Note: Descriptions are shown in the official language in which they were submitted.
~26i666Z
TECHNICAL FIELD
This invention relates to a novel radioactive 2-
iodospiroperidol and a process for preparing the same.
The compound of this invention is a novel compound
not disclosed in any literature. This compound has a
; strong affinity for dopamine receptor and is very useful as
a nuclear medical diagnostic agent of dopamine receptor and
as a radioactive medicine~
~.
BACRGROUND ART
It has recently been found that in the pathological
state of brain (such as Parkinson's disease or schizophrenia)~
the amount of dopamine receptor is different from that
in~ the normal person, and attention has become directed
to the relation between dopamine receptor and various
: :
kinds of brain diseases in the fields of medicine and
pharmacology. Under these circumstances, the advent of
,,
a nuclear medical diagnostic agent and a radioactive
mediclne targeting dopamine receptor has been strongly
` desired.
20 DlSCLOSllR3~ OF IN~ENTION ~
The present inventors~have made extenslve studies
alml~ng at obtaLning~a~dopamine receptor-tropic diagnostic
;agent and ~a radioactive medicine having radloactive iodine
: :: ,,: ,,, j ;;;.. :; : :,, ,. , :
, ",:
:~26~
1 in its molecule, and have found that the novel iodine
compound represented by the formula (I) shown below has a
strong affinity for dopamine receptor and can be specifical-
ly combined therewith.
5According to this invention, there is provided
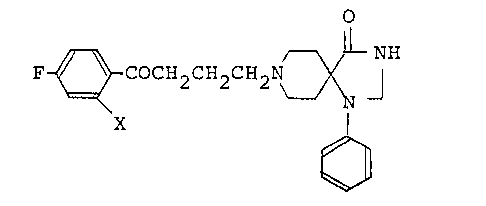
a novel radioactive 2-iodospiroperidol represented by the
formula (I):
F ~ COCH2CH2CH2N ~ ¦ (I)
X
.~ ~
wherein X represents a radioactive iodine atom, and a process
for preparing the same. The compound (I) of this invention
has a much stronger affinity for dopamine receptor than
p-iodospiroperidol which is a known iodine compound dis-
closed in llterature [J. Nuelear Medieine, 23(5), 100 (1982~],
and possesses very exeellent properties as a dopamine
reeeptor-tropie radioactive diagnostic agent or radioactive
;15 medieine.
Therefore, proper application of the eompound (I~ -
:
~ of th1s invention makes it possible not only to non-invasive-
:
ly detect the presenee of dopamine receptor in the brain
and other internal organs and tissues of human beings and
animals but also to dynamically measure the change of amount
~ of the reeeptor, and thus, this compound is greatly useful
; _ 2 _
" ,
., , , . . ,~ ,
:. : . : :. ,:
: ~ ~ .: ... :: , . ..
1~66~
1 for the nuclear medical diagnosis and treatment of brain
troubles and other diseases. Also, in view of the relevancy
of dopamine receptor to cancers such as breast cancer and
the like, the compound is also useful in application to this
field.
BEST MODE FOR CARRYING OUT THE INVENTION
The method for the preparation of the compound of
this invention will be described below.
The compound of this invention represented by the
above-mentioned formula (I) can be produced by a conven-
tional method for the synthesis of radioactive iodine
compounds. For instance, it can be produced according to
either Process A or Process B shown below.
, ~
., ,
~; [Process A]
:
~ 15 ~ An amino compound represented by the formula ~
O
F ~ COC~Cd2CI~ ~ (II)
NH2
:,~, : : , ,
is~reacted with~an alkali metal salt of nitrous acid in a
suitable~solvent~such~as tetrahydrofursn, dioxsne, sceton-
itr~ile~or~;the like ln the presence of~an acld such as diluted
sulfuric acid or~diluted hydrochloric acid to form a
20 ~dlazonium salt represented by the formula lIII):
:. , ~, :
~26~
~ H
F ~ -COCH2CH2CH2N ~ ~ (III)
I
~-N
z
1 wherein Z represents an anion represented by a halogen
ion or the formula HSO4 .
Then the compound (III~ is reacted with a radio-
:~: active iodine metal salt to obtain the compound of the
; .5 formula (I). The above reactions are carried out at a
temperature in a range~ of -5 to 30C.
,
The compound (I) obtained can be purified by a
: conventional method:such as thin layer chromatography ITLC)
: or high-performance liquid chromatography ~HPLC).
10~ [Procesa B]
halogeno compound represented by the~formula
2~cH2cH2 ~ ~ ~ (IV~
., ~ , , ;, ~,,, ~; ~; . , , - ~
wherein~Y:~:represents~a halogen atom is subjected~to~an ex
hange~re~ct on with a~radio~c~l~e l-d.ne netal;salt ln
-
~Z6~
1 ~ suitable solvent such as acetonitrile, dimethylformamide,
ethylene glycol, an ether derivative of ethylene glycol, an
ether derivative of diethylene glycol, water or the like at
a temperature of 50 to 180C. The compound (I) obtained
can be purified by a conventional method such as TLC or
HPLC.
In the process of this invention, for example,
I-123, I-125, I-131, I-132, etc. are exemplified as the
ra~ioactive iodine atom, and I-123, I-125 and I-131 are
preferred. The radioactive iodine metal salt means a metal
salt of the above radioactive iodine, and may be any of those
capable of providing a radioactive I ion, though alkali
metal salts such as, for example, sodium iodide, potassium
- iodide and lithium iodide are preferred. As the halogen ion
in the formula (III), anions of chlorine, bromine, iodine
and the like are exemplified, and as the halogen atom in
the formula (IV), there can be exemplified, for example,
fluorine, ch1orine, bromine and iodine atoms.
By intravenously injecting the radioactive 2-iodo-
spiroperidol (I) obtained by this invention into patients
and taklng a sclntigram with the lapse of time, or by measur-
lng the radioactivity b~ the probe~method and determining the
intake o~ the~compound (I) in a speciflc organ, it is
possible to simply and appropriately determine the site and
25~ scope of the~focus and the degree~of affection.
,
The radioactive 2-iodospiroperidol according to
thi~s invention is thus useful for the diagnosis of the brain
dlseases, breast cancer and the like, but this compound is
5 -
~: : :
, ~ . , ,
- `
1 also useful for the diagnosls and treatment of other
various diseases resulting from a change of dopamine receptor.
The present invention will further be specifically
described below referring to Examples.
S REFERENTIAL EXAMPLE
Preparation of 8-[4-(4-fluoro-2-iodophenyl)-4-oxobutyl]-
l-phenyl-1,3,8-triazaspiro[4,5]decane-4-one(2-iodo-
spiroperidol~
2-Aminospiroperidol (410 mg) was suspended in 2 N
hydrochloric acid and acetonitrile, and to the resulting
suspension was added dropwise an aqueous solution of sodium
nitrite (95 mg) with ice-cooling. The resulting mixture was
stirred at a temperature of 5C or less for 30 minutes and
an aqueous solution of potassium iodide (166 mg) was added
dropwise to the diazonium salt produced with ice-cooling,
after which the mixture obtained was further stlrred at the
same temperature for 1.5 hours. After the reaction, the
react1on mixture was made alkaline and~subjected to a solvent
extraction, and the solvent was then removed by distil1ation
; 0~ to~obtain~a;crude product. This was purified by silica gel
column chromatography to obtaLn~2-iodospiroper1do1 ~427 mg).
Melting point: 170-175C.
IR (CHC13)~ om : 1710 (C=0~
H-NMR~(CDC1~3)~ ~(ppm):~1.40-3.18 ~/14H,~m,~ methylenej,
25~ 4.72 ~2H,~s,~-~HCH2N~), 6.31-7.79
(8H, m,~ benzene r~ing H).
Mass~spectrum~ 70 eV) m/e:~521 ~M 1, 244.
6 -
i6~;~
1 EXAMPLE 1
Preparation of [ I]-8-[4-(4-fluoro-2-iodophenyl) 4
oxobutyl]-l-phenyl-1,3,8-triazaspiro[4,5]decane-4-
one([l25I]-2-iodospiroperidol)
The diazonium salt prepared by using 2-aminospiro-
peridol (40 ~gl, 2 N sulfuric acid and sodium nitrita was
reacted with Na 125I (2 mCi) with ice-cooling in the same way
as in the Referential Example, and the resulting crude product
was purified by HPLC (column: Licrosorb RP-18) to obtain
[125I]-2-iodospiroperidol (1.4 mCi). This product was
identical with the specimen obtained in the Referential Example
in Rf values of TLC and retention time of HPLC.
EXAMPLE 2
Preparation of [1 3I]-2-iodospiroperidol with Na 123I
In the same manner as in Example 1 using Na 12 I
(5 mCi), there was obtained [123I]-2-iodospiroperidol
(1.4 mCi). This product was identical with the specimen
obtained in the Referential~Example in Rf values of TLC and
: retention time of HPLC.
EXAMPLE 3
Preparation of ~125I]-2-iodospiroperidol by exchange
method
:::
Dimeth~lformamide (10 ~1) and Na I (2.2 mCi)
: were added to 2-iodospiroperidol (3 ~g) synthesized by the
: 25 method of the Referential Example, and a slight amount of
0~1 N sulfuric acid was ~urther added thereto, and
- 7 -
i:: :
': :
!
'. ~ ' ,
,~ ' ' .
:,
~6~i66~
1 the resulting mixture was heated. After the reaction, the
resulting crude product was purified by HPLC to obtain
[125I]-2-iodospiroperidol (0.9 mCi). This product was
identical with the specimen obtained in the Referential
Example in Rf values of TLC and retention time of HPLC.
' '
.
.
, ~
: . :
~ 8 -
,,, , ~
~: : : - :
~-: ~ .; . - . .