Note: Descriptions are shown in the official language in which they were submitted.
DNA VECTORS AND THEIR USE IN RECOMBINANT DNA TECHNOLOGY.
Field of t~le Invention
This invention relates to DMA vectors, their
production and -their use in recombinan-t DNA techno-
logy. The invention also relates -to the control of
replication of DNA vectors in the cells of host
organisms, and to the production of gene produc-ts.
Bac~ground of the Invention
DNA vectors, such as plasmids, are normally
circular, extrachromosomal DNA molecules which replica-te
autonomously within the cells of host organisms. The
cells of many unicellular organisms, including some
bacteria, contain naturally-occuring wild-type plasmids
which contribute ~arious functions to the host cells
5 SUC~l as antibiotic resistance and fertility. These
~ild--type plasmids and deriva-tives of them are the basic
tools of recombinant DNA technology, providing vehicles
for the transformation of the cells of host organisms
with foreign DNA sequences whichcode for product on,~
within the transformed cells, of corresponding foreign
polypeptide and protein products. Thus, in recombinant
DNA techniques, plasmids are cu-t open at specific sites
using restric-tion enzymes and recombined in vitro with
additional DNA sequences,including genes coding for
desired foreign products, -to give recombinant plasmids
which may be used to transform appropriate host cells.
These recombinant plasmids, similar to the parent
plasmids from which they are derived, are capable of
autonomous replication within host cells, and on re-
3~ plication reproduce no-t only the DNA sequences of the
parent plasmid bu-t also the inserted additional DNA
sequences, including the foreign genes. During protein
synthesis, transcription and translation of the DNA
sequences of the recombinant plasmids carried within
transformed host cells give rise inter alia to the
~,,
~2~21~13
- 2 -
synthesis of foreign products corresponding -to the
inserted foreign genes.
One factor which affects the yield of synthesised
foreign product is the number of copies of the foreign
gene which are present within the transformed cells, i.e.
the copy number at which the recombinant plasmid is
maintained within the host cells, this being defined
normally as the number of copies of the plasmid per
host genome. Generally speaking, the higher the copy
number of the recombinant plasmid the greater is the
yield of foreign product. Both low copy number plasmids,
us~lally maintained within host cells at about 1-10 copies
per genome, and high copy number plasmids, usually
m~intained at from 11 up to severalhundred copies per
genome, are known. The copy number of a given wild-
type replicon is controlled by DNA sequences surrounding
and including a DN~ sequence which defines the origin
of replication. Thus hereinafter we refer to high copy
number~and low copy number origins of replica-t-on.
High copy number plasmids have been used in re-
combinant systems with a view to obtaining good yields of
for~ign products. This can lead to undesirable results,
however, since many such high copy number plasmids -tend
not to be maintained stably within transformed cells
and may be lost from the cells before they can be grown
to sufficien-t levels to permit bulk production of foreign
produc-ts. For example, the foreign produc-t may inhibit
propaga-tion of the transformed cells or the high copy
number plasmids themselves may be inherently unstable.
3~ It is known that the copy numbers of some plasmids
can be amplified above normal levels by inhibition of
protein synthesis; for instance, by addition o-f protein
synthesis inhibitors such as chloramphenicol to the
fermentation medium. However, protein synthesis is
required for production of most gene products, and
therefore the inhibitor must be removed before synthesis
- 3 -
of foreign gene products can take place. This removal
of inhibitor requires complicated manipula-tions and is
not always possible.
Various other solutions have beenproposed to over-
5 come the problem of s-table maintenance of high copy
number plasmids in host cells. For example, in UK
Patent Specification No:1,557,774 it has been proposed
to use mutant plasmids having a temperature-dependent
plasmid copy number pattern such that the plasmid shows
a controlled constant plasmid copy number when host
bacteria carrying the plasmid are cultivated at one
temperature, but an altered plasmid copy number pattern,
allowing a much higher or to-tally uncontrolled copy
number, when the host bacteria carrying the plasmid are
.15 ~rown at a different temperature. Thus cells may be
propagated to desired production size cul-ture at one
temp~erature at which the plasmid replicates at low copy
number and at which its gene products do not signifi-
cantly-inhibit cell growth. The temperature ~y then
~ be altered, greatly increasing the plasmid copy number
and also the corresponding production of gene products.
The introduction of copy number temperature dependence
in such mu-tant plasmids, however, may introduce a source
of instability into the plasmid, and i-t is likely that
~5 these mutant plasmids may be unstable or subject to loss
when cells carrying them are propagated over a prolonged
period of time.
The replication of plasmids is controlled by
nucleotide sequencescontained within the overall DNA
3~ sequence of the plasmid. These sequences include a
sequence defining the origin of replication at which
DNA replication is initiated and often also associated
sequences which control the :initiation of replication
at the origin and the copy number at which the plasmid
is maintained. For example, certain plasmids, of which
ColEl is a typical example, have plasmid replication
systems having a number of features in common, These
systems comprise a DNA sequence defining an origin of
replication and upstream thereof a DNA sequence coding
for transcription, in opposing directions 9 of two RNA
species, RNAII and RNAI. The RNAII species provides
an RNA primer which forms a complex a-t or near the
origin from which DNA synthesis is initiated; -the ~NAI
species interferes with the formation of this initiation
comple~, Transcription of the two RNA species is con-
trolled by separate promoter sequences associated with
the DNA sequences which code for their transcription.
In ~ddit.ion there is a small polypeptide (the rop
protein) which is believed to interact with the promoter
for RNAII; this polypeptide is not essential for re-
plication and its role is unclear. The origin of
replication, the RNA coding sequences and associated
promoters together provide an internally self-regulated
system,.which controls the replication incompatibility
?O and the copy number of these plasmids. Certain other
plasmids, exemplified by RI and some Staphyloccocal
plasmids, also control replication initiation at the
transcriptional level, but by a messenger RNA species
whose product provides an initiation factor, probably
~5 a polypeptide, which is involved in DNA replication.
It is an object of the present invention to
provide new DNA vectors which have controllable copy
number patterns and therebyovercome problems associated
with stable maintenance of vectors which replicate at
hi~h copy number only, which new vectors will not be
subject to the potential instability of previously
described mutant plasmids which have temperature-
dependent copy number patterns, and which, furthermore,
will have the advantage that their copy number can be
controlled by agents other than temperature, e.g.
metabolite concentration.
-- 5 --
Accordingly, in a first embodiment the invention
provides a DNA vector comprising two replication systems;
a first origin of replication resulting in a low copy
number and stable inheritance of the vector and a second,
high copy number origin of replication at which replica-
tion is directly controllable as the result of replacement
or alteration by DNA manipulation of the natural vector
s~uence(s) which control replication at said origin.
By means of the invention, when host cells carrying
the vector are propagated under a first set of conditions,
replication takes place mainly, and preferably exclusively,
from the low copy number origin, and when the cells are
pro~agated under a different set of conditions, replication
takes place at high copy number at the second origin.as
1~ well and the production of large amounts of foreign gene
products encoded by the vector is induced.
In a preferred embodiment the invention provides
that the second, high copy number, origin of replication
comprises an origin of replication and an associated DNA
-~ se~uence encoding an RNA species which provides a primer
or i~nitiation factor (e.g. a polypeptide) which initiates
DN~ replication by formation of a complex at or near the
origin of replication, in which transcription of said RNA
species i~5 directly controllable such that, when host
~ells carrying the vector are propagated under selected
conditions, replication takes plase at high copy number
~rom the ori~in and the production of large amounts of
Eoreign gene products encoded by the vector is initiated.
~he invention also includes a method for the
3~ preparation of a vector according to the first aspect,
~:72~3
- 5a -
comprising including in the DNA sequence coding for
the second replication system a DNA sequence which per-
mits direct control of replication at the second
origin.
.. ..... . . .. .... . . . .. . . .. . . . . . . . . .... . . . . .
. ~ ....... .. . .
v~
Methods for the preparation of vectors according
to the flrst aspect of the invention suitably com-
prise ligating a first DNA sequence coding for the re-
plication system comprising the first origin wi-th a
second DNA sequence coding for the secondary replication
system. The DNA sequence which permits direct control
of replication at the second origin may be incorporated
into the second DNA sequence either before or after
ligation with the first DNA sequence. Thus the inven-
tion further provides methods for the preparation ofvectors according to the second aspect of the inven-tion 9
comprising including in the second DNA sequence a DNA
s~uence which permits control of replication at the second
origin by controlling transcription of the RNA species.
~5 The invention further includes a process for the
production of a polypeptide, protein or other gene product
which comprises transforming host cells with a vector
according to the first aspect of the invention, in
which said vector contains a gene sequence coding for
~0 production of said polypeptide, proteinor other gene product,
propagating said transformed cells under a first set
ofconditions at which replication takes place at low
copy number mainly, and preferably exclusively,
from the first origin, and then propagating said
~5 transformed cells under a second set of conditions at
which replication takes place at high copy number also
(or exc-lusively) from the second origin and the expression of said
polypeptide, protein or other gene product is induced.
By means of the process of the invention, trans-
formed cells are propagated to give the large scale
culturesrequired for economic production of polypeptide~
protein or other products under conditions where the
vector replicates at low copy number and the instability
problems associated with high copy number vectors are
avoided, followed by propagation under different condi-
tions where the vector replicates at high copy number
..~
~ 2~zle~3
wth concomi-tant high yield of polypeptide, pro-tein or
other products.
In particular it has been found, when the ex-
pression of the gene product is under the control of a
promoter which is regulated by cytoplasmic levels of a
repressor, that the increase in vector copy number leads
to outstripping of the repressor control and high level
expression of the gene product. This is so even in the
case when the synthesis of the repressor is autoregulated,
e.g. when the promoter/repressor system is that of the
tryptophan operon.
The control systemswhich are used to con-trol the
copy number of the vectors of the invention may comprise
anyof the control systems which are known for controll-
ing replication (and/or expression) in recombinant DNAtechnology. In particular, the copy number of the
controllable origin of replication may be controlled by
temperature or one or more metabolites or metabolite
analogues. Examples of metabolite-dependent sys~ems
which may be used include: tryptophan, lactose,
galactose, arabinose or any other metabolite or meta-
bolite analogue, the presence, removal or further
metabolism of which can be used to activate transcrip-
tion from a given promoter.
~5 The controllable replication system used in the
vectors of the invention may be derived from high copy
number cloning vectors, such as ColEl-like plasmids,
e.g. pAT153, NTPl, CloDF13, RSF1030 or P15A, which ha~Je
eopy number control systems which involve transcription
3~ o~ RNAII or a similar RNA species which provides a primer
which initiates DNA replication by formation of a
complex at or near the origin of replication. In addi-
tion other plasmid replication origins whose replication
is controlled by an mRNA species and/or its product~s~
may provide the controllable replication systems used in
the vectors of -the invention. Such replication origins
~7~L3
- 7a -
may be obtained from Gram ve bacterialspecies and are
exemplified by Rl, R6, R100, RP4, or Gram +ve bac-terial
species, which are exemplifiedbypUB100, pC194 and
certain other Staphylococcal plasmids. Furthermore,
bacteriophage origins may be used for secondary con-
trollable origin, e.g. those from ~, T3, T4, T7,
M13,~X174, SPPl, SP02 etc.
In an important embodiment of the invention, the
controllable replication systems may be prepared from
such high copy number cloning vectors by replacement of
1~ the
-- 8 --
natural promoter, which pr~motes transcription of the RNA species,
by a contr~llable promoter, such as the PL promoter, PR prorroter,
Pre pr~moter, P'R prornoter, T71ate pr~moters, t~p prorr~ter, tac
proToter, lac promoter, gal pr~rnoter~ara promoter or _ecA promoter
5 (the origin OI replication in such a system is tenned a "hybrid
origin"). Altematively the natural promo-ter may be used and trans-
cription of the RNA species made controllable by incorpc)rating a
regulating f~nction, such as an operator sequence, e.g. the lac
operator or OL or OR operators of phage lambda~into the replication
systems.
The plasmid pMG9 (containing an Xho I linker DNA
sequence inserted as described by K Tatchell e-t al,
Cell, Vol 27, pages 25-35, November 1981 (Part 2))
provides a convenient starting material for preparation
15 Of a controllable replication system based on the ColEl
replication origin. This plasmid has a unique Xho I
restriction site, close to the start of the sequence
coding for transcription of the RNAII species, which
we have found may be used for insertion of operator and
20 controllable promoter sequences, e.g. ;tPL, to give a
directly controllable replication system. Plasmid
pM&9 was deposited at the National Collection of Type
Cultures, Central Public Health Laboratory. Colindale
Avenue, London NW9 5HT on 24 ~. rch 1983, under NCTC
No.11539, as a culture of cells of E coliK12, strain DHl,
containing the plasmid pMG9.
The replication system comprising the first
origin of replication may be obtained from any suitable
low copy number plasmid. For example, a replication
30 system comprising the pSC101 origin may be used, and
the plasmid pHSG415 (T Hashimoto-Gotoh et al~ &ene, 16
(1981) pages 227-235) provides a convenient source for
such a replication system. It will be appreciated
that pHSG415 has a temperature sensitive replication
35 origin, but that this origin may be replaced by its
wild-type temperature-stable counterpart from pSClO1.
pHSG415, however, provided a convenient, temperature
~ 272~L~3
sensiti~e replication origin for use in the exarnples
hereinafter described.
Controllable functions may be incorporated into
high copy number replication systems, high copy number
and low copy number replication systems may be ligated,
and foreign genes may be incorporated into the vectors
to produce vectors according to the invention using
techniques which are known and understood by workers
skilled in the recombinant DNA art. The resultant
vectors may then be used to transform suitable host
cells usina standard procedures -to produce foreign poly-
peptide, protein and other products.
The host cells may comprise eucaryotic cells,
includir.g yeast cells e.g. ~.cerevisae, or, more usually,
bacterial cells, of species such as B.subtilis or,
especially, E.coli.
I
.
Tne invention is furche} ~escribea vy way of illustration only in the
following E.samples, Examples 1-7. These Exan~ples relate to the
construction of spacific dual origil~ plasmids according to the invention,
and to studies of copy number control, heterologous ~ene e~pression and
stability of tllese plasmids. It will be appreciated that the invention is
not limited to the specific plasmids and methods described.
Thesa E~amples refer to tne accompanying diaDrams in which:
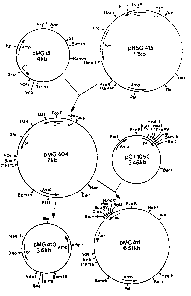
~ iguro 1 shows plas~id restriction maps and indicates the DNA
~aniplllations which were used to prepare a dual origin plasmid according to
th-e il~vention, p2lG411;
Finure 2 is an agarose gel oL DNA isolated fro~. c~ltures of a p~7G411
trans~ormaint of E.coli QY7 ta~en at hourly intervals following a
tenperatLre shift from 30C to 42C (lanes 1-7, hours C-6);
Figuro 3 snows plasmid restriction maps and indicates the DNA
~anipulations used to prepare two furtller dual origin plasmids according to
ehe invention, p~iGl~9 and p~lG16~;
~ i~ur3 ~l is a graph of copy nu~ber induction following a te~perature
shi~t (30C to 42S with subsequent incubation at 37C) of trans-
~ormants of ~.coli carrying pr.l&lS9, pMGlbS, pb!G168 and p~ l69;
~ igure 5 shows plasmid restriction maps and i~.dicates tl~e DNA~anipulations used to prepare a metabolite controllable dual origin ~ector
according to the present invention, pPIG427;
~72~3
Figure S sho-Ys plasmid restriction maps and indicates the DNi~
~anipulations used to prepare two f~rther metabolite controllable dual
origin vectors according to the invention, p~lG415 and p.~.iG416;
Figure 7 is a lG.~o polyacrylamide gel of protein ~ro~ total cell
e~tracts prepared from E.coli E103(S) transforrJed by p~lG169 after
tenperature shift treat~ent, samples being taken at hourly intervals ~lanes
1-8, hours 0-7);
~ igure 3 is a graph showing incrcase in plas~id copy nu~ber and
~hlorn~pllenicol acetyltransferase activity for cultures of E.coli
El03(S) transforrled by pi'>lG169 after teDperature shock treat~ent, and
Figure 9 is a 10~ polyacrylamide gel of protein from total cell
e~tracts of cultures of p~lG169 transformed E.coli ElU3(S) at hourly
intervals over a perio~ of S hours after te~perature snoc~ treatment (lanes
6-l, hours 0-5).
ElV~lPLE 1
Constr~ction of ~ Dual-origin Plasmid ~ith Copy Number under Pl Control
(a) Construction of a Dual-origin Plasmid pMG4U4
Plasmid p~Gl5 (Figure l) is a derivative of ~Sa-12 (pBR322 replicon)
which has constitutively high copy nunber (approxirnately 300 copies per
chromosor.e) due to the insertion of a XhoI linker (CCTCGA~G) into the
ori~in region (Table l) (Tatchell et al, 1981)o During construction of
~S~ the ro~ gene was lost by spontaneous deletion extending beyond the
~S~1I site in the tet gene, bllt reLnining the Bami1I site. Tne
position of the hoI linker ~vas determined by inserting the l.lkb
~hnI-P~m~1I DNA fragment of p~-!Gl5 into bacteriophage ~.113mp~ and DN~
sequencin~ from the XhoI site by the method of Sanger et al, (1977).
Ins_rtion of the linker was at a point 30 bases do-N~s~raam of the
tra1lscriptional start of ~AII (Table l). ~lis ~hoI linker provided a
~iquc clcavn~e site close to the S' end of the ~lAII transcript, such that
th~ l.lkb ~hoI-Xa~-lI fragment isolated from p~lGl5 carried a promoter-
lcss P'~AII sequence (Figure l). To determine if this seq-~ence could
fl~ction as n primer of DNA replication when coupled to another promoter,
the l.lkb fra~ment was inserted downsteam of the ~m resistance gene
(~rl~) nromoter of pl~s~id pT1S~4lS (Tlashimoto-Gotoh ~ al, 1901)
~Fi:~urc 1). p~SG415 is a stable low copy number Apr~ ~m~ Cn1~ plasmid
whoso replicQtion origin is derived from pSClOl; this plasmid replicates at
30~C b~lt not ~t 42C.
- 13 -
TABLE 1
Site of Insertion of XhoI Linker near the Origin
WILD TYPE S~nUE~'CE
~h'AII
~' CTTGC~-~ACA~A~ CCACCGCTACCM CGGTGGTTT
~555
p`~lG15 SEQUENCE
P~NAII
5' CTTGC~U~C.~A~ ACCACCGCTACC~C~GTCCTCGAGG&&TT
I XhoI Linkei
-~55
Conditions for restriction enzyme digestions, DNA ligations, and
sg3rose gel cl3ctrophoresis were as described in ;~;ianiatis et al (1982).
Insertion of the XhoI-~amEI frag~.ent fro~ pilG15, coupling the
pIomoterless ~'~AII sequenca to the ~mP~ promoter, gave tne dual origin
plas~id pr~lG404 (Figur~ l~. Analysis OI the plasmid pilG404 by restriction
en7~e dig~stion verifie~ its structure (results not shown), and plasmid
r~ yields demonstrated that its copy number was considerably higher than
thnt o~ p~iSG415, In addition, pdlG404 was maintained in bac,eria at 4~C
wll~r3ns p}iSG4l5 was lost due to its in3bility to replicate at that
~p3ratur-e, It was conclllded tnat the hybrid 'ColEl origin' w~s
functioning in prllG404, da~onstrating that the ~ gene pro~oter could
substitute for tne ~NAII promoter,
Confirmation of the functioning of the hybrid origin was obtained by
digesting p~.G404 with BalI and recircularising the larger fragment
.. . . . ..
~2~
- 14 -
co~ltaining onl~ t~e hy~rid ori~in. This ~eneraced a plasr,~id ~L~ which
~vas capablc of antono~ous replication at both 30~ and ~2C,
clemonstrating thæt tne hybrid origin was functional.
(b)Construction of Dual-origin p~G411
Plasmid p~lG404 has two origins of replication: the lo~v copy number
origin of pSC101 and the 'Col~l' hybrid origin. The pSC101 origin should
ensura stable replication of the plasmid at 30C (~shi~oto-Go.oh et
ll, l9~1), allosYing the construction of derivati~es with the 'ColEl'
hybrid Ol`i~iU driven by a contro]lable pror,~oter. A 0.5~b SalI-~coRl
`ra~ment carryin~ .he Pl promoter OI bac~eriopnage ~ was isolated from
the plasmid pCT10~0 (Figure 1) and ligated to the 5.5kb EcoRl-XhoI
fr~gment from p~lG404. The ligation mixture wæs used to transform E
coli ~Y7 (l cI~57 defective lysogen) and D~ll at JO~, and the Apr~
.ransfor~ants screened for CmS clones. One of these, prlG411 was chosen
~or further st~dy (Figure 1). Plasmid phG411 transformants of Dl~l and QY7
~rew well under selective conditions and copy n~mber determinations were
m~de ~t a variety of gro~vth temperatures.
~c)Copy Number ~leasurements of phlG411
Plasmid copy num3er ~vas determinecl by tl,vo mothods. One aepended upon
tho separation of chromosomal and supercoiled DNA by eaesiu~ chloride
eentri~ug~tion~ Tho second method depended upon the separation of
Ghromosomal and plasmid DNA by agarose gel electrophoresis. Cells were
grown overnight in L-broth containing ampicillin, and diluted one hundred
fold into minimal ?,'9 salts medium containing a~picillin. At an OV~60 of
0.~, 2-deo:cyadenosine (200 ~g/ml) thymidine (1 ~g/ml1 and ~3il]-thymidine
.
IZ7~ 3
~ ~g Ci/ml) wera a~ded, and incu~ation continued for 2 hours. Cells wcrc
centrifllg-d, ~Yashed and resuspended in an equal volume of 50 ~F,J tris L~Cl
7.~ sucrose (-~/v) and lysozyme (200 ~g/ml). After incubation for
minutes at 0C, EDTA y1as added to a concentration of 10 ~I and
incubation contined for 10 minu~es, and finally sarkosyl ~0.4~ w/v) ~as
sdded. Chromosomal DNA in the lysate was sheared by 6 passages through a
1~ ga~ge needle, and eell debris removed by centriEugation. ~NA
preparations were futher purified by a single phenol and chloroform
e~traction and ethanol precipitation. This labelled DNA prepaIation was
th~n u~ed for eopy number determination by either method.
For the ngarose gel method, samples were electrophoresed on a 0.7$
a~arose gel (in 0.04 ~i tris acetate, 0.001 ~I EDTA, plI 7.9) for 12 hours at
~0 V. DNA bands were visualised by stai~ins in ethidiu~ b~omide. Tke
chromosomal~ and plas~id DNA bands were cut from the gel, dissolved in
saturated sodiu~ iodide and the DNA precipitated by the addition of 10~
(wlv) triehloroacetic acid (TCA). Precipitates were collected on GF/C
lihat.~lan filters, ~ashed in 1~ (w/v) TCA, ethanol and then air dried.
~adioactivity was determined by counting in a liquid scintillation
counter,
~ or eopy nl~er determinations by caesium chloride ce~trirugation DNA
s~rl~lcS ~vere eentrif~lged to equilibrium in caesius chloride-ethidiu~
bro~ a in a r.ec~man TI50 rotor at 4S,000 rpm for 24 hours. The gradients
~ere fraetionated, and the fractions precipitated witn TCA onto GF/C
~`~hatman filters, washed and processed as described above.
~ 3
-- 1 6
PlasmiG GOpy l~r~nbers were determined fro~. pIIGl5, pAT153 and p~iIC411 at
30C, 37C and ~C and the results obtained are given in Table 2.
. . .
Variation of Plasmid Copy N~bar with T~mperat~re
PlasmidBacterial Strain Percenta~c of Copies pRr
(growth temperature) total DNAChromosome
n~;Gl~~101 (37C) 33 309
p~l`l5~ ~E101 (37C) 5.~ 59
~:G~llQ~'7 t3noc) 0.7 4
~t~741.' ~Y7 (37C) 13.~ 7
p~lG411 QY7 (42C) 24.~ 143
I~Ieasurenents of p~lG411 copy nt~ber in strain QY7 ~l lyso~en) at various
te~ceratures de~onstrated that tne A repressor controls pi~G411 cop~ number.
After Drowtlt at 30C, copy n~ber was estimated at 4 per c~ro~.osome,
~hilst at 37C it had increased to 78 and at 42C it had increased to
1~.3 ~er chror~.oso~e (Table 2).
To de~onstrate that the hybrid 'ColEl' origin in p~'G411 could be
s~vitched OII by inactivatino the ~ repressor, a transform~tnt of QY7 was
~ro~vn at 30C~ and then the tempe~ature raised to 42C durin~
o~ponantial ~rowth. The plasmid copy number increase ~as followed by
agaroso gcl analysis of D~IA isolated from 1 ml cultures ~Figure 2).
Samplas wera taken at 1 hour intervals over a period of 7 hoursO As
prodictod, an increase in pbG~ll copy number was observea over the 7 hour
period.
- 17 -
EX~IPLE 2
Constrllction of Dual Origin Plasmids with Ind~cible Copy
Number under Pr Control
~)Construction of Dual Origin Plasmids pblG159 and pMG165
Plasmid p~lG411 has two origins of replication, the low copy n~ber
origin of pSC101, and the Pl-driven ColEl origin. Since the repressor
gene, cIgs7 is resuired for copy number control~ this limits the use of
p`l.'G411 to lysogenic bacterial strains~ To overcome this constraint, a
du~l-ori~,in plasmid carrying the cIgs7 gene was constructed.
~ irstly, to facilitate subsequent cloning of foreign genes an oligo-
nucleotide comprisin~ a SalI and nindIII restriction site was inserted
into the a~I site immediately downstream of the hybrid origin in
p~ ll; this gave p~rsl53 (Figure 3). The 5.9'~b EcoRl-Ea~II fragnent of
p~ 71:;3 w~s purified and ligated to a l.lkb ~col~-Ea~lI fragnent of
p~V2 (Queen, 198~), to give pi.lG159. The EcoRl-Za~I fragment of pCQV2
cnrries both Pr and the cIs~7 gene, and on insertion into p~C153,
r~places the Pl containing fragment, fusing the ColEl origin RNAIT Seqnence
to Pr. Note that the repressor gene is transcribed away fron tne origin
IFi~ure 3). To increase plas~id stability (see bclow) when the dual-origin
v~ctcrs are maintained at 30C, the pS~101 2.85kb ~incII fragnent
~nrryin~ the ori~in of replication, replication protein and par sequence,
~las introduced into p.,lG159, by replacine the 3.25kb EcoRl-BalI fragment
w~ith the pSC101 l~incII fragment with an EcoBl linker at one end. This
~ave ph'&l65 ~Figure 3).
- 18 -
(b)Copy Number Control of Pr Driven Dual ~rigin Plasmids
To demonstrnte t~at the 'Col~l' hybrid origin driven 'Dy Pr was
controlled by tl1e ter.~per~ture sensitive repressor expressed fro~ ~he cloned
cIs~7 gene, p~'GlS9 and plUGl65 were transfo~med into E.coli El03(S).
Copy number determinations at 30C were made as previoasly described from
31~-thymidine labelled cells (Table 3). Copy number induction after
temperature sihift to 42C was followed by agarose gel electrophoresis.
To quantitate the copy n~ber change and kinetics of induction in L-brot~,
a 'spot' hybridisation method was used.
T.~BLE 3
Variation o~ Plas~id Copy Nu~ber with T3mperaturs
Pl~smidB~cterinl Strain Copies p~r Chromosome
(g.owth temperature)
p~ 9~103(S) (30C) 3-4
p~ 103tS) (30C) 3-4
~Gl5gElO3(3) (~2C~ ~ 320
p'~ l5~El03(S) (4~C) ~ 248
~ or the 'spot' hybridisation method 32P-labelled probe DMA was
pre~a~ed by n-cl~ translation (-`~aaidtiS et al, l~?). D~A samples were
orepared by tne al~aline lysis method (Ish-~orowitz and Rurke, lgSl) from l
ml of cu1ture takel1 at various times after the temperature shift. Samples
Or thc D~A preparations were treated with P~!ase at 37C for 5 minutes,
follo~Yed by incuDation with restriction enzy~e ~a~I for 30 ~inutes, and
héat denaturation at lO0C for 3 minutes. Varying amounts of the
digested ~IA preparations were spotted onto nitrocellulose filters, which
- 19 -
were then dried at 70C, ~.nd subseq~ently hybridised ovcrnight (15 ho~rs)
with t'ne den~tured probe Di`~A at 37C in 2 x SSC/50~ for~amide. ~ilters
~vere thell washed twice in 2 x SSCtS0~ formamide and once in 2 x SSC, and
then air dried, ~adioactivity was determined by co~ttin~ in a liquid
scintillation counter. The values obtained were corrected for cell growth
durin~ induction, and the magnitude of the eopy nu~ber change over the
uni~dueed value was determined.
~ otll p~i~l5~ and pi.G165 sho~led a rapid copy number induction ~ollowing a
t~p~ratuxe shift to 42C and continued incubation at 37C. Uninduced
valuas of 3-~ copies per chromosome rising to 90-100 copies after 2 hours
inlluetion, and up to 300-400 eopies after 4-5 hours induction were obtained
(Fi~u~e 4), clearly de~onstrating the eontrol of copy n~ber e~erted by the
eloned Pr promoter and cIgs7 gene.
EXA~LE 3
Construction of Dual Origin Plas~ids with Inducible Copy
N~nber under Ptrp Co~trol
The t~o ~bove Esatnples of dual origin plas~nids with eontrollable eopy
nl~ber, emI)loyed the use of temperature as the indueing agent, this ~sample
desGri'~es 'clle use of a ,metaboii~e to control copy nu~b~r. '~he 6.2~b
llindIII-E~lII fra&ment of p~iG411 was ligated to the 0~65kb ~indfII-
~a~I fra&~ent o~ pCT54 (Figure 5) using standard burfers and techniques
('~!nniatis et al, 1982) to give pMG426. This plas~id was unstable even
under a~pieillin seleetion and witll tryptophan present in the medium (whicn
should repress transeripcion fro~ Ptrp). It ~as concluded that the
instability was due to the inability to repress completely transcriptin
L3
- 20 -
~IOm Ptrp, tnereby giving a high copy nl~mber. To decrease transcription
tllrough the origin, a transcriptional ,erminator ~as inserted behYeen the
Ptrp nlld the 'ColE1' origin sequence. Such terminators reuuce levels o.
transcripLion appro~i~ately 10-fold. ~ l~Obp AluI fragment from
bncteriopll3~e T7 DNA, carr-~ing the early transcription terminator was
lignted to DNA lin~ers converting the termini to ~indIII recognitin
sites (Emtaoe et al 1983). This fragment was then lioated to ~indIII
di~ested pP~Gl~ DNA to give p?'G427 (Figure 5). p`&427 was more stable than
p~iG4~5 and when tranfor~ants were gro~Yn in ruedi~ containing tryptophan
~00 ~ it e~Yhibi~ed a low copy number. '.'1hen such a culture was
~llift~ in~o ~ediu~ lac~ing tryptophan, the copy number increasea rnpidly.
~nis demoilstratcs that ~ II transcription can be controlled by the levels
r.~tn~olites or chemicals in the external medium, and that controlled
copy ~I~.ber chQn~es can be effected by agents other than temperature.
~LE
.
Construction of Dual Osigin Piasmids with Inducible Copy
Number U~der Control of the 'tac' Promoter
In this E~nmple a vector ~ns constructed where the ~II promoter was
r~placed ~y the tnc promoter tPtac) (Rllssell and Bennct 1982), s-ch that
~py n~ber wns controlled by the addition or removal of lactose or a
~a~tose nnnlo~ue. l~ 121bp Ba~ E,coRl DNA fra~ment carrying the Ptac
~ns purificd fro~ pD~5~0. This fragment was ligated to Bam~I-Eco~l
di~ested p;~G40~ D~A> and a~picillin resistant, chloramphe~icol sensitive
transformants of ~.coli D~l were isolated. Plasmid DNA fro~ one such
~rnnsformant was isolated, analysed by restriction en~yme digestion and
shown to contain the 121bp promoter fragment inserted into the chlor-
. .,
- 21 -
ampllenicol resistance gene (pl,.G421) (Figure 6). p;;lfi421 ~N-~ was digested
~ith ra,,llI, treated with calf intestine alkalinc phosphatase, and ligated
to a l.~'ib Bam~II proL~oterless origin fragt~ent isolated from p-l~411.
~picillin resistant transfor~tants of E.coli D.~l were obtained when the
tr~nsEorDation mi~ture was plated at either 30C or ~2C. At 42C,
~he low copy nu1~be. origin is inactive. Two distinct plasmid types were
identified fro~t the transformants (p~,G415 and pi-lG416) ~Figure 6). pMG415
car~ied 3 single BamLTI origin fragment in the correct orientation,
d~nstr~m of the t~lc, whereas p.~S~416 carried 3 copies oE tke origin
~x~ e~.lt as direct repeats, also orientated for e~pression frott~ Ptac
II`C 6).
Plasmids p~ 415 and pli~416 were transformed into E.coli D900
~I CIsq)~ a strain which overproduces t e lac repressor, the
controlling element of the Ptac promoter. Induction of Ptac can be
~ffdct~d by the addition of the lsctose analogue IPT~ (isopropylthiogalac-
toside~. p~.~G41~ transformants of D900 grown in L-brot~ had a low copy
num~r as judged by agarose gel electrophoresis, but this was not increased
hy the addition of IPTG. p~lG416 transformants of D900 grown in L-broth
~lso h~d a low copy number, but this increased quic~-ly on the addition of
~ to the culture, demonstrating controllable copy number induction of
p.`~ lu f`ro-~ Ptac.
- 22 -
E~ iPLE 5
The E3pression of the Calf Stomach met Prochymosin Gone
Cloned into a Dual-origin Plasmid
(~)Construetion of p~lG168
To demonstrate that dual-origin plasmids ,vere useful for the e~pression
of clotled heterologous genes in E.coli, a plasmid ~vas constructad
carryin~ the calf storiach met-prochymosin gene. pCT70 (~mtage et al
19~3) was digested ivitll llindIII and SalI alld a ~ 2.41cb fragment
e~rryin~ the mat~proc3lymosin gene under Ptrp control was isolated. This
~ra~ont ~as li~atA(l to t~vo DN~ fragments isolated from p'liil65. the 5.6kb
~atl-~stI ra~rlellt and the llcb IlindIII-PstI fragment ~Figure 3~.
Tho r~sulting plasmid (p~lG168) isolated from transformants OI E.coli
~i~l, coDprised the cloned gene do~-nstream of .he origin of replication,
such th-qt any transcriptional read through f~om Pr. ~vould lead to
~dditional transcriptio~t of the met-procnymosin gene (Figure 3).
(b~pression of ~iet-prochymosin Protein from prlGl6~
p'lCl6~ was trausfori,ied into E,coli E103(S) and met-procnymosin
P~prassion nn~lysed by polyarrylaDIide gel electrophoresis. E.coli
l'.lG3ts) trnnsfor~iants were grown in L-broth at 30C to an OD600 o-4,
ranol s'rift_d to 4 C. Cultures ~vsre tihen incubated at 37C w;tih
shil~in~, and samplcs removed at hourly intervals for analysis of eopy
number and protoin e~pression. DNA copy number ~Yas l~setermined as deseribed
~or p;~G159 and pi`~!G165. For analysis of protein, each cultu~e sa~iple ~vas
eontrifu~ed, the pellet collected and resuspended in stop bllffer (1i~J SDS,
lO i~l tris ~ICl p~ 7.5) and an equal volluiie oE sample buffer (0.12 rl tris
slCl pll 6.~, 2G5 glycerol, 1,2 ~ mercaptoethanol, 6ri'~ SDS) a~id boile~L for 3
~i.tut~s.
- 23 -
I'oly~cyla~ 1e ~el electropilorcsis, stainin~ and dcst~ lin~ r,.etnous ~Yerc
essentially as described i1~ ~'aui2tis et a1 (l9P2). ';taine(l polyacry-
larlidc ~els ~vere sca1lncd usins a Jo~cc 1,oc~1 Chro~oscan at 530 1~m. 130th
D~r~ pl~smia copy nu.-lbcr an~ mct-prochy~osin protcin levels werc ~reatly
increased following a ~emperature s21ift from 30C to 42C (Table 4,
~igures 4 an~ 7).
Copy nu~ber ir~ere3sed rapidly durin~ a 90 ~1inute post induction period
~t 37~C, ~hilst ~et-prochy~osin acc~:1~latio~ was ~ore gradual. On the
basis ~ polyaeryl~ide gel seanning, t~e recor.bin~nt gene pro~uct
~ccumul~ted to 2t least lr~ of total extractable protein by 4-S hours after
tine tempera.ure shift. Induced copy number values for p~;~l6P, uere lo~_r
thln for the parent dual ori~in plasmid (p~'G165). but still increased fro~
3-~ per c'~rorosome to 120-150 per chror.osome. Cell viability fell
fo1lo-~in~ induetion of p';Gl6 bu~ not of p~Gl65. It ~as coneluded that
th;s loss in ~iability resulted fro~ the to~ic aeeurlulation of reeo~binant
~ene psoduct.
1`Jt~
Incrc~se in hlot-Prochymosin Gene ETpression
Timd after Induction ~ ~otal Protein as
at 37C (hrs) met-prochymosin
O < 0.5
3 7,08a
I 9.09
10.25a
a) Tnese measureQents are from ~el scans; t1:e unin~uce(l levels o~ protein
are di~ficult to measurc accurntcly 1"~ tllis mct1loa.
I`
L3
- 2~ -
~L~ 6
Expression of the Chloramphenicol Asetyl Transferase G~ne
Cloned onto a Dual Origin Plasmid
(~L~ Construction o phlGl60
Bac~use of the liraitations in the accurate quantitation of stained
protein b~nds on polyacrylamide gels, cloned ~ene e~pression on dual origin
vncto~s ~s further quantitated by assaying the increased activity of
c`nlor~ph2nicol acetyl transferase following copy nur.~iber induction of
~lasl~id p.~ l69. Plas~id p~;G169 was made Erom p~lG16~ in an analagous ~ay to
cha e~onstruction of p~lG16. (Example 5), except that the purified ilindIII-
1 rar~ent carried the structurial gene Cor chloramphenicol acetyltr:~nsf-ar~se ~nder control of Ptrp (Fi~ure 3).
b)~Y~r~ssion of Cloned Chloramphenicol Acotyl Ts~nserase
p~ rl~9 W~S transformed into F.coli ~103(S) and chloranphenicol
acctyl transIernse levels Laeasured by polyacrylaL~ide gel electrophoresis
an~ zyne assay (Sllaw 1975). E.coli E103(S) ~ransform~nts were grown
n~ i~dllced by te~,perature shift to 47.C, followed by continued
ine~ubation at 3?C as described for p..lG163 transformants in Fxa~ple 5.
Sa~l~s were removed at hourly intervals and plasmid copy n~aber determined
~ ur~ 4, ~)~ chloraripher.ico'L acetyl trar3ferase specific aci~ivities
dat~rL~inad (Sllaw 1975, ~ead and h'orthcote 19~1), (Figure 8, Table ~), and
3~ples run on poly3crylamide gels (Pigure 9). The plas~;d copy number
incre~sed with similar kinetics to that of p~i'G168, and with si~ilar
absollte v~lues (Figure 4). Chloramphenicol acetyl transferasè iLssays
àa~onstrated that the spccific activity of the en~y~ie in crude e~tracts
increased appro~ iately 80-fold as a res-llt oE the copy nu~ber induction
2~
- 25 -
~igere ~.). C~lc~la~i~as o~ t~e pLorortio~ oL ~A;tl-3c,~ble ~rotcin preseIIt
as chlorallphenicol acetyl transferase were madc from a ~noiwled~e of the
specific activitj of the pure protein (Table 5~. Uninduced levels
c~pressed from p`;~l69 represented 0.25~ extracted protein, whilst afteI 6
ho~-rs induction tnis had risen to 21.S5.
TABLE 5
Increase in Chloramphenicol Acetyl Transferase Specific Ac~ivity
Tlm~ ~t~r Induction a) ~ Total Protein as
at 37C (hrs) ChloramphenicoI
acetyl transfe~ase
0 ~.25
5.6
2 15.5
_ 18.1
18.5
21.~
6 21.9
7 20.9
e~e vall~es were calculated from the l~own specific activity of pnre
c]~lorampllenicol ncetyl transfernse ~195 units pcr mg protein).
-
~A~PLE 7
Plasmid St~bility S~udies of Dual Origin Plasmids
Plasmids p~iG165 and p~i!G16~ were transformed i~to E.~Q~ P~Y30~.
.nd p~;G163 into E.coli E103(S) to study their stability at 30Cder conditions of chemostat growth without antibio~ic selection. All
cxperimen,s were started from a single colony o~ the appropriate E.
. . ~ . .
- 26 -
Goli strain, ta~c~ iro~ an antibiotic-containing a~ar plaLe and
inoculated into t00 ~ls of L-broth i~ a 250 ml conical flask with steel
spring baffle. The culture W2S incubated in an orbital shaker (37~`, 240
rpm) until stationary phase was reache&. ~ne cells were harvested,
resuspended in sterile defined medi~ minus glucose and inoculated into a
fer~enter vessel. ~le defined medi-~ was glucose 4 gl~ ~4/2S04
5 g 1~ a2HP04 7 gl-l; ~2P0~ 3 gl-l; proline 200 ~g 1-1;
leucina, 100 mg 1-1; thiamiile 10 mg 1-1; rlgS04.7H20 200 mg 1-
C~Cl~,6~0, 5 mg 1-1, ZnS0~.7~0 20 ~g 1-1 1iinSO~.4H20 2 mg
~ m~ ; CuS0~,5~20, 5 mg 1-1, CoC12.6H20, 0.5 mg 1
1; ~cS0,t.7~0 100 m~ ; NaCl 200 mg 1-1; E~TA ~ia2 600 mg 1-
1; ~a0H 1~0 ~g 1-1]. Tnis nedium was supplemented with tryptophan 100
~g 1-1 unless othen~ise stated. Anti-foam (polypropylene-glycol 2000)
present in the ~ediu~ at 0.001r,~ v/v, Tlle fermenter cell population wns
allo~Yed to grow 2S a closed batch system until the biomass ~as at least 605'~
of the maxi~um supported by the medium. The pump was then turned on and
t~a ~ystem run as a cher,~ostat. When the total bio~ass i3 the fermenter was
constnnt, it was assu~ed that the initial transient gro~vth phase had ceased
~nd thera~fter the number of generations in the steady state was calculated
usin~ tha formula~ n(nur.~ber of generations) = ~tlln2 whe~e ,u = growth rate
whic~ is aqual to the dilution rate under steady state conditions, and t =
t.ima,
S3m~1es wc~e withdrawn from the continuous cultures, diluted and plated
onto L-a~a~. 100 single colonies were picked onto both antibiotic
supplc~entcd agar and L-agar as a control. The numoer of colonies
resistant to the antibiotic was expressed as a percentage of the number
growing on the L-agar plate, and taken as representillg t~e proportion of
~iL2721~3
tha popul2Li~n carryin~ the plasmid. Sa;nples were re~oYcd periodically and
plas~lid I,NA prepa~ations ~ade and annlysed for 2ny gross alte}ations.
Cha~ostat analysi3 of stability of p~'G165 and pi;'G16S in E.coli
~V30S havs demonstrated that these plas~ids are completely stable for at
leas, 6S generations (Table 6) ~Yith tryptophan in the ~edium. Under these
conditions transcription of met-prochy~osin gene on p~G168 was repressed.
Plasmid Stability
E.coli Plasmid Stability Numb~r of ~e~erations
St~in % ~ Trp - Trp
r~v3o~ p~ l65 100 72.5 30
~V3~ p`;G16S 100 68 ~n
~lOa (~)p~I~168 105 20
Stability analysis is still in progress for grovth in the abse..ce of
~ry~tophanl but a~ter 30 generations no plasmid loss has been detected.
~tability analysis of p~l&l68 in E. coli E103(8) is st~ -cgress
but nftar 20 ~en~rations no pl2s~ cs had been obser~ed. The dual-
ori~in plasnids appear ~o be s-table ~nder conditions of lo~v copy nu~ber,
.?~.~ raplication being directed frorl the par~, pSC101 ori~in.
.. .. ... .. .. ..
Yt~3
- 2~
REFF.RENCES
Enta~e, J.S, Angal, S, Doel, ~I.T, liarris, T.J.P. Jen~ins, B, Lilley, G
and Lo~Ye, P.A. Proc Natl Acad Sci U~A 80 (19S3) 3671-3675.
llashi~oto-Gotoh, TJ Fran1~1in, F.C.~, Nordhei~, A and Tim~is, ~.N. Gene
16 ~19Sl) 227-235.
Ish-~orowitz, D and Bur~e, J.F. Nucl Acids ~es 9 (1981) 2989-2998.
iatis, T, Fritsch, E.F and Sambroo~, J. Molecular Cloning Cold
Sprino ~arbor E3boratory (1982).
t~een, C. J ~ol and Applied Genet 2 (1983) 1-10.
r~ad, S.~i alld ~30rthcote, D.~. Analytical Biochem 116 (1981) 53-64.
Russell, ~.~ and Bennett, G.N. Gene 20 (1982) 231-243.
San~r. F, ~ic~len, S. and CoulsPn, A.r~. Proc Natl Acad Sci USA 74
(1977), 5~63-~4S7.
Sha~, '.~.V. ~'etho~s in ~nz~ology 43 (1975) 737-755.
Tatch~ , Nas~yth, ~.A and ~all, B D. Cell 27 (1981) 25-35.
. ~ , .
~ ~2~-~ j
_ 29 -
List of Escherichia coli strains mentioned in the Examples
Strain Genotype Reference or Source
DHl F ,rec~l,endAl,gyrA96, Man~atis et al (1982)
thi-l,hsdR17(rk,mk)
supE44,~
HB101 F ,hsdS20(rB,mB),recA13,Maniatis et al (1982)
aral4,proA2,1acYl,galK2,
rpsL20,(Smr),xyl-5,mtl-1,
supE44,~
RV308 ~ ,F ,Smr,gal305 a)
ATCC 31608
D900 F'i q,~p ,proA B /laci , J.R.Sadler (Denver,
laco+,lac~+,lacy ,proB, USA)
Sm .
QY7 F ,lacam,trpam, ~bio256- S.Brenner (Cambridge
-cI857~ Hl, ~uvrB. UK)
El03S L.D.Simon (New
Jersey, USA)
~)
ATCC is the Rmerican Tylce Culture C~llection
desi~nation .
. . . ... . . . ..... ... . .
~7~
- 30 -
The present inventlon makes possible -the crea-tion
o~ a new l~ind o~ plasmid in which the copy number can be
deliberately controlled by regula-table promote~ such as
PL or PR`.
The presence of the XhoI linker 30 base pairs from
the 5' end of RNA II allows the replacement of the
natural promoter for RNA II by other promoters. Thus,
plasmid pMG404 put RNA II under the control of the
kanamycin resistance gene promoter on the plasmid
1~ pH~'15 it is believed that both originsin pr~404 func-
ti~n, the teMperature sensitive pSC101 origin from
~H~G41~ ~nd the ColEl origin under control of the KmR
promoter. Although the exact point of DNA initiation
h~s rot been determined in these plasmids, -the function-
ing o~ the hybrid origin is indicated by the highercopy number of pMG404 than pHSG415, and the replication
o~ p~IG40~ at 42C. The construction of pMG410, a
recircularised BalI fragment carrying the hybrid origin
but n~ the pSC101 temperature sensitive originj is
~0 ~dditional evidence that the hybrid origin is functional.
The properties of pMG404 demons-trate tha-t the RNA
II promoter and the first 30 bases of RNA II can be re-
placed by another promoter without abolishing the
initiation of DNA replication. However the Km
~5 promo-ter is constitutively expressed and it is not
there~ore possible to alter the copy number of pMG404.
Several well-defined controllable promoters exist which
~unction in E.coli; the PL promoter from bacteriophage ~
w~s used to construct plasmid pMG411, with a ColEl hybrid
origin under direct control. pMG411 retained the origin
from pHSG415, and was maintained at a low copy number a-t
30 in a strain carrying a ts ~ repressor gene (QY7).
~lhen the ~ repressor was inactivated at 42, -the copy
number increased, indicating that replication of the
ColEl origin was being driven by PL. It is therefore
clear that the copy number of such plasmids can be
- 31 -
deliberately controlled from regulated promoters and
this opens the way to constructing plasmids whose copy
number can be controlled either by tempera-ture (as
wi~h PL in pMG411~ or more importantly by the altera-
5 tion of the concentration of metabolites such as trypto-
phan or lactose.