Note: Descriptions are shown in the official language in which they were submitted.
HOECHST AKTIENGESELLSCHAFT HOE 85tF 217 Dr.KH/mu
Sucrosetricarboxylic acid, process for its preparation,
and its use
The present invention relates to sucrosetr;carboxylic
acid and its sacondary products such as salts, lactones~
esters, amides and the like, to a process for preparing
and uslng the same.
It is known that the catalytic ox;dation of carbohydratesl
specifically that of the primary hydroxymethyl groups con-
tained therein, proceeds slowly and in most cases non-
uniformly. For instance, oligomeric carbohydrates such as --
branched arabinoxylan from rye flour are oxidized in 4 days
at 65C in only 4% to uronic acids. It is true that the
oxidation of sucrose has also been mentioned, but state-
ments about the ;solation of the reaction products were
not mentioned at the time. It was merely stated that it
~as found in a subsequent total hydrolysis that the hydro-
lyzate contained only small amounts of glucuronic acid.
The oxidation of sucrose with a platinum-alumina catalyst
and oxygen has also been previously described (German
Patent 886,305, Example 3 and the corresponding US Patent
2,845,439~ Example 4). After a 6-hour treatment this
oxidation gave only 60% of the theoretically expected yield
of the corresponding glucuronic acid derivative. However,
the isolation of this compound is not described.
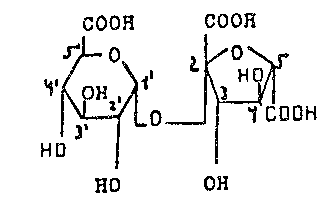
The invention provides, then, sucrosetricarboxylic acid
(i.e. (~-D-arabinofuranaric-2-hexulosyl)-~-D-glucopyrano-
sid~ronic acid) of the formula
COOH COOH
,o ~OOH
HO
~ OH
~ '7~
-- 2
The invention further provides a process for preparing
sucrosetricarboxylic acid, ~hich comprises o~idizing suc-
rose with oxygen, if desired in a mixture with inert gases,
for example in the fsrm of air, by means of a signif;cantly
more effect;ve catalyst than plat;num/alumina. Su;table
catalysts are those of platinum metals, such as paLladium,
but in particular platinum itself, on activated carbon, in
part;cular the more active types. These generally contain 5
- 10% by ~eight of metaL, in particular platinum. In addit-
ion to a distinct increase in the rate of oxidation, an ;n-
creased selectivity of the oxidation bet~een primary and
secondary hydroxyl groups of the carbohydrates, in particu-
lar of nonreducing carbohydrates, is achieved. Preferably
the oxidation is carried out by treating ~he solid catalyst ~n an
aqueous reaction medium ~ith gaseous oxygen, i.e. in a three-
phase reaction. Th;s three-phase reaction is carried out for
example in a bubble column reactor ~hich can be operated not
only batch~ise but also continuously. In preferred embodi-
ments, highly concentrated oxygen is used and the reaction
solution is recycled, ~hich ~acilitates setting, and keeping
constant, the pH. It is within the capacity of the skilled
~orker to set the reaction solution to a selected and suit-
able reaction temperature and to optimize the concentration
ratio of substrate/catalyst or substrate/oxygen.
The process according to the invention is generally carried
out at 30C to the boil, preferably at 50 to 95, in particu-
lar 60 to 90C. In general, atmospheric pressure is em-
ployed, but it is also possible to employ superatmospheric
pressure, ~hich is a way of increasing the supply of oxygen
and/or the reaction temperature. In the process according to
the invention9 maintaining certain sucrose concentrations is
part;cularly advantageous; concentrations below 5~ by weight
easily give rise to an over-oxidation and above 20~ by weight
only to comparatively low conversions under atmospheric
pressure. In general, pH values of 5 to 9, preferably 6 to
8, are used. The course of the reaction can be monitored by
~B
`, ~ ..
- 3 -
sampling~ for example by means of gas chromatography
analysis of the derivatized, for example silylated, pro-
ducts.
The sucrosetricarbo~ylic acid formed ;s generally obtained
in a mixture with less oxidized intermediates, i.e. vari-
ous monocarboxylic and/or dicarboxylic acids. The oxida-
tion can be discontinued at as early a stage as a tri-
carboxylic content of at least 20%. However, the oxid-
ation is generally cont;nued until at least 30 or 40 andpreferably more than 60 or 70% o~F sucrosetricarboxylic
acid has been Formed. This sucrosetricarboxylic acid can
be concentrated and isolated out of the reaction mixture in
a convent;onal manner.
The process according to the invention can be carried out,
for example, in a jacketed reactor which holds the sus-
pension of the catalyst in the aqueous medium and wh;ch
conta;ns at the bottom a frit or another correspondingly
Z0 suitable porous membrane and through which a gas stream,
very finely divided by this separating membrane, flows from
the underside. For economic and safety reasons, the oxygen
is expediently passed through the reaction medium at such a
rate that the catalytically activated oxygen is just con-
sumed at the upper end of the bubble column. To improvethe degree oF mixing and to prolong the time of exposure to
the oxygen, it can be advantageous to stir the reaction
mixture.
On account of its chemical structure or structural ele-
ments, the sucrosetricarboxylic acid formed is suitable
as such or in the form of its immediately obtainable reac-
tion products, i.e. in particular mixtures with the inter-
mediates, for applications in the field of complexing
agents, for example analogously to gluconic acid and
glucaric acid in washing agent formulations, as food
additives, for example for the applications customary for
citric acid, as a polyfunctional reactant (crosslinking)
and also as a starting material for chemical reactions
~hydrophili~ing reagent).
8:~ -
-- 4
The process according to the invention has made it possible
to carry out the oxidation of sucrose ;n such a way as to
form the h;therto unknown sucrosetr;carboxylic acid.
Examples
_
1) In an externally heated, upright glass tube (dia-
meter: 50 mm, length: 80 cm) having a frit bottom and,
installed at a point slightly thereabove, a discharge means
for the reaction mixture, a stream of oxygen (about 25
liters (S.T.P.)/h) flows upwardly through a solut;on of 1209
of sucrose ;n 1.2 l;ters of ~ater and also 60 9 of added
platinum catalyst (5% of Pt/active carbon). The acids
formed by the oxidation are wholly or partly converted ;n-
to the sodium salts either by feeding in sod;um hydroxidesolut;on, ~ith the attendant Poss;b;l;ty of pH control, or
by means of the correspond;ng molar amounts of ;nit;ally
introduced sodium hydrogencarbonate. At a eemperature of
80C ;n the ox;dation and neutralizat;on and a pH of 6.5
held constant dur;ng both stages, the products have after
s;lylation and according to gas chromatography analysis
the follo~ing composit;ons as a function of the reaction
time (see Table 1):
5 Table 1 Composition of oxidation products as a function
of reaction time t;n percent)
6 h 12 h 1~ h
. ~
Sucrose consumed - -
Monocarboxylic ac;ds 20.9 5.2 1~5
30 (2 isomers)
Dicarboxylic acids 38.9 39.6 2917
t2 isomers)
Tricarboxylic acid I 5.1 22.3 35.3
The reaction solution ;s filtered to remove the catalyst,
and the filtrate is concentrated in a thin film evaporator
and freeze-dried. Y;eld 1û4 9 (86.7% by weight). The
total acid content is 7.18 mEqtg (theoretical value for
sucrosetricarboxylic acid 7.80 mEq/g).
To characterize the sucrosetricarboxylic acid (STA), the
reaction mixture is converted by means of commerc;ally
ava;Lable cation exchanger ;nto the free acids and freeze-
dried after f;ltration. After complete acetylation w;th
an excess of ace~ic anhydride and equimolar amounts of
p-toluenesulfonic acid (20C, 20 h), the sirupy residue is
chromatographed over silica gel (eluent: methylene chloride/
methanol 10:1 v.v.). The pentaacetylated sucrosetricarb-
oxylic acid obtained last with polar eluent in the chromato-
graphy is converted with catalytic amounts of sodiummethanolate in methanol into the trisodium salt.
1H-NMR (D20, 400 MHz): ~= 5.45 (d,H-1',J1 ,2 = 3.75 Hz~,
4.23-4.12 (m, H-5',H-3,H-4,H-5), 3.82, (struct. t, H-3'),
J3-,4-= 9.5 Hz, J2',3'= 9.8 Hz), 3.54 (dd, H-2', J1' 2~=
3.75 Hz, J2' 3~= 9-8 Hz), 3.4S (struct. t,H-4',13 4-=
J4 ,5~=9.5 Hz).
Z0 GC-MS (gas chromatography/mass spectroscopy) (after s;lyl-
ation, chemical ionization ~ith isobutane) m/z = 961 (M~1-
fragment of the 8-fold silylated compound, relative inten-
sity about 0.1%).
FA~-MS (fast atom bombardment) (sodium salt used, glycerol
as matrix) m/z = 451 (MH~, relative intensity 36X).
2) 9y the method of Example 1, 120 9 of sucrose, dis-
solved in 1.2 liters of water, are oxidized at 60C and
pH 7.5 in a stream of oxygen. Table 2 shows the composi-
tions determined by GC analysis.
-- 6 ~
Table 2 Composition of oxidation products as a function
of reaction t;me (in percent)
8 h 28 h 52 h 84 h
_ _ . . . , .. . . _ _
Sucrose 1.1 - - -
S Monocarboxylic acids 28.9 10.3 5.7 4.7
(2 ;somers)
Dicarboxylic acids 35.8 36.1 27.3 23.0
(2 isomers)
Oxalic acid 5.9 10.8 12.6 10.9
10 Tricarboxylic acid 1 4.6 20.7 32.7 40.9
Filtration to remove the catalyst and freeze-drying the
filtrate leaves 93.8 9 (78.0% by weight).