Note: Descriptions are shown in the official language in which they were submitted.
-- 1 --
C2 ADDITION TO SUPPRESS H2S EVOLUTION
IN ABSORPTION-DESORPTIO~ PROCESS
Background o~ the Invention
This invention relates to an improved methanol
scrubbing process for removing sour gases, especially CO2
and H2S, from gaseous mixtures wherein the methanol con-
tains alkaline compounds, and after absorption of the sour
gases, is subjected to expansion, stripping and/or thermal
regeneration.
It is known to use physical solvents for the puri-
ication of raw synthesis gases by removal of CO2, COS and
H2S. These scrubbing steps are performed at an optimally
` low temperature since the absorption capacity of the phy-
sical solvents, such as methanol, for example~ increase
with decreasing temperature. With the use of methanol,
the scrubbing temperatures employed are generally well
below 0C. Regeneration of the physical solvent loaded
with the scrubbed-out gaseous components is conducted
primarily by a combination of pressure reductlon and/or
stripping and/or thermal regeneration. During these pro-
; ~ cesses, the absorbed gaseous components are desorbed. The
regeneratéd solvent is then recycled into the scrubbing
step.
In order to protect against corrosion it is common
; to maintain in the methanol cycle about 100-1000, prefer-
ably 300-800 ppm by weight of an alkaline-reacting com-
pound, e.g., NH3, an amine, or an alkali salt. These
~ . . . .. . . . .
~2~ 2~
compounds, for example ammonia, however, tend to bind H2S
thus forming NH4HS and make it difficult to strip out the H2S
in the reg~nerating column. To compensate for this tendency,
the regenerating column must be e~uipped with a greater
number of plates, or a larger amount of steam must be
utilized to prevent any H2S from remaining in the thermally
regenerated methanol and passing into the scrubbing column.
It is also known that C02 reacts with the NH3 and NH4HS
dissolved in the methanol to form ammonium carbamate. Since
C2 is a stronger acid than H2S, the NH4HS is decomposed to
form H2S which then enters into the scrubbed gas as well as
into the C02 or C02/stripping gas fraction. This deleterious
evolution of H2S into the scrubbed gas does not occur in the
scrubbing step if the scrubber can be operated so that there
are at most traces of C02 (e.g., 1-50 vppm [parts per million
by volume]) at the head of the column, but the release of H2S
becomes considerable if the C02 is not completely scrubbed
out at the head of the column. Since it is expensive and
sometimes otherwise unnecessary to remove the C02 to less
than 50 vppm, the evolution of H2S has become a serious
problem in those systems employing an alkaline-reacting
compound to prevent corrosion in the methanol cycle. For a
description of other specific alkaline-reacting compounds,
attention is invited to the following reference: U.S. Patent
4,250,150.
Summar~ of the Invention
An object of the invention is to provide an improved
process of the type discussed above, especially in those
systems where the purified gas contains above 50 vppm of C02.
Another object is to provide an improved system for
removing H2S from th~ loaded methanol in a regenerating
"",
. ~: -- ...
' : '
: . ~ - -
':
~27~ 4
column, and especially to prevent sul~ide compounds from
remaining in the regenerated methanol.
To attain these objectæ, CO2 is introduced into the
cycle methanol at a point in the cycle where the latter is
otherwise free of CO2 iD order to prevent sulfide com-
pounds from remaining in the regenerated methanol.
According to this invention, CO2 is introduced into
the methanol in a ~uantity such that the H2S can be
stripped out in the regenerating column up to a concen-
tration at which it is no longer troublesome in thescrubbed gas, but so that the CO2 scrubbing-out step is
still sufficient.
In this process, CO2 is preferably introduced into
the H2S-loaded methanol during regeneration. The presence
~15 of CO2 leads to the aforementioned formation o ammonium
`~carbamate and thus to the liberation of H2S. This H2S is
then discharged together with the remaining H2S overhead
from the regenerating column. ~he ammonium carbamate is
sufficiently soluble in methanol even at the lowest tem-
perature of the process and is recycled to the regenera-
;~tion column where it is decomposed in the upper region of
the column.
~;The required amount of H2S-free CO2 can be advanta-
geously supplied as pure gaseous CO~, as a CO2-containing
gaseous mixture (e.g., intermediate expansion gas from the
scrubbing operation proper), or as CO2 dissolved in metha-
nol.
Consequently, with the use of the process of this
invention, the practice of operating a methanol scrubbing
step with methanol that contains NH3, an amine, or an
alkali can be continued, which -- as mentioned above -- is
advantageous for corrosion considerations~ In contrast to
prior conventional processes, it is herein unnecessary to
equlp the regenerating column with a largar number of
` 35 plates, or to utilize more steam. ~oreover, there is no
'` ~ `'
j`:
~: ,
- . . . .. . .
, . . . , - : . . . .
; .: ..
7~2~
entrainment of H2S into the CO2 or CO2/stripping ga~
fraction any longer.
As another aspect of this invention, it is known
that methanol is likewise used conventionally for preven-
tion of ice formation during the cooling of a gas prior toa scrubbing step. Accordingly, this invention also has
applicability to the cooling to H2S- and water plus metha-
nol-containing gases, especially prior to a scrubbing
step, wherein the methanol is separated from the absorbed
water in a methanol-water separ~ting column and wherein an
alkaline-reacting compound is present in the methanol, and
the latter is reused. By introducing an alkaline-reacting
compound, such as sodium hydroxide solution, for example,
into the methanol used to prevent ice formation during the
cooling step of the crude gas, traces of organic acids,
such as, for example formic acid, are in most cases also
entrained and absorbed by the methanol together with the
water before the actual scrubbing step. In the methanol-
water separating column, these acids would otherwise cause
a drop in pH but for the addition of alkaline solutions.
However, in the presence of an alkaline solution, NaHS and
- Na2S, for example, are formed as undesirable by-products
and are withdrawn in dissolved form in the aqueous bottoms
of the separating column. If such an aqueous stream, as
~; 25 i5, or diluted with more water, is utilized for scrubbing
out methanol vapor from a CO2 or CO2/N2 fxaction, then --
similarly as in the synthesis gas scrubbing column -- the
alkali sulfides are reacted with CO2 to form carbonates
with the evolution of H2S which is passed into the - -
3a scrubbed gases. In the same way, if the aqueous bottoms
are discharged into the environment or into a wastewater
stream, the CO2 contained in the air can effect release of
H2S into the atmosphere. To prevent H2S from entering
into either a CO2 fraction or into the atmosphere, the
methanol~water separating column is modified by passing
i
: ~ ., -
: . , ,
:: : , . .
-: , : , . , . . : . ,
- :
, :- , : . : :
:: - : ,
~7~12L~
-- 5 --
C2 or C02-containing gases into same preferably into the bottom section
thereof, especially below the first plate.
Broadly stated, the invention lS an improvement in a
methanol cycle scrubbing process for the removal of sour gases comprising
C2 and H2S, from a gaseous mixture, wherein the methanol contains at
least one alkaline-reacting compound and, after absorption of the sour
gases, is regenerated for reuse by expansion, stripping and/or thermal
regeneration. The improvement comprises introducing C02 into the methanol
cycle at a point where the methanol is normally essentially free of C02 ,
in order to suppress any reaction between said alkaline-reacting compound
and H2S.
Brief Description of thè Drawings
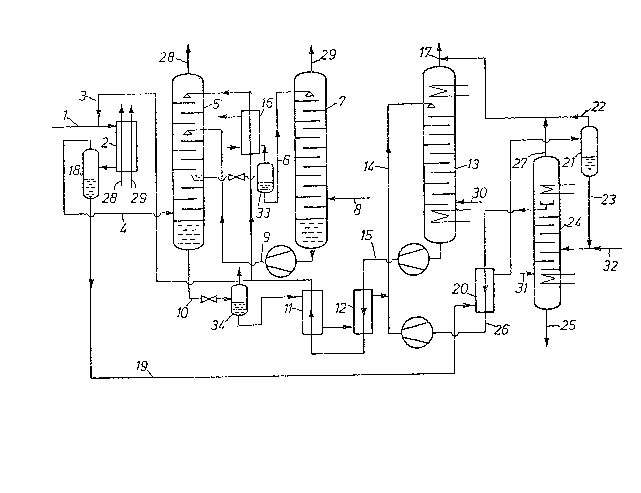
The figure is a schematically illustrated preferred
~;~ comprehensive embodiment of the invention.
` 15 Detailed Descrlptlon of the Drawlng
At 1, synthesis gas to be purified is fed in an amount
- of 100,000 Nm3/h at a temperature of about 30C and under a pressure
:,,
~ of 70 bar into a precooler 2. The synthesis gas contains
:~ H2 64 vol-%
.
~ 20 C0 33 vol-%
; ~ 2
C0 2 vol-%
H2S 1 vol-%
H20 80 kg/h
formic acid traces
: : -
.:
~ .
~ .
.
. ~. ~,.. . .
:' ~ ' ' . ' ' . . '
~L~ 7
- 5a -
In precooler 2, the gas having a relative humidity of
about 100% is cooled -to about -30C by indirect heat exchange with
gaseous fractions returned -From the scrubbing step v;a conduits 28
and 29. (These gaseous fractions will be described -Further below.)
In order to prevent the occurrence of solid obstructions from ice
formed from condensed water, 160 kg/h of regenerated methanol are
injected into the precooler 2 via conduit 3. The thus-precooled
synthesis gas passes via a phase separator 18 and conduit 4 into
a methanol scrubbing column 5 to remove the sour gases, i.e., C02
and H2S. In this column, sulfur compounds are scrubbed out in the
lower section with a partial amount of methanol. In the middle
section of the scrubbing column 5, a rough C02 scrubbing step
is performed with the primary quantity of methanol, and a
final C02 purification is conducted in the upper section. In
the middle section of the scrubbing column 5, scrubbing is
.
~'
.", ~ .
`:~
~;
.
~`
:
~ ~.
, i
.,, :
~ :
,.~. .
~,
- .
.
conducted with partially regenerated methanol, and in the
upper section wi-th completely regenerated methanol. The
thus-scrubbed gas is passed via conduit 28 to precooler 2
and then to further processing.
A CO2-loaded methanol stream is conducted via con-
duit 6 to an intermediate expansion tank 33 wherein con-
comitantly dissolved H2 (49 vol-%) and CO (1 vol-%),
remainder CO2, are removed in gaseous form. The resultant
methanol is further regenerated in a regenerating column
7u to remove additional but not all the CO2 therefrom by
stripping with an auxiliary gas, e.g., N2, supplied via
conduit 8. The resultant methanol is recycled via conduit
9 into the scrubbing column 5. The thus-liberated gases,
primarily CO2 stripping gas, are removed overhead from
column 7 and passed via conduit 29 to precooler 2.
The H2S- and CO2-containing methanol collected in
the bottom of the scrubbing column 5 is conducted via
conduit 10 into an intermediate expansion tank 34 wherein
a gas is obtained similar to that from tank 33, however
containing H2S. The resultant methanol is then passed via
heat exchangers 11 and 12 into a regenerating column 13.
~-~ In heat exchanger 11, the methanol is heated to about
ambient temperature, then in heat exchanger 12 to about
65C from where it passes via conduit 14 into the regene-
rating column 13. In the latter, the methanol is entirely
desorbed by boiling, and the regenerated methanol is re-
moved from the sump of regenerating column 13 via conduit
15 and cooled in heat exchanger 12 to 30C and in heat
exchanger 11 to -10C. After further cooling to about
-40C in a refrigerant evaporator 16, the methanol is re-
~ cycled into the scrubbing column 5. The CO2 and H2S frac-
-~ tion released in the thermal regenerating column 13 is
passed via conduit 17 overhead out of the column and can
be ~urther treated to obtain elemental sulfur.
The water-loaded, cold (-30C) methanol injected
.
,
,
. ~ .
; . ~ ~ ''. .: ~ ' ' ' - " `
~: .
~` ': , ~ ` - .' . ` '
: :. : , . . .
~ '.; ~' ' .
73~2~L
-- 7 --
into precooler 2 is passed into phase separator 18, con-
duit 19, a countercurrent heat exchanger 20 where it is
heated to ambient temperature, then passed into a phase
separator 21 wherein the primary amount of dissolved gas-
es, especially CO2, is separaked by expansion to 2 bar
~the expansion valve not being shown). These gases are
admixed via conduit 22 -to the CO2 and H2S Eraction rom
regenerating column 13. The methanol-water mixture passes
via conduit 23 into the water-methanol separating column
24 equipped with a steam-heated reboiler and a water-
cooled condenser. Traces of formic acid are still present
in the water. For neutralizing this acid, an alkaline
solution, e.g., 8% by weight streng~h NaOH, is introduced
via conduit 32 in an amount of 4 l/h. The addition of
alkali has the effect of balancing the pH, which would
- otherwise decrease because of traces of the acidic com-
pounds. Via conduit 25, a mixture of water, formic acid
and alkaline solution is then withdrawn from the base of
the column; and via heat exchanger 20 and conduit 26, H2S-
and CO2-containing methanol is withdrawn rom the upper
section of column 24 and then conducted to the methanol
regenerating column 13. Inert gases can exit rom the
- column by way of con~uit 27.
On account o the ammonia contained in the methanol,
aS removal by stripping i~ impeded in regenerating column
13 to such an extent that some H2S (100 Nl/h~ will remain
in the thermally regenerated methanol and will pass into
column 5. In the latter, the CO2 (2 vol-%) let in the
~` column head reacts with the NH3 and NH4HS dissolved in the
methanol, with formation of ammonium carbamate and release
of H2S into the methanol circuit. O thls amount, 13 l/hl
corresponding to 0.2 vppm, of H2S is transferred dele~eri-
ously into the scrubbed gas.
In order to avoid this problem, the invention pro
vides that preferably about 1/4 to 10, more preferably
,- ~ . . . . .
: . - , . . . .
'L'~7~
-- 8 --
about 1/2 to 1, mole of CO2 is blown into the lower sec-
tion of column 13 via conduit 30, per mole of N~3 con-
tained in the bottom section of the regenerating column.
This CO2 can be taken from the liquid phase of the inter-
mediate expansion tank 33, said liquid containing a gasrich in CO2, free of H2S, and under a sufficient pressure.
The alkaline solution present in the water-methanol
separating column 23 has the effect of forming NaHS, Na2S,
NaHCO3 and ~a2CO3. In order to prevent the sulfur com-
pounds from leaving the column with the a~ueous bottomsolution, the invention provides that CO2 is introduced,
preferably as a gas, via conduit 31 into the column 24 in
an amount of about 100 l/h.
It is clear that the principles of this invention
are also applicable to other physical scrubbing agents
wherein it is desired to suppress the reaction of H2S with
the alkaline-reacting compounds, or stated in another way
to prevent the formation of sulfide compound with alkali
cations.
.
~ .
:`
~`:
~:``
-:
~'~
::
:~ . , . -
.
, .
.
.
-