective if the complex sulfide ore to
be dressed contains secondary copper minerals such as
bornite and chalcocite. The second technique using sodium
sulfide and sulfur dioxide in combination depresses zinc
sulfide and iron sulfide minerals by the same degree, and
in the subsequent step oE zinc activation, part of the
iron sulfide minerals is also activated to produce a zinc
concentrate of low grade. In order to avoid this problem,
the second technique is conventionally performed by the
procedure shown in the flowsheet of Fig~ 1. The ground
ore is stripped of gangue minerals by bulk flotation,
and the iron sulfide in the resulting bulk copper-zinc
concentrate is removed prior to separating copper from
zinc. The results oE ~lotation performed in this
conventional method are summarized in Table 1.
-- 2 ~
i5~7~
o u~ ~ U~ ~ ~ ~ ~r ~
~o o a~ co co ~ co o
C: ........
o ~ o CO ~ ~ o ~ U~
r~ ~ O ~ ~D
o ~ ~ I~ ~ ~ oo ~ ~r
dP O Lr) a~ o ~ ~ a~
~n ........
o U~
C~
o o~ oIn 1
~ ~r ~ ~ o
~ . .. ~ . . .. .
~ u~ ~ o~r ~~ o o
Tl~ ~ NLO
U~
U~
0~ O ~ ~ ~ ~ ~ ~ ~ C~
~O ~ CO ~O ~ ~ ~ ~ O
~ ........ .
o ~ o o
. . . . . . . .
~rl d~ O ~ ~ ~ O ~ ~D ~r co
a~ o ~ ~)
~I
_
a
~I r~ al
O ~ C~ = = O
., a) oa) o
O a~
o
h ~)4~ ~
M
O~r~Orl ~
tl) r~ rl Orl
O ~ HC~
S~6
As the data in Table 1 reveal, even if the froth
obtained in the copper-zinc roughing flotation is
ade~uately cleaned prior -to Cu-Zn separation as shown in
Fig. 1, sinks with high proportions of valuable consti-
tuents are removed as tailings from the Cu-Zn cleaning.
Since these tailings have high distributions of
copper and zinc, the process depicted in Fig. 1 suffers
an appreciable loss in the amount of minerals recoverable.
SU~MARY OF TH~ INVENTION
The principal object of the present invention is
to eliminate the problems shown above and provide a
`~eneficiation method capable of effective recovery of
separate co-æper and zinc concentrales from complex sulfide
ores containing secondary copper minerals such as bornite
and chalcocite. In`the method of the present invention,
iron sulfide minerals are depressed by a greater degree
than zinc minerals so that in the subse~uent step of zinc
activation only the zinc minerals are activated while the
iron sulfide minerals are not readily activated.
By so doing, the need to incorporate the step of reducing
the content of iron sulfide minerals in the bulk copper-zinc
concentrate prior to copper-zinc separation is eliminated.
As a consequence, the overall process of beneficiation is
simplified while, at the same time, the loss of valuable
copper and zinc components is held to a minimum and t~e
~5~
grade of the zinc concentrate obtained in the subsequent
step is appreciably increased.
The stated object of the present invention can
be achieved by a method of recovering copper and zinc
concentrates separately from complex sulfide ores
containing sulfides of copper, zinc, iron and other
minerals by difEerential flotation, ~aid method com-
prising adjusting to 45 - 80 wt% the pulp density in a
mineral pulp composed of water and as-ground feed ores
or a mineral pulp of the bulk concentrate of copper and
zinc sulfide minerals obtained as a froth in the bulk
diEferential flotation of the copper and zinc minerals
present in the first mineral pulp; conditioning either
mineral pulp in the presence of sodium sulfide in an
amount of 0.2 - 3 kg per ton of the ore; further
conditioning the mineral pulp in the presence of zinc
sulfate in an amount 1.5 - 3.5 times the weight of the
sodium sulfide and 1.5 - 10 kg of sulfur dioxide per
ton of the ore, while blowing at least 50 m3 of air
per ton oE the ore; and subsequently adding collectors,
frothers and any other flotation reagents so as to
~j5~ 7~i
separately recover copper and %inc concentrates by
~lotation .
DE_AILED DESCRIPTION OF THE INVENTION____ _ ____ ___ _ _ _
The method of the present invention may be
directly applied to ground complex sulfide ores contain-
ing sulfides of copper, zinc, iron and other minerals.
The copper sulfide mineral is obtained as a froth whereas
the ~inc sulfide mineral, iron sulfide mineral and gangue
minerals remain in the pulp as a sink. The froth and the
sink are respectively cleaned to recover copper and zinc
concentrates. Alternatively, the ground ore feed is first
subjected to bulk differential flotation wherein the
gangue minerals are discarded as a sink and the copper
and ~inc minerals and part of iron sulfide minerals is
recovered as a froth and subsequently treated by the
method of the present invention.
For the purpose of the present invention, it is
necessary that a mineral pulp of the as-ground feed ores or
a mineral pulp of the bulk concentrate obtained by subject-
ing the ~irst mineral pulp to bulk differential flotation
be adjusted to have a pulp density of 45 - 80 wt~. If the
pulp density in either mineral pulp is less than 45 wt~,
"~
S~37~
the reagents used in the subsequent conditioning step are
unable -to exhibit their intended effects. If, on the
other hand, the pulp density is more than 80 w-t%, the
pulp is unable to maintain -the normal suspension of mineral
particles during the conditioning s-tages.
The amount of sodium sulfide that should be added
in the first conditioning step varies with the mineralogical
composition of the ore feed and the dgree of oxidation of
the ore. The higher the proportion of secondary copper
minerals such as bornite and the greater the extent of
oxidation of the feed ore, the greater the amount of
sodium sulfide which should be added, i.e., 0.2 - 3 kg
per ton of the ore.
After the addition of sodium sulfide, the pulp is
conditioned for at least 5 minutes so as to achieve
satisfactory contact with the mineral particles.
~inc sulfate is then added in an amount 1.5 to 3.5 -times
the weight of the previously added sodium sulfide.
If the amount of zinc sulfate added is less than ~.5 times
the wei~ht of sodium sulfide, the zinc sulfide mineral is
not effectively depressed, and no corresponding increase
in the depressing effect is achieved even if more than 3.5
times the weight of sodium sulfide of zinc sulfate is used.
Sulfur dioxide is added simultaneously with zinc sulfate.
~ 7~;
The sulfuI div~ide may be added irl the gaseouC
st~te or in the state of aqueous solution. ~he ~nount of
the sulfur dio~ide to be added varies with the ~ineralogical
composition of the ore feed and otheI factors, but it should
be added in an amount ranging from 1.5 to 10 kg per ton of
the ore. If less than 1.5 kg of sulfur dioY~ide is added,
the zinc sulfide mineral cannot be effectively depressed
and if more than 10 kg of sulfur dioxide is added, not
only the zinc sulfide mineral but also the copper sulfide
mineral is depressed. ~he second conditioning step must
be carried out under air blowing. ~he amount of air to be
blown varies with the amounts of sodium sulfide and zinc
sulfate used, but at least 50 m3 of air must be blown per
ton of the ore. Blowing more than 200 m~ of air does not
yield a commensurate improvement in the effe-ct of condi-
tioning the pulp. ~he duration of the conditioning step
is governed by the volume of air being blown and must be
long enough to ensure the supply of the necessary volume
of air.
After conditioning the mineral pulp under air
blowing, conventional flotation reagents such as pH
modifiers, collectors and frothers are added so as to
perform differential flotation wherein the copper sulfide
mineral is caused to float while depressing the zinc
sulfide and iron sulfide minerals. Both the froth and
-- 8 --
5876
sink are sub~ected to scavenging flotation, cle~ling
lotation and any other necessary treatments, so as to
recover the desired copper and zinc concentrates.
The present invention is hereunder descri~ed in
greater detail by re~erence to the following ex~lples
which are given here for illustrative purposes only and
are by no means intended to limit the scope of the invention.
Example 1
A sample of the complex sulfide ores taken at
mine A, Canada having the chemical assays listed below was
processed in accordance with one embodiment of the present
invention by the procedure shown in the flowsheet in Fig. 2.
Ore composiion (wt%):
Cu Zn Pb S ~e SiO2 A1203 CaO MgO
1.66 2.23 0.06 31.26 27.21 12.8 3.75 1.78 3.85
About half of the copper present in the ore is
comprised of bornite and chalcocite and it is ~mpossible
to depress the zinc minerals by a conventional differential
~lotation technique using sodium cyanide and zinc sulfate.
rthermore, as already shown in ~able 1, another convention-
al techni~ue using sodium sulfide and sulfur dioxide produces
a copper concentrate (23.42% Cu) at a recovery-of 52.57%
and a zinc concentrate (50.35% Zn) at a recovery of 62.76%.
~herefore, the recoveries of copper and zinc and the grade
:
7~
of the fin~l zinc concentrate are very lo~J. l~s i5
su~ested by -these dat~, the comple~ sulfi~e ores having
the composition shown above have been considered fairly
difficult to dress in cor~mercial operation.
In this EY~ample, the sample ore was treated
il~mediately by the differential flotation method. ~irst,
the ore was subjected to the primary grinding step wherein
it was wet-ground in a ball mill to -44 ~m 93%. The ground
ore was concentrated to produce a mineral pulp having a
pulp density of 60 wt%. The pulp was miY~ ed with 0.65 k~
of sodium sulfide per ton of the ore and conditioned for
10 minutes. The pulp was conditioned for another 10 minutes
under air blowing (10 m3/min. t) while 1.1 kg of zinc sulfate
(about 1.7 times the weight of the sodium sulfide) per ton
of the ore and an aqueous solution of 4.2 kg of sulfur
dioxide per ton of the ore were added. Thereafter, the
pulp was adjusted to a pH of 6.5 with slaked lime and
subjected to copper flotation for 20 minutes in the
presence of a collector (ethylisopropyl thionocarbamate
available from The Dow Chemical Company, U.S.A. under the
trademark Z-200) and a frother (methylisobutylcarbinol
which is hereunder referred to as MIBC). The froth was
subjected to the secondary grinding and cleaned to produce
a copper concentrate. The sink in the copper flotation and
part of the sink in the cleaning flotation were treated
_ 10 -
:
.~
lZ~513~
with copper sulfate in order to activate and float the
zinc minerals. The froth was cleane~ to produce a zinc
concentrate, and the sink was discarded as a tailing.
The results of the above beneficiation process
are summari2ed in Table 2.
Table 2
Weight Assays Distribution
Product % Cu, % Zn, % Cu, % Zn, %
Ore 100.00 1.66 2.23 100.00 100.00
Cu concentrate 5.53 24.76 5.74 82.71 14.22
Cu mlddlings0.24 3.27 4.65 0.47 0.50
zn concentrate 3.03 1.76 ~6.84 3.27 78.43
2n miadlings3~98 1.59 1.62 3.82 2.89
Tailings 87.17 0.18 0.10 9.73 3.96
As l'able 2 shows, the method of the present invention
pèrformed in accordance with the flowsheet in Fig. 2 produced
a copper concentrate having a Cll content of 24.76% and a Cu
recovery of 82.71%, as well as a zinc concentrate having a
Zn content of 56.~4~ and a Zn recovery of 78.43%.
In comparison with the data shown in Table 1 that were
obtained by the process outlined in the flowsheet of Fig. 1,
the method of the present invention produced Cu and Zn
concentrates of higher grade, with an appreciable increase
in their recovery.
~ 1.1 -
~ .
~ ~5 ~'7
Example 2
A sample of the complex sulfide ores taken at
mine A, Cc~lada having substantially the s~me chemical
ass~ys as shown in Example 1 was first subjected to the
primary grinding, wherein i-t was wet-ground to -74 ~m 75%.
After pH adjustment to 12 with slaked lime, the ground ore
was subjected to differential flotation in the presence of
a collector (ethylisopropyl thionocarbamate) and a frother
~MI~3C). The bulk concentrate made of the copper and zinc
s~l~ide minerals and part of the iron sulfide mineral was
recovered as a ~roth, whereas the remaining iron sulfide
and gangue minerals were discarded as a sink. The bulk
concentrate was subsequentl-~ treated by the method of the
present invention outlind in the flowsheet in ~ig. 3.
The bulk concentrate was subjected to the secondary
grinding step wherein it was ground to -44 ~m 80%~ and the
ground concentrate was thickened to form a mineral pulp
having a pulp density of 60 wt% The pulp was mixed with
1.0 kg of sodium sulfide per ton of the bulk concentrate
~d the mi~ture was conditioned for 10 minutes. ~he pulp
was conditioned for an additional 10 minutes under air
blowing (10 m3/min. t) while 1.7 kg of zinc sulfate
(1.7 times the weight of the sodium sulfide) per ton of
the bulk concentrate and an aqueous solution of 5.8 kg of
sulfur dioxide per ton of the bulk concentrate were
_ ~2 -
ILZ~S87bi
added After adjustment to a pH of 6.5
with slaked lime, the pulp was subjected to copper flotation
for 20 minutes in the presence of a collector (z-200) and a
frother (~lIBC). The froth was subjected to the tertiary
grinding and cleaned to obtain a copper concentrate.
The sink in the copper flotation and part of the sink in
the cleanin~ flotation were treated with cop~er sulfate
to activate and float the zinc minerals, which were cleaned
to produce a zinc concentrate. The sink was comprised of
an iron sulfide concentrate.
The results of the beneficiation so performed are
summarized in Table 3.
Ta.ble 3
Weight Assays Distribution
Product % Cu, ~ Zn, %Cu, %Zn, %
Ore 100.00 1.65 2.28100.00100.00
Cu concentrate 5.62 24.70 3.43 84.13 8.46
Cu middlings0.15 2.93 11.900~27 0.78
Zn concentrate 3.12 1.82 60.14 3.44 82.34
Zn middlings3.31 1.65 1.993.31 2.~9
Iron sulfide28.08 0.35 305.96 3.69
concentrate
Tailings 59.72 Ø08 0.072.89 1.84
- 13 -
As the data in Table 3 show, the ~etho~ of benefi-
ciation in accordance with the flowsheet in Fig. 3 produced
a copper concentrate having a Cu content oI 24.70% and a Cu
recovery of about ~4%, as well as a zinc concentra-te having
a ~n content of 60.14% and a ~n recovery of abou-t 82.~4%.
In comparison wi-th the results shown in ~able 1 -that were
obtained by the conventional techni~ue outlined in Fig. 1,
the method of the present invention produced copper and
~inc concentrates of higher grade, with a signiicant
increase in their recoveries.
As wiIl be apparent from the foregoing description
the present invention provides a significant improvement
in the beneficiation of complex sulfide ores and offers
great economical advan-tages in that it produces copper and
~inc concentrates of higher grades and achieves an
appreriable increase in the recoveries of the respective
minerals.
- 14 -