Note: Descriptions are shown in the official language in which they were submitted.
~7~L151
BACKGROUND OF THE INVENTION
The present invention relates to a process for
the treatment of used rubber tires by vacuum pyrolysis
to produce liquid and gaseous hydrocarbons and a solid
carbonaceous material.
The accumulation of large quantities of scrap
tires has become a major environmental problem. Because
of their resistance to biodegradation, used automobile
tires provide a favourable environment for vermin,
rodents and fire. Environmental regulations, on the
other hand, prohibit the disposal of such waste materials
- by burning outdoors or by burial underground.
One possible solution to the above problem is
to convert the tires into fuels and other useful hydro-
carbon products, for instance by thermal decomposition.
In order to avoid side reactions and cross-reactions
among product species when heating the tires under atmos-
~: :
pheric or superatmospheric pressure, which results in
~'
a very inefficient conversion process, U.S. Patent No.
4,235,676 has proposed to conduct the pyrolysis of rubber
tires under sub-atmospheric pressure. According to this
patent, the vacuum pyrolysis of tires is effected by moving a
mass of shredded tires through an elongated tubular mem-
ber maintained at a temperature between about 400~ and
,::
~ 800C, in the absence of air and/or oxygen, with the
material being turned or stirred as it passes through
. ~ :
the tubular member, and withdrawing the gases and vapors
produced by means of a vacuum of from about 4 inches to
about 6 inches of mercury (i.e. an absolute pressure of
from about 608 mm Hg to about 658 mm Hg). The process
conditions, however, are such as to promote the forma-
~1.;;~7~151
tion of gaseous hydrocarbons to the detriment of-the
more highly desirable hydrocarbon oils.
SVMMARY OF THE INVEN'rION
It is therefore an object of the present
invention to carry out the pyrolysis of used rubber
tires under conditions to promote the formation of
liquid hydroearbons and to thereby yield higher amounts
of hydrocarbon oils.
In accordance with the invention, there is
thus provided a process for the treatment of used
rubber tires by vacuum pyrolysis in a reactor to pro-
duce liquid and gaseous hydrocarbons and a solid carbona-
~ ceous material, wherein the pyrolysis of the tires is
: carried out at a temperature in the range of about 360C
to about 415C, under a sub-atmospheric pressure of less
than about 35 mm Hg and such that gases and vapors pro-
duced in the reactor have a residence time of the order
of a few seconds, whereby to increase the yield of the
; ~ liquid hydrocarbons~and lower the yields of the gaseous
-: : 20 hydrocarbons and solid carbonaeeous material.
~. .
` :~ ; It has been unexpeetedly found, according to
the invention, that by seleeting a pyrolysis tempera-
~; ~ ture of about 360C to about 415C, preferably of about
380C to about 400C, a sub-atmospheric pressure of less
.
than about 35 mm Hg, preferably of less than about
; 30 mm Hg, and a residence time of the gases and vapors
: in the reactor of a few seconds, preferably 1-3 sec.,
and eondueting the process with sueh seleeted parameters,
the yield of the highly desirable liquid hydrocarbons is
signifieantly inereased while the yields of the less
;~ : desirable gaseous hydrocarbons and solid carbonaceous
,: ~
- 3 -
;
.
: ;- - :
.
- .
711S~
material are lowered, thereby enabling hydrocarbon oils
to be obtained in substantially maximum yield. Indeed,
it has been observed that if the tires are treated at a
temperature above 415C, there is a gasification of the
residual solid carbonaceous material, producing more
gaseous hydrocarbons without formation of any
further liquid hydrocarbons. On the other hand,operat-
ing under a sub-atmosphexic pressure greater than
35 mm Hg has been found to promote the formation of
gaseous hydrocarbons to the detriment of the liquid
hydrocarbons; a too long residence time of the gases
and vapors in the reactor, i.e. exceeding a few seconds,
, ~
- also has the same detrimental effect.
The used rubber tires, prior to undergoing
pyrolysis, are preferably shredded into cuttings. Such
tire cuttings may have a mesh size of about 5-15 mm,
for example.
-; According to a preferred embodiment of the
invention, the reactor used for carrying the pyrolysis is
;~20 a multi-tray reactor having a plurality of spaced-apart
heated trays arranged above one another and each adapted
to receive a bed of the tire cuttings for subjecting
:. -i~:
same to the pyrolysis. The trays are heated at tempera-
~ tures to provide a vertical temperature gradient between
;j25 uppermost and lowermost trays with the lowermost tray
being heated at a temperature higher than the uppermost
~-tray. For example, the uppermost and lowermost trays
may be heated at about 250C and about 500C, respect-
ively: it should be understood, however, that the bed
Of tire cuttings even lf heated by means of a tray main-
tained at a temperature of about 500C is not allowed
- 4 -
.
~L2~ 5~
to reach a temperature exceeding about 415C and this
may be achieved by controlling the residence time of
the tire cuttings on such a tray.
Such a multi-tray reactor is advantageously
provided with a plurality of discharge outlets each
associated with a respective tray for discharging gaseous
- hydrocarbons and condensable hydrocarbon vapors generated
in the reactor. These gaseous hydrocarbons and condens-
able hydrocarbon vapors are withdrawn from the reactor
through the discharge outlets and passed through heat
exchanger means for condensing the condensable hydrocarbon
vapors to thereby obtain the liquid hydrocarbons. To
this end, the discharge outlets are connected via the
heat exchanger means to vacuum means for maintaining the
sub-atmospheric pressure in the reactor and causing the
,~
gaseous hydrocarbons and condensable hydrocarbon vapors
to flow out of the reactor through the discharge outlets.
,
Preferably, the heat exchanger means include
primary and secondary heat exchanger means, the primary
heat exchanger means comprising a plurality of heat
exchanger elements each connected to a respective dischar-
ge`outlet. The heat exchanger elements are maintained
at temperatures to provide a vertical temperature gra-
dlent between uppermost and lowermost heat exchanger
~ 25 elements with the lowermost heat exchanger element being
;~ mdintained at a temperature higher than the uppermost
~ -:
heat exchanger element. For example, the uppermost and
lowermost heat exchanger elements may be maintained at
about 10C and about 40C, respectively. The secondary
heat exchanger means, on the other hand, may comprise
a plurality o~ condensation traps in fluid flow communi-
.
:: _ 5
, ,. :,
~ ~: , . - . . . .
, - . - - - ,
Li5~L
cation with one another. Thus, the gaseous hydrocarbons
and condensable hydrocarbon vapors after having passed
through the prlmary heat exchanger means are passed into
the condensable traps from one to another. For example,
the gaseous hydrocarbons and condensable hydrocarbon
vapors may be first passed into a condensation trap main-
tained at a temperature of about -20C and then into the
other condensation traps which are maintained at a tempe-
- rature of about -80C.
; 10 As indicated above, the process according to
~` the invention enables used rubber tires to be converted
into high amounts of liquid hydrocarbons. Typically,
about 60 weight ~ liquid hydrocarbons, about 38 weight
solid carbonaceous material and about 2 weight ~ gaseous
hydrocarbons can be produced from used rubber tires by
, ~ ~ the~process of the invention. The liquid hydrocarbons
- ~ produced in accordance with the invention have a calorific
va~lue of about 10,200 kcal kg 1 and are thus suitable for
; use as heatlng fuel.
BRIEF DESCRIPTION OF THE DRAWINGS
~: : :
Further features and advantages of the inven-
tion will become more readily apparent from the following
descrlptlon of preferred embodiments thereof as illustra-
ted by way of example in the accompanying drawings, in
~ which
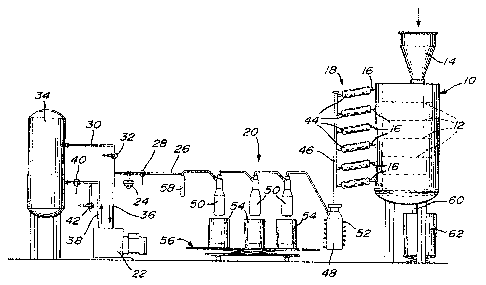
I Fig. 1 is schematic illustration of an apparatus
for carrying a process accordlng to the invention'
Fig. 2 is a plot of the product yield as a
~ : :
function of temperature; and
Fig. 3 is a plot of the yield of liquid hydro-
carbons as a function of pressure.
:
~ 6 -
~L2'7~ 3l51
DESCRIPTION OF PREFERRED EMBODIMENTS
, ., . _ _
- Referring first to Fig. 1, there is illustrated
an apparatus for carrying out the vacuum pyrolysis o~ used
rubber tires in the form of cuttings, comprising a multi-
tray reactor 10 having a plurality o~ spaced-apart heated
_ .
trays 12 arranged abo~e one another and each adapted to
receive a bed of tire cuttings charged into the reactor
_
via the hopper 14 and transported from an upper to a lower
tray by conventional means (not illustrated,) for subjecting
the tire cuttings to pyrolysis. The trays 12 are heated
at temperatures to provide a vertical temperature gradient
between the uppermost and lowermost trays with the lowermost
tray being heated at a temperature higher than the uppermost
tray. Typically, the uppermost and lowermost trays are
heated at about 250C and about 500C, respectively. The
heating of the trays 12 and the residence time of the tire
cuttings thereon are such that the tire cuttings when
~-~ reaching the lower portion of the reactor (i.e. the two
..~
lowermost trays) are treated at a temperature of about 360C
~ .
to about 415C and that the temperature of the tire cuttings
does not exceed about 415C.
The reactor 12 is provided with a plurallty of
discharge outlets 16 each associated with a respective
; tray~12 for discharging the gaseous hydrocarbons and
condensable hydrocarbon vapors generated in the reactor.
.::
~ ~ The discharge outlets 16 are connected via primary and
..
secondary heat exchangers 18 and 20 to a vacuum pump 22
for maintaining sub-atmospheric pressure in the reactor 12
and causing the gaseous hydrocarbons and condensable
hydrocarbon vapors to flow out of the reactor through the
~; ~ discharge outlets. A sub-atmospheric pressure of less
than about 35 mm Hg is maintained in the reactor 12 by
: ' -
-- 7
.:
~: . . .
.
.
~7~
means of a vacuum control device 24 connected to the
vacuum line 26 and adapted to set a predetermined sub-
atmospheric pressure. The vacuum line 26 which is pro-
vided with a valve 28 is bifurcated into two lines, a
first line 30 provided with a valve 32 and connected to
a gas reservoir or tank 34 for storing the gaseous
hydrocarbons produced in the reactor 12, and a second
line 36 leading to the vacuum pump 22. A further line
38 provided with valves 40 and 42 interconnects the
vacuum pump 22 and gas tank 34, the valve 42 being a
vent valve.
The primary and secondary heat exchanger 18 and
20 through which the gaseous hydrocarbons and condensable
hydrocarbon vapors are passed are adapted to condense
the condensable hydrocarbon vapors to thereby obtain
the desired liquid hydrocarbons. The primary heat
exchanger 18 comprises a plurality of shell and tube
.,
heat exchanger elements 44 each connected to a respective
discharge outlet 16. The heat exchanger elements 44 are
.~ :
maintained at temperatures to provide a vertical tempera-
ture gradient between the uppermost and lowermost heat
exchanger elements wlth the lowermost heat exchanger
element being maintained at a temperature higher than the
uppermost heat exchanger element. Typically, the upper-
~ ~ 25 most and lowermost heat exchanger elements are maintained
'~ at about 10C and about 40C, respectively. About 70
-~ of the total condensable hydrocarbon vapors produced
are condensed by means of the primary heat exchanger 18.
,
The gaseous hydrocarbons and remaining conden-
sahle hydrocarbon vapors leaving the heat exchanger
" :: :
- 8 -
:. ~ : , '
S~
elements 44 are collected by means of the collecting
conduit 46 and then passed through the secondary heat
exchanger 20. The latter comprises a plurality of con-
densation traps 48,50 in ~luid flow communication with
one another. The first condensation trap 48 is advanta-
geously maintained at a temperature of about -20C by
means of a refrigerant coil52 in which an aqueous solution
of ethylene glycol is circulated. The other condensa-
; tion traps 50, on the other hand, are maintained at a
temperature of about -80C by being immersed in
acetone/C02 baths contained in thermos containers 54
supported on a wheeled vertically displaceable platform
56. A filter 58 comprising glass wool is provided for
filtering the gaseous hydrocarbons from which have
e~tracted any condensable hydrocarbon vapors, prior to
, ~
- the gaseous hydrocarbons being sucked via lines 26,36
~ .
through the vacuum~pump 22 and directed into the gas
tank 34 via line 38.
The solid carbonaceous material which is pro-
20 ~ duced in the reactor 12 as a result o~ the pyrolysis
: ..
- of the tires is discharged via the bottom outlet 60 into
a suitable container 62 placed underneath.
-
At the start of the process, the gas tank 34
is first evacuated by closing the valves 28,40 and open-
ing the valves 32,42 so that any air or other gas con-
tained in the tank 34 is sucked via lines 30,36 through
the vacuum pump 22 and vented to the atmosphere via line
,: : -
~ 38 through the vent valve 42. Once the gas tank 34 has
:: :
~ ~ 30 been evacuated, the valvès 32,42 are closed and the
;~ valves 28,40 are opened so as to establish the necessary
~: .
~ .
r ~ ~^ ' , ~ 9
' ~
' ~ ' . ' - . ' ,
~, ' ' ' ' '' , . ..
~71~1
vacuum throughout the system and direct the gaseous
hydrocarbons produced through the vacuum pump 22 and
into the gas tank 34.
Using the apparatus illustrated in Fig. 1 and
sampling the pyrolytic products for their composition
as a function of temperature provided the following
results:
Table 1
Temperature Yields ~weight %)
Ex. No. ( C) Oils Char Gases
. ._ . -.- _ _
;~ 1 250 8.9 91.1 0.0
2 310 20.2 79.5 0.3
~` 3 335 29.5 68.8 1.7
`~ 15 4 363 51.5 45.6 2.9
415 61.2 36.6 2.2
~ ~ 500 60.~ ~ 5 4.3
., ~ .
The above data are reported in Fig. 2, in which
the symbols (o), (~) and (~) represent the following:
~- 20 O : liquid hydrocarbons
.
: char (solid carbonaceous material)
: gaseous hydrocarbons.
As shown in Fig. 2, when the pyrolysis temperature
exceeds about 415C, the yield of liquid hydrocarbons
is lowered. This is due to a gasification of the char
~-~ or solid carbonaceous material, which produces more
: . - ,
~ ~ gaseous hydrocarbons. As it is apparent, the optimum
- ; temperature range for a maximum production of liquid
:
~ hydrocarbons is about 360 - 415C.
~ .
:
::
-- 1 0
.
,~
.: ~ .. ,
~ .
~27~5~
The tire cuttings used in these experiments had
the following characteristics:
Elementary Analysis:
C: 85.7%
H: 7.5%
N: 0.3%
: 5.1%
S: 1.4%
Volatile matter: 65.2%
Fixed Carbon: 28.7%
Ashes: 6.1%
Calorific Value: 8,787 kcal kg 1
Size: ~ " Tyler
The hydrocarbon oils produced from such tire cuttings
; 15 had the following characteristics:
Elementary Analysis-
C: 87.1%
H: 10.5%
N: 0.2%
O: 1.4% :~
: ~ S: 0.8%
. Calorific Value: 10,200 kcal kg 1
~- : Density: 0.95 g ml 1
~: .
: ~ Humidity: 0.15%
: :
Dynamic Viscosity (21C): 168 cp
: :~ : Dynamic Viscosity (49C): 46 cp
Three additional experiments were carried out
with~a view to illustrating the effect of pressure on :
: the yield of the Iiquid hydrocarbons. The results
~ 30 obtained are as follows:
: '
:: :
: -- 1 1 -
~'7~51
TABLE 2
_ .. ~
Absolute Pressure Yields (weight %)
Ex. No.(mm Hg)Oils Char Gases
. I
7 15 57.2 38.4 4.4
8 35 56.9 37.5 5.6
:~ 9 ~__ 54.5 39.8 5.7
:: .
:~ The above data for the liquid hydrocarbons
only are reported in Fig. 3. As shown, when
-~ the sub-atmospheric pressure is greater than about 35
mm Hg, the yield of liquid hydrocarbons is lowered. As
it is apparent from Table 2, operating under an absolute
pressure above 35 mm Hg promotes the formation of
gaseous hydrocarbons to the detriment of the liquid
~' hydrocarbons. The sub-atmospheric pressure must there-
- ~ 15 fore be maintained under about 35 mm ~g to provide a
~ maximum production of liquid hydrocarbons.
;
' :,
' ~ '
`~, : '
:
- :
~:'
,.j :
: :~
~'
~ : :
:;. - 12 -
.~ ,.~.
:~ ..: - .
' :: ~ . : , .