Note: Descriptions are shown in the official language in which they were submitted.
3[35
The invention relates to a novel intermediate
for use in the production of thiote-tronic acid.
It has been reported that thiotetronic acid may
po58e5~ utility as an lntermediate product for the
production of (t) thiolactomycin, which is an antibiotic
having a broad effective spectrum. Tetrahedron Letters,
Vol. 25, No. 46, pp 5243 to 52~6, (1984). From E. BenarY,
Chem. Berichte 46, 2103, ~1913), it i5 known to produce
thiotetronic acid starting from acetyl thloglycoyl
chloride through reaction thereof with sodium malonic
ester and subsequent ring closure and water treatment.
D.B. Macierewicz, Rocz. Chem. 47, 1~35, (~973), reproduced
the reaction of E. Benary and obtained thiotetronic acid
in a yield of 30.3 percent, based on the actyl~hioglycoyl
chloride used. Another possibility for syn~hesis is set
out in J.Z. Mortensen et al., Tetrahedron Letters, 2~,
3~39 (1971). Startin~ from 2,4-dibromothiophene, the
thiotetronic acid is obtained in a yield of 46.2 percent
by way of three steps as a result of reaction with butyl
lithium and t-butylperbenzoate.
However, in the case of all of the above-
identified traditional syntheses, the yields are much too
low for a technical or commercial process. Moreover, the
processes are characterized by cumbersomeness, expensive
educts and by reagents that are difficult to handle.
An object of the invention is to provide a novel
intermediate compound which can be used in the production
of thiotetronic acid by a process which is distinguished
by high yields and high purity of thiotetron1c acid,
3~ ~avorable educts and simple procedural steps.
Accordin~ly, one aspect of the invention
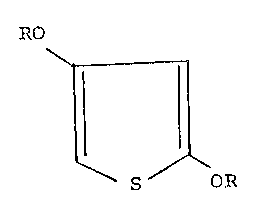
provides 2,4-diacetoxythiophene of the formula:
o
11 ' .
CH3-C-~ ~ 0
0-C-CH
~6~
Another aspect of the lnvention provides a
process for preparing 2,4-diacetoxythiophene, which
comprlses reactin~ 4-chloro-A-chloromethyloxetan-2~one
wlth hydrogen sulfide in the presence of an amine and
treating the reaction mixture with ketene.
This application is a divisional of our
copendin~ Application Serial No. 499,621, filed January
15, 1986, which describes and claims a process for
preparin~ thiotetronic acid, in which 4-chloro-4-
0 chloromethyloxetan-2-one is reacted with hydrogen sulfide
ln the presence of an amine to form thiotetronic acid
directly, or, alternatively, the reaction solution is
reacted prior to isolation of the thiotetronic acid with
ketene, the resultant diacetoxyth~ophene is separated and
the diacetoxythiophene is finally converted into
thiotetronic acid using mineral acid.
The 4-chloro-4-chloromethyloxetan-2-one startins
material can be produced in a simple manner according to
published European Patent Application 60,808 and after
~0 flash distillation can be used for the conversion
accordin~ to the invention.
The hydrogen sulfide is conveniently used in
~aseous form.
As suitable amines, advantageously primary,
secondary or tertiary amine~, ammonia and also guanidine
can be used. Tertiary amines, such as triethylamine, are
particularly ad~antageous.
The reaction is conveniently carried out in a
solvent or solvents. As compared to the reactive educt,
inert solvents such aR halo~enated hydrocarbon~, ether or
carboxylic acid esters are used. For example, methylene
chloride, chloroform, ethereal solvents, such as
tetrahydrofuran, and also acetic acid ester can be used.
Particularly preferred, however, as the solvent i~
tetrahydrofuran.
The educts are used effectively in a mole ratio
of 4-chloro-4-chloromethyloxetan-2-one to hydrog0n sulfide
~6~
to amlne of from 1:2:2 to 1:4:3, and preferably between
1:2.5:2 and 1:3.5:2.5.
Preferably the reaction i8 operated at a
temperature of from 0 to -40C, more preEerably between
-10 and -25C.
Effectively, the method proceeds in such a way
that the educt solution is sa-turated with the hydrogen
sulfide and the amine is added subsequently over a period
of 30 to 120 minutes.
After complete addition of the amine, the
processing oP the thiotetronic acid can take place by
~ tering off the precipitated salt and subsequently
concentrating the solution. For the separation of small
quantities of dimeric anhydroth~otetronic acid, the
residue can be absorbed in ethereal solvents, ~uch as
diethyl ether, tetrahydrofuran (THF) or dioxane,
preferably in diethyl ether, and can be filtered by way of
an absorption agent, such as silica ~el. After renewed
concentration by evaporation of the solvent, the
thiotetronic acid is obtained in crystalline form and in
good yield.
After recrystallization in an aromatlc
hydrocarbon, preferably in toluene, -the thiotetronic acid
is subjected to additional purification.
In order to obtain diacetoxythiophene, after the
addition of the amine, without lsolation of crude
-thiotetronic acid, the lat-ter i5 treated with solution
with ketene at a temperature of -10 to ~5C,
advantageously 0C. Based on one mole of 4-chloro-4-
chloromethyloxetan-2-one used, ketene is effectively used
in a quantity of 2 to 4 moles, preferably 2.5 to 3.5
moles.
The novel intermediate compound 2,4-
diacetoxythiophene havin~ the formula:
R~
~r :ll
~2~
wherein each R is CH3b_, results. The 2,4-
diacetoxythiophene can be purified by distillation in a
simple manner.
By treating the 2,4-diacetoxythiophene with a
non-oxidizlng inorganic mineral acid, pure thiotetronic
acid is obtained. Hydrochloric acid or sulfuric acid,
preferably hydrochloric acid, can be used in aqueous
~olution ln a concentration of 10 to 30 percent. The
conversion temperature is generally between 0 and 30C,
preferably 15 to 25C. The reaction time can be 2 to 5
hours.
After concentration, preferably using high
vacuum, this procedure provides practically colorless
thiotetronic acid in high yield with a purlty greater than
96 percent.
As used herein, all parts, percentages, ratios
20 and proportions are on a weight basis unless otherwise
stated herein or otherwise obvious herefrom to one skllled
in the art.
The following Examples illustrate the invention
or are included for reference purposes.
~5 EXAMPLE 1
15.5 g (0.091 mole) of 4-chloro-4-chloromethyl-
oxe-tan-2-one was cooled to -20C in 300 ml of
tetrahydrofurane and was saturated with gaseous hydrogen
sulfide. Subsequently, a solution of 20.2 g (0.2 mole) of
triethylamine in 100 ml of tetrahydrofuran was added
dropwise at -15C over a period of one hour. The
temperature of the reaction solution was allowed to rise
to ambient temperature, the solution was ~iltered off from
the salt obtained and the solvent was evaporated on a
rotation evaporator. The residue was filtered through a
column filled with silica gel, using 300 ml of ether as
eluent. 9.0 0 of a yellow colored crystalline product
having a melting point of 115C to 11~C was obtained.
The conten-t (HPLC~ amounted to 89.3 percent.
This corresponds to 8.0 g of 100 percent product = 75.~
percent yield. 7.5 g of the crude thiotetronic acid was
recrystallized hot from 350 ml of toluene. 6.6 g of pale
rose-colored microcrystalline product having a meltiny
point of 120C and a content (HPLC) of 96 percent was
obtained. This corresponded to 6.3 g of 100 percent
product = 94 percent yield or ~1.2 percent based on the
oxetanone used.
EXAMPLE 2
Thiotetronic acid was produced as described in
Example 1. However, the thiotetronic acid was not
isolated but the tetrahydrofuran (THF) solutlon was
concentrated to 50 ml and over a period of one hour 0.3
mole of ketene was fed into this solution at 0C.
Subsequently, the temperature of the reaction solution was
allowed to rise to ambient temperature and the solvent was
distilled off usin~ a rotation evaporator. The residue
was subjected to high vacuum distillation. 13.7 g of 2,4-
diacetoxythiophene was obtained, content 95.6 percent,
b-~-o 25 105C, which corresponded to 13.1 g of 100
percent product (yield 65.2 percent).
Spectroscopic data:
H-NMR (300 MHz, CDCL3) ~ 2.24 (s, 3H), 2.30 (s, 3H),
~5 6~5~ ~dr J=2.5 HZ, lH), 6.61 (d, lH).
MS (~OeV) m/z=200 (+, 3), 158 (M~-CH2=C=0, 10), 116 (M+-2
CH2-C=0, 35) 43 (100)
l.B1 g of the 2,4-diacetoxythiophene wa~ reacted
with 3.5 ~ of 20 percent hydrochloric acid and the mixture
~0 was stirred at ambient temperature for 3 hours. After 1
hour, a clear solution developed from the initial
emulsion. This colored solution was evaporated under high
vacuum. 1.0 y of practically colorless crystalline
product was obtained having a melting point of 119 to
121C. (HPLC content: 96.1 percent), This corresponded
to 0.96 ~ of 100 percent thiotetronic acid = 96 percent
yield.