Note: Descriptions are shown in the official language in which they were submitted.
i 2 684~j~
-?-
BACKGROU~D OF THE INVENTION
The present invention relates to free nitroxideradicals and their production process. These radicals,
which are stable in solution, are used in magnetometry
and gyrometry in weak magnetic fields, using nuclear
magnetic resonance procedures with dynamic polarization
of the nuclei of a solvent.
It is known that in earth's field magnetometers
and gyrometers using nuclear magnetic resonance, the
main problem is that of polarization, i.e. the-
forced orientation of the spins, or the angular
moment vectors of the nuclei, particularly protons
of the solvent in which the radical is dissolved in
an appropriate proportion.
This polarization can be obtained according
to French Patent 1,174,136 entitled "Improvement to
methods for measuring weak magnetic fields by
magnetic resonance", filed on April 6th 1957 in
the name of the present Applicant, by coupling the
unpaired electron of a free radical, whose electronic
paramagnetic resonance line is saturated by an
appropriate high frequency field which has nuclei
of the solvent.
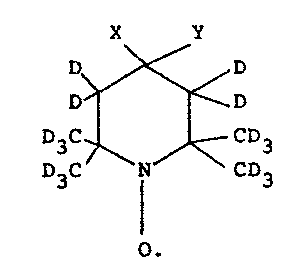
The hitherto used free nitroxide radicals
had a nitrogen 14 atom. Among these radicals are
those having the following developed formula:
- B 7028.3 LC
. . : " : .
: ,
,, , : .: ~ . :- :
- 1268464
--2--
D ~ D
D3C N CD3
In this formula, X represents D, Y represents
D or OD or X and Y represent O bonded to the cycle
of the radical by a double bond. The application to
weak field magnetometry of deuterated 2,2,6,6-
tetramethyl-4-piperidone-1-oxyl, currently called
deuterate TANO, is described in French Patent
2,063,416 entitled "Polarization of the nuclei of
a solvent by electronic pumping" filed in the name
of the present Applicant on October 15th 1969.
In a high magnetic field (3000 Gauss), the
diagram of the electronic levels of a l4N nitroxide ~ ¦
radical is such that three transitions are permitted
giving-a hyperfine structure with three resonance
lines.
In a very weakmagnetic field (below lO Gauss)
the operational range of magnetometers, only two
transitions are permitted. The resonant frequency
of these transitions saturated to bring about the
25 dynamic polarization effect of the nuclei of the ~ ~
solvent is a function of different parameters ~ ~,
~nature of the solvent, nature of;the radical).
Moreover, the phase of the nuclear slgnal obtained
by saturation of the "high line" is~in opposition to
that obtained~by saturation of the "low line". This
: ~: '
- :
.. . .
: : - ;, ~ .. . . . ...
- :, ,,.. ,-. , ' , ~:
- ~ ' - ', :
,
.
.
~268~6
--3--
effect is utilized in gyrometer and magnetometer
probes.
Such devices are described in French Patent
2,098,624, filed in the name of the present Applicant
on July 22nd 1970 and entitlecl "Nuclear magnetic
resonance magnetometer", as well as in a further
French Patent 2,213,500~ filed in the name of the
Applicant on September 20th 1972 and entitled
"Process for measuring a rotation speed and gyrometer
performing the same".
SUMMARY OF THE INVENTION
.
In order to increase the dynamic polarization
coefficient of the nuclei of a solvent, particularly
protons and consequently obtain an equal output signal
with a reduced high frequency power, the invention
envisages the replacement of nitrogen 14 by nitrogen
15 in nitroxide radicals of the type having the
developed formula:
X~ y
D ~ D
~1 I~D
D3C N CD3
O.
: 25
in which X represents D, Y represents D or OD and
in which X and Y represent 0, bonded with the cycle
of the radical by a double bond~
For simplification purposes throughout the
:'
.... ..
.: :: .
. ,,,. ~
~26846~
remainder of the text, the deuterated 2,2,6,6-
tetramethyl-4-piperidone-1-oxyl radical, nitrogen
15, according to the invention will be called
deuterated 15N TANO (TANO being the abbreviation
of triacetonamine nitroxide); the deuterated 2,276,6-
tetramethyl-piperidine-l-oxyl radical, nitrogen 15,
according to the invention will be called deuterated
15N TANANE; whilst the deuterated 2,2,6,6-tetramethyl-
4-piperidinol-1-oxyl, nitrogen 15, according to the
invention will be called deuterated 1 N TANOL.
The diagram of the electronic levels of a
nitrogen 15 nitroxide radical is such that in a
high magnetic field only two transitions are permitted,
giving a hyperfine structure with two resonance lines
with a nitrogen 15 nuclear single electron coupling
differing from the nitrogen 14 nucleus single electron
coupling.
This offers several advantages in magnetometry
and gyrometry. In particular, the ele~tronic spectrum
in the very weak field is always composed of two
lines. However, the latter are finer and of easier
saturatDn, which leads to a significant energy gain.
Moreover, through the nitrogen 15 radicals and ~lvents
this gives access to a different electronic resonant
frequency range.
The invention also relates to the process
for the preparation of the aforementioned nitroxide
radicals, as well as théir application to magnetometry
and gyrometry with nuclear magnetic resonance with
dynamic polarization of the nuclei of a solvent.
' . ' ~ :''..,,.,,'' ~ .
' ' '~ . . .
~2~4~
BRIEF DESCRIPTION OF THE DRAWINGS
The invention is described in greater
detail hereinafter relative to non-limitative
embodiments and the attached drawings, wherein show:
Fig 1 the weak field resonance lines of the deuterated
N TANO radical, i.e. the curve of the nuclear signal
S as a function of the frequency ~ (expressed in
megahertz - Fig la) and the deuterated 1 N TANO
radical (Fig lb) dissolved in methanol at a molar
concentration 10 3.
Fig ~ diagrammatically, a magnetometer probe without
a forbidden axis.
Fig 3 the high frequency power gain, i.e. the curve
of nuclear signal S as a function of the high frequency
power P(expressed in watt) for the deuterated 14N
TANO radical (Fig 3a) and the deuterated 1 N TANO
radical (Fig 3b).
DETAILED DESCRIPTION OF THE INVENTION
. . .
Fig la shows the weak field resonance lines
of the deuterated 14N TANO radical and Fig lb the
weak field resonance lines of the deuterated 15N
TANO radical, the two radicals being dissolved in
a concentration of 10 3M in methanol. On the basis
of these curves, it can be seen that the resonance
lines of deuterated N TANO are finer and better
resolved than those of deuterated 14N TANO and are
consequently more easily saturated. This leads to
a significant saturation power energy gain. In
addition, there is a frequency displacement of the
resonance lines.
:
- ,-. . -, -- . - :.:
,.. .. .
,
,
8 ~
Thus, the range of polarization frequencies
of the nuclei extends from 60 to 70 MHz for
deuterated N TANO and 55 to 65 MHz for deuterated
l~ TANO, as a function of the solvent used. Thus9
by using the "high line" of one of the two radicals
and the "low line" of the other to obtain a more
extensive "frequency crossing" range than when using
only nitrogen 14 nitroxide radicals.
The increase in the frequency crossing range
is advantageous in gyrometry, where working takes
place in an artificially created field, the directive
field, whose direction is that of the axis about
which the rotation is measured. Thus, it is important
that the earth's magnetic field is as small as possible
compared with the directive field. For this reason,
use is made of a directive field of min 2 Oersted
(2.10 4 Tesla).
The nitrogen 15 nitroxide radicals can be
advantageously used, like the nitrogen 14 nitroxide
radicals, dissolved in most organic solvents not
destroying the radical. The use of these radicals
in water or in water doped with lithium chloride is
also possible.
Fig 2 shows a probe of a magnetometer without
a forbidden axis containing numerous elements described
in French Patent 2,213,500.
Fig 2a is a diagrammatic section of a probe.
The probe comprises two similar assemblies 2 and 4,
aligned along axis ZZ'. Each assembly comprises a coil
6,6' surrounding samples 8, 8'. The cross-section of
'~
.
684
--7--
coils 6,6' can in particular be triangular. The
samples are contained in two independent containers
10, 10', which can advantageously be shaped like
two symmetrical bottles respecti~ely 14, 15 and
14', 15', in such a way that when juxtaposed they
define a space in which coils 6, 6' can be arranged.
The exciting cavity resonator comprises a conductor
12 connected to the initial conductor of coaxial
cable 13 and an outer envelope 18.
In uniaxial probes, like that shown in
Fig ~2a,coils 6, 6' are wound in opposite directions,
in such a way that the unwanted signals introduced
there mutually compensate one another. However, the
useful signals from the electromotive forces produced
by nuclear resonance phenomena are summated. Such a
winding procedure is shown in Fig 2b.
It should be noted that the above result
is only achieved if the macroscopic resultant of the
magnetic moments of the nuclei of one of the samples
is opposite to the macroscopic resultant of the
magnetic moments of the nuclei of the other sample.
In the illustrated embodiment, having a single very
high frequency excitation source, it is necessary for
these opposing effects to take place in response to
an excitation occurring at the same frequency for
the two samples.
In order to obtain such a result, it is
possible to use e.g. a sample 8 constituted by
deuterated 1 N TANo dissolved, at a molar concentration
, in a solvent formed from 92% by volume dimethoxy
.. ..
, ~ ": ; ; ,
, . .
,... ; ::, .. ... .. . .
--8--
ethane and 8% water and a sample 8' constîtuted
by deuterated N TANO dissolved, at a molar
concentration lO 3 in methanol.
In the case of such a magnetometer probe,
it is the "high line" of sample 8 which is excited,
whereas it is the "low line" of sample 8' which is
excited.
The "frequency crossing" of these two
transitions takes place for a 0.4 Oersted field
with an electronic pumping frequency of 58.9 MHz.
In the case of magnetometer probes, through
using nitrogen 15 nitroxide radicals according to
the invention, it is possible to obtain a high
frequency power gain, as illustrated by the curves
of Fig 3.
Figs 3a and 3b respectively represent the
nuclear signal S obtained at the terminals of
coils 6, 6' of the probe, as a function of the high
frequency power P, for the deuterated 14N TANO
radical and the deuterated N TANO radical. It
can be seen that for the same value of the nuclear
signal, the high frequency power value is lower
for solutions containing deuterated 1 N TANO, which
corresponds to a higher dynamic polarization co~
efficient of the nuclei of the solvent.
The processes for producing the deuterated
N TANO, the deuterated 5N TANOL and the
deuterated 15N TANANE~radicals will now be described.
These radicals are obtained from nitrogen 15 deuterated
triacetonamine. This is obtained by reacting in the
. .~, .
.. - : . .: :.
. . . :
...
,-
:3L26~34~
g
presence of a dehydrating and complexing agent
such as calcium chloride, deuterated acetone with
ammonia, whose nitrogen is nitrogen 15 in accordance
with the following reaction diagram:
o
~, CD3 15NH D~D
H
The nitrogen 15 deuterated triacetonamine
obtained in this way is separated from the reaction
mixture and then purified.
In an exemplified manner, information will
be given on the operating conditions for obtaining
nitrogen 15 deuterated triacetonamine~ 25g of
acetone and 8g of calcium chloride are introduced
into an autoclave, which is immersed in liquid
nitrogen and placed under vacuum. 3.5cc of liquid
15N ammonia are introduced into it. After 48 hours
- at 50C, the reaction product is placed in a 50cc
round-bottomed flask and heated for 5 hours at 70C.
The red-brown liquid phase is then collected
and to it is added 0.5cc of water. The nitrogen 15
deuterated triacetonamine hydrated obtained in this
way precipitates on cooling the liquid to -15C,
whilst vigorously stirring. The nitrogen 15 deuterated
. - ,
.. . .
~2~fl6~
-10-
triacetonamine is purified by sublimation. In
this way, 15.lg are obtained, i.e. a 30% reaction
yield.
The pure nitrogen 15 deuterated triacetonamine
can also be obtained by chromatography of the red
liquid on a neutral alumina column eluted by a
mixture of ether and petroleum ether.
A description will now be given of the
production of deuterated 1 N TANO and deuterated
N TANOL radicals from nitrogen 15 deuterated
triacetonamine.
The deuterated 15N TANO radical is produced
by oxidizing nitrogen 15 deuterated triacetonamine
with hydrogen peroxide in the presence of phosphotungstic
acid. The deuterated lSN TANO radical is then extracted
from the reaction mixture.
Thus, the obtaining of deuterated 15N TANO
radical can be represented by the following reaction
diagram:
~
O :::
~ IO) ~ D
D3C 5 ~ 3 ~ D3C ~ 15 ~ CD3
I
~: O.
:~
.. . . . .
: . , . .~ :-:: , .
: :: : `~,;
.. -
.. ..
6~4~
-11
For example, details will be given of
the operating conditions for obtaining the deuterated
15N TAN0 radical from nitrogen 15 deut~rated tri-
acetonamine.
lg of nitrogen 15 deuterated triacetonamine
obtained as described hereinbefore, lOmg of phospho-
tungstic æ id and 1.5cc of hydrogen peroxide with
110 volumes are dissolved in 5cc of water. After
reacting for 2 hours 9 ether extraction takes place
of the deuterated N TAN0 radical. The organic
phase is washed with normal sulphuric acid, then
water and is then dried on sodium sulphate. The ether
is evaporated and the radical recrystallized in
petroleum ether. In this way, 0.75g of deuterated
15N TAN0 radical is obtained, i.e. a 75% reaction
yield.
As certain methylenic deuteriums in " ~ "
of the ketone function have exchanged with protons
during oxidation or extraction, an isotopic exchange
in heavy water in a basic medium is necessary in
order to obtain a total deuteration.
For this reason, the 0.75g of previouslY
obtained 15N TAN0 radical are dissolved in 20cc of
heavy water, whose pH is raised to 13 by adding
potassium carbonate. This solution is stirred for
2 hours at ambient temperature and then the radical
is extracted with ether. The organic phase is dried
on sodium sulphate and the ether evaporated.
The deuterated 15TANoL radical is prepared
by reducing the ketone function~ the deuterated 15N
'
.,
.
, ~ . . .
~L~ ~ 8~ ~
TANO radical in anhydrous ethyl ether by lithium
tetradeuteroaluminate. The reaction product obtained
is hydrolyzed and then purified after being separated
from the reaction mixture.
S The obtaining of deuterated N TANOL
radical can be represented by the following reaction
diagram:
oll D ~ D
D ~ ~ D LiAlDD ~ ~ D
D ~ 15 ~ CD3 D3C ~ lS ~ CD3
O. O.
In an exemplified manner, details will be
given of the operating conditions for obtaining the
deuterated 15N TANOL radical from deuterated 15N
TANO.
To a s~ution of lg of deuterated 1 N TANO
in 200cc of anhydrous ethyl ether are added 140mg
of lithium tetradeuteroaluminate. After reacting for
2 hours at oC~ hydrolysis takes place by the successive
addition of lcc of water, lcc of 15~ soda solution and
3cc of water. The flocculent precipitate is filtered,
the filtrate dried on sodium sulphate and the ether
evaporated. The deuterated M TANOL radical is
collected with a quantitative yield.
With regards to the preparation of deuterated
.
-- - .
.. . . .
.. .
~, : :; ~
;. . ...
:. . .. .
12 ~ 8
-13-
TANANE, this takes place by reducing the ketone
function of nitrogen 15 deuterated triacetonamine
in deuterated diethylene glycol by deuterated
hydrazine. Sodium is reacted on the product obtained,
which makes it possible to obtain deuterated 2,2,6,6-
tetramethylpiperidine.
The amino function of the deuterated 272,6,6-
tetramethylpiperidine is then oxidized in ethyl ether
by metachloroperbenzoic acid. Following neutralization
of the acid, the deuterated N TANANE radical is
extracted following its purification.
The obtaining of the deuterated 1 N TANANE
radical can be represented by the following diagram:
~ ~ ~D3
In an exemplified manner, information will
be given on the operating conditions for obtaining
the deuterated N TANANE radical.
The reduction of the ketone function of
nitrogen 15 deuterated triacetonamine takes place in
.--
.. .
3L2~8~6
- 14 -
deuterated diethylene glycol by deuterated hydrazine,
so thatit is previously necessary to deuterate the
diethylene glycol and the hydrazine.
Preparation of the deuterated hydrazine:
A solution of 5g of hydrazine hydrated in
lOcc of heavy water is refluxed for 12 hours, followed
by distillation of the water. This exchange is repeated
three times under the same condition.
Preparation of the deuterated diethylene glycol:
A solution of lOcc of diethylene glycol in
lOcc of heavy water is refluxed for 12 hours and
then the water is distilled. This exchange is repeated
three times under the same conditions.
Obtaining nitrogen 15 1 N TANANE radical
2.5g of nitrogen 15 deuterated triacetonamine
are added in small portions over 2 hours to a solution
of 3g of deuterated hydrazine hydrate in lOcc of
deuterated diethylene glycol, in a 50cc round-bottomed
flask, equipped with a condenser and heated to 80C
on an oil bath. After 12 hours reaction, the condenser
is replaced by a distillation bridge and the excess
hydrazine and water are eliminated. This is fol~owed
by the cold addition of 1.5g of sodium. The condenser
is then replaced and the oil bath heated to 160C.
After 30 minutes, the nitrogen 15 deuterated 2,2,6,6-
tetramethylpiperidine is sublimated and starts to
deposit on the walls of the condenser. After 6 hours
reaction, this gives 1.7g of nitrogen 15 deuterated
2,2,6,6-tetramethylpiperidine hydrate.
A solution of 1.5g of metachloroperbenzoic acid
;
. ~ . . ..
-
.
:, . -
,.
~26~46~
in lOcc of ether is then added dropwise to a ~ution
of lg of nitrogen 15 deuterated 2,2,6,6-tetramethyl
piperidine hydrate in 20cc of ethyl ether. After
reacting for 1 hour at 0C, th~e metachloroperbenzoic
acid is neutralized by a 5N soda solution. The organic
phase is washed with water and then with a few cc of
lN sulphuric acid in order to ~extract the amine which
has not reacted. This is followed by washing twice
with water, drying on sodium sulphate and slow
evaporation. This gives 0.62g of deuterated 1 N
TANANE radical3 i.e. a 71% reaction yield.
:' ;
- - ,
~ .. . . .