Note: Descriptions are shown in the official language in which they were submitted.
~ 2r'7~.~.~ GCl83a
..
AZETIDINES
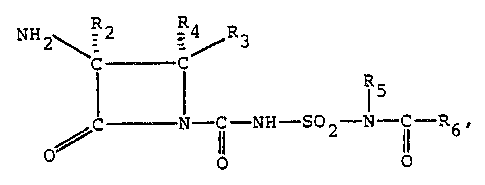
B-Lactams having the formula
I R-2 -4
R -NH-C - C-R3 IR5
~C - N-C~-NH-SO~-N -i~-R
O
and salts thereof, have antibacterial activity.
~ In formula I, and throughout the specification,
; lO the symbols are as defined below.
Rl is acyl;
R2 is hydrogen or methoxy;
i R3 and R4 are the same or different and
each is hydrogen, alkyl~ alXenyl, alkynyl,
cycloalkyl, aryl or a 5,6 or 7-membered
heterocycle or one of R3 and R4 is hydrogen
and the other is azido, halomethyl, dihalomethyl,
trihalomethyl, alkoxycarbonyl, 2-phenylethenyl,
` 2-phenylethynyl, carboxyl, -CH~X1, -S-X2,
~'
GCl83a
--2--
3 I 3
--X2 ~ -O-CI-X~ , -S~C-X4 , or -A-C-NX6X7 ;
X5 X5
X1 is azido, amino (-NH2), hydroxy, alkanoyl-
amino, alkylsulfonyloxy, arylsulfonyloxy, aryl,
cyano, -S-X2 or -O-X2 ,
X2 is al~yl, substituted alkyl, aryl, arylalkyl,
alkanoyl, substituted alkanoyl, arylcarbonyl or
heteroarylcarbonyl;
one of X3 and X4 is hydrogen and the other
is hydrogen or alkyl, or X3 and X4 when taken
together with the carbon atom to which they are
attached form a cycloalkyl group;
X5 is formyl, alkanoyl, arylcarbonyl,
arylalkylcarbonyl, carboxyl, alkoxycarbonyl,
aminocarbonyl (NH2-C-), (substituted amino)carbonyl,
or cyano (-C--N) ;
A is -CH=CH-, -CH2-CH=CH-, ~(CH2)~m ~ -(CH2)m,-O-,
-(CH2)m,-NH , -(CH2)m,-S-CH2-, or -(CH2)m,-O-CH2-;
~ m is 0~ 1, 2 or 3;
:~ m' is 1 or 2;
X6 and X7 are the same or different and each
is hydrogen or alkyl, or X6 is hydrogen and X7
is amino, substituted amino, acylamino or alkoxy;
R5 is hydrogen, alkyl or aryl;
R6 is hydrogen, alkyl, aryl, a 5, 6 or
7-mem~ered heterocycle, -NR7R8, or - (CH2) n~x
wherein n is 1,2,3 or 4 and X is halogen, aryl,
alkoxy, aryloxy or -NRgRlo ;
R7 and R8 are the same or different and
each is hydrogen, alkyl or aryl, or R7 is hydrogen
and R~ is a 5,6 or 7-membered heterocycle or
-(CH2)n-Y wherein n is 1,2,3 or 4 and Y is
alkoxy, amino(-NH2), alkylthio or halogen; and
'7~ ;` GC:L8 3a
Rg and R1o are the same or different and
each is hydrogen or alkyl, or R9 i~ hydrogen
and Rlo is a 5,6 or 7-membered heterocycle.
Listed below are definitions of various
terms used to describe the ~-lactams of this
invention. These definitions apply to the
terms as they are used throughout the specifi-
cation (unless they are otherwise defined in
specific instances) either individually or
as part of a larger group.
The terms "alkyl" and "alkoxy" refer to
both straight and branched chain groups. Those
groups having 1 to 10 carbon atoms are preferred.
The terms "cycloalkyl" and "cycloalkenyl"
refer to cycloalkyl and cycloalkenyl groups
- having 3,4,5,6 or 7 carbon atoms.
The terms "alkanoyl", "alkenyl", "alkynyl",
"alken-l-yl" and "alkyn-l-yl" refer to both
straight and branched chain groups. Those groups
ha~ing 2 to 10 carbon atoms are preferred.
The term "halogen" refers to fluorine,
chlorine, bromine and iodine.
The term "protected carboxyl" refers to
a carboxyl group which has been esterified with
a conventional acid protecting group. These
groups are well known in the ar~; see, for
example, United States patent 4,144,333,
issued March 13, 1979. The preferred protected
carboxyl groups are benzyl, benzhydryl, t-butyl,
and ~-nitrobenzyl esters.
The term "aryl" refers to phenyl and phenyl
substituted with 1,2 or 3 amino(-NH2), halogen,
hydroxyl, trifluoromethyl, alkyl (of 1 to 4 carbon
atoms), alkoxy (of 1 to 4 carbon atoms) or carboxyl
3S groups.
GC183a
7X'~f~;
The expression "a 5, 6 or 7-m~mbered
heterocycle" refers to substituted and unsubstituted,
aromatic and non-aromatic groups containlng one
or more nitrogen~ oxygen or sulfur atoms. Exemplary
substituents are oxo(=O), halogen, hydroxy, nitro,
amino, cyano, trifluoromethyl, alkyl of 1 to 4
carbons, alkoxy of 1 to 4 carbons, alkylsulfonyl,
phenyl, substituted phenyl, 2-furylmethylenei~ino
( ~ ~, phenylmethyleneimino and substituted alkyl
groups (wherein the alkyl group has 1 to 4 carbons).
One type of "5,6 or 7-membered heterocycle" is
the "heteroaryl'' group. The term "heteroaryl"
refers to those 5,6 or 7-membered heterocycles
which are aromatic. Exemplary heteroaryl groups
are substituted and unsubstituted pyridinyl,
furanyl, pyrrolyl, thienyl, 1,2,3-triazolyl,
1,2,4-triazolyl, imidazolyl, thiazolyl, thiadiazolyl,
pyrimidinyl, oxazolyl, triazinyl, and tetxazolyl.
Exemplary nonaromatic heterocycles (l.e., fully
or partially saturated heterocyclic groups) are
substituted and unsubstituted piperidinyl,
piperazinyl, imidazolidinyl, oxazolidinyl,
pyrrolidinyl, tetrahydropyrimidinyl, and dihydro-
thiazolyl. Exemplary of the substituted 5, 6 or7-membered heterocycles are 2-oxo-1-imidazolidinyl,
3-alkylsulfonyl-2-oxo-1-imidazolidinyl, 3-benzyl-
imino-2-oxo-1-imidazolidinyl, 3-alkyl-2-oxo-1-
imidazolidinyl, 3-aryl-2-oxo-1-imidazolidinyl,
3-(2-hydroxyethyl)-2-oxo-1-imidazolidinyl,
3- L (l-methylethylidene)amino]-2-oxo-l-imidazolidin
G~183a
3-benzyl-2-oxo-1-imida~olidinyl, 3-(2-aminoethyl)-2-
oxo-l-imidazolidinyl, 3-~2-(alkylamino)ethyl~-2-
oxo-l--imidazolidinyl, 3-[2-(dialkylamino)ethyl]-2-
oxo-l-imidazolidinyl, 3-amino-2-oxo-1-imidazolidinyl,
3-ureido-2-oxo-1-imidazolidinyl, 3-[(alkoxycarbonyl)-
amino]-2-oxo-1-imidazolidinyl, 3-[2-~(alkoxycarbonyl)-
amino]ethyll-2-oxo-1-imidazolidinyl, 2-oxo-1-
pyrrolidinyl, 2-oxo-3-oxazolidinyl, 4-hydroxy-6-
methyl-2-pyrimidinyl, 2-oxo-3-pyrroldinyl, 2-oxo-3-tetra-
hydrofuranyl, 2,3-dioxo-1-piperazinyl, 2,5-dio~o-1-
piperazinyl, 4-alkyl-2,3-dioxo-1-piperazinyl,
and 4-phenyl-2,3-dioxo-1-piperazinyl.
The term "substituted alkyl" refers to
alkyl groups substituted with one, ox more, azido,
amino(-NH2), halogen, hydroxy, carboxy, cyano,
alkoxycarbonyl~ aminocarhonyl, alkanoyloxy, alkoxy,
aryloxy, a 5,6 or 7-membered heterocycleoxy,
mercapto, alkylthio, arylthio, alkylsulfinyl,
or alkylsulfonyl groups.
The term "substituted amino" refers to a
group having the ormula -NYlY2 wherein Yl is
hydrogen, alkyl, aryl, or ary~alkyl, and Y2 is
alkyl, aryl, arylalkyl, hydroxy, cyano, alkoxy,
phenylalkoxy, or amino(-NH2).
The term "substituted alkanoyl" includes
within its scope compounds havin~ the formula
o
(substituted alkyl)-C- (wherein "substituted
alkyl" is de-fined above) and phenylalkanoyl.
~l27~726 ,~ .
6-
, ... ..
The term "acyl" refers to all organic radi-
cals de:rived from an organic acid (l.e., a carboxy-
lic acid) by removal o the hyd.roxyl group. Cer-
tain acyl yroups are~ of course, preferred but this
preference should not be viewed as a limitation of
the scope of this invention. Exemplary acyl groups
are those acyl groups which have been used in the
past to acylate ~ lactam antibiotics includins 6-
aminopenicillanic acid and derivatives and 7-amino-
cephalosporanic acid and derivatives; see, for ex-
ample, Cephalosporins and Penicillins, edited by
Flynn, Academic Press (1972~, German Offenlegungs-
schrift 2,716,677, published October 10r 1978, Bel-
gian patent 867,994, published December 11, 1978,
Vnited States patent 4,152,432, issued May 1, 1979,
United States patent 3,971,77~, issued July 27, 1976,
United States patent 4,172,199, issued October 23,
1979, and British patent 1,348,894, published March
27, 1974. The following list of acyl groups is pre-
sented to further exemplify the term "acyl"; it should
not be regarded as limiting that term. Exemplary acyl
groups are:
(a) Aliphatic groups having the formula
R -C-
wherein Ra is alkyl; cycloalkyl; alkoxy; alkenyl;
~æ ~7Z~ GC183a
--7--
"
cycloalkenyl; cyclohexadienyl; or alk~l or alkenyl
substituted with one or more halogen, cyano,
nitro, ~lino, mercapto, alkylthiot or cyanomethyl-
tllio groups.
(b) Car~ocyclic aromatic groups having the
formula
Rc
(CH23n~
CH-C- ,
Re
Rb ~Rd
~=7 C~2
c
Rb ~ C 2
~ 72 ~ ~, GClg3a
--8--
-C~-C- or
S
~H2
wherein n is 0, 1, 2 or 3; ~, Rc, and Rd each
is independently hydrogen, halogen, hydroxyl,
nitro, amino, cyano, trifluoromethyl, alXyl
of 1 to 4 carbon atoms, alkoxy of 1 to 4 carbon
atoms or aminomethyl; and Re is amino, hydroxyl,
a carboxyl salt, protected carboxyl, formyloxy,
; a sulfo salt, a sulfoamino salt~ azido, halogen,
hydra~ino, alkylhydrazino, phenylhydrazino, or
[(alkylthio)thioxomethyl~thio.
Preferred carbocyclic aromatic acyl groups
include those ha~ing the formula
.
HO ~
~H2-C-,
~ 2 2
,:
~z~ GC183a
~ ~ ~9._
HO ~ ~ Re is pre~erab~y
: ~e
: 5
a carboxyl salt or sulfo salt) and
O
~ 7~-C- (Re is preferably
Re
a carboxyl salt or sulfo salt).
(c) Heteroaromatic groups ha~ing the
formula
o
R~-(CH~)n-C-t
Rf-CH-C-
; Re
O
R E--O--CH2--C-- ,
~:, , O
-S-CH2--C- r
O O
R~-C -C- ~
wherein n is 0, 1, Z or 3; Re is as defined
above; and R~ is a substituted or unsubstituted
5-, 6- or 7-membered heterocyclic ring contamlng
3n 1,2,3 or 4 (preferably 1 or 2) nitrogen, oxygen
~ and sulfur atoms. Exemplary heterocyclic
:;
a 2~92 ,~ GC183a
-ln-
rings are thienyl, ~uryl, pyrrolyl, pyrid.inyl,
pyra~lyl~ pyrazinyl, thiazolyl, thiadiazolyl~
pyrimidinyl ancl tetrazolyl. :Exemplary suhstit:uents
are halo~en, hydroxyl, nitro, a~ino, cyano,
trifluoromethyl, alkyl of 1 to 4 carbon atoms,
alkoxy of 1 to 4 carbon atoms, or
; HOOC-CH-C~-O-C-N~-.
NH2
Preferred heteroaromatic acyl groups
include those groups of the above formulas
wherein Rf is 2-amino-4-thiazolyl, 2-amino-5-
halo-4-thiazolyl, 4-aminopyrimidin-2-yl,
5-amino-1,2,4-thiadiazol-3-yl, 2-thienyl,
? 2-furanyl, or 6-aminopyridin-2 yl.
(d) [[(4-Substituted-2, 3-dioxo-1-piper-
a~inyl)carbonyl~amino~arylacetyl groups ha~ing
the ~ormula
~ 20 e '1~
-C-CH-NH-C-N N -Rh
Rg O ~ ~O
wherein Rg is an aromatic group (including
carbocyclic aromatics such as those of the
formula Rc
Rb` ~ d
30 and heteroaromatics as included within the
definition o Rf); and Rh is alXyl, substituted
2 27 ~; GC183a
~L 7 ~
alkyl (wherein the alkyl group is s-~stituted
with one or more halogen, cyano, nitro, aminc
or mercapto groups), arylrnethyleneaminQ (i e.,
~N=C~-Rg wherein Rg is as defined above),
~ylcarbonylamino (i.e., ~N~~C~Rg wherein Rg
is as defined abo~e) or alkylcarbonylamino.
Preferred ~[~4-substituted-2,3-dioxo-1
piperazinyl)carbonyl]amino]arylacetyl groups
include those wherein ~ is ethyl, phenylmethylene-
amino or 2-furylmethyleneamino.
(e) (Substituted oxyimino)arylacetyl groups
ha~ing the formula
O
--C--C=N--O-R
Rg
wherein R is as defined above and Ri is hydrogen,
alkyl, cycloalkyl, alkylaminocarbonyl, arylamino-
O
carbonyl (i~e., ~C~NH~Rg wherein Rg is as defined
above) or substituted alkyl (wherei~ the alkyl
group is substituted with 1 or more halogen,
cyano, nitro, amino, mercapto, alkylthio,
aromatic group (as defined by Rg), carboxyl
(including salts thereof), amido, alkoxycarbonyl,
phenylmethoxycarbonyl, diphenylmethoxycarbonyl,
hydroxyalkoxyphosphinyl, dihydroxyphosphinyl,
hydroxy(phenylmethoxy)phosphinyl, or dialkoxy-
phosphinyl substituents)~
~ 7~ GC1~3a
-12-
Preferred ~substituted oxy ~ino)aryl.ac~tyl
group~ include those wherein Rg is 2-amino-4-
thia~olyl. Also preferred are those groups
wherein Ri is me~lyl, ethyl, carboxymethylt
l-carboxy-l-methylethyl or 2,2,2-trifluoroe~hyl.
~ f) (Acylamino)arylacetyl groups having
the formula
O O
-C-CH~NH-C-R
: Rg
wherein R is as deined above and Rj is
R
Rb ~ (CH2)n-0-, amino, alkylamino, (cyanoalkyl~-
amino, amido, alkylamido, (yanoalkyl)amido,
-CH2--NH--C~ -CH-cH2-c-N~-cH3~
.~
HO
~2--N (CX2'-CH2 OH)2, ~CH3
OH OH
~?~ O
~7~7~,~ GC183a
Preferred (acylamino) ~rylacetyl groups o~
the above formula include those groups wherein
R. is amino or amido. Al~o preferred are
those groups wherein Rg i~ pheny~ or 2-thienyl.
s (g) 1 113-Substitu~ed-2-oxo-1-imida~oli-
dinyllcar~onyl~amino~arylacetyl groups having
the formula
R
o ~ ~c~
-C-C~-NH-C-N N-Rk
Rg CH CH~
wherein ~g is as defined above and ~ is
hydrogen, alkylsulfonyl, arylmethyleneamino
(i.e., ~N-CH~Rg wherein Rg is as defined
above), -C-Rm (wherein Rm is hydrogen, ~lkyl
or halogen substituted alkyl), aromatic group
(as defined by Rg above), alkyl or substituted
alkyl (wherein the alXyl group is substituted
with one or more halogen, cyano, nitro, amlno
or mercapto groups).
~ Prefexred ~[3-substituted-2-oxo-1-imida~oli-
; ainyl3carbonyl~amino~arylacetyl groups of the
a~ove formula include those wherein Rg is phenyl
or 2-thienyl. Also preferred are those sroups
wherein Rk is hydrogent methylsulfonyl, phenyl-
methyleneamino or 2-furylmethyleneamlno.
C183a
The compounds oE this invention form basic
salts with various inorganic: and organic bases
which are also within the scope of this invention.
Such salts include ammonium salts, alkali metal
S salts, alkaline earth metal salts, salts with
organic bases, e g., dicyclohexylamine,
benzathine, N-methyl-D~glucamine, hydrabamine
and the like. The pharmaceutically acceptable
salts are preferred, although other salts are
also useful, e.~, in isolating or purifying
the product.
Some of the compounds of this invention
may be crystallized or recrystallized from
solvents containing water. In these cases
water of hydration may be formed. This invention
contemplates stoichiometric hydrates as well as
compounds containing variable amounts oE water
that may be produced by processes such as
lyophilization. R R5 1
~-Lactams having a -C-NH-S02-N ~ C-R6
substituent in the l-position and an
acylamino substituent in the 3-position contain
at least one chiral center - the carbon atom
(in the 3-position of the ~-lactam nucleus)
to which the acylamino substituent is
attached. This invention is directed to
those ~-lactams which have been described above,
wherein the stereochemistry at the chiral
center in the 3-position of the ~-lactam nucleus
is the same as the configuration at the carbon
atom in the 6-position of naturally occurring
C183a
penicillins (e.g., penicillin G) and as the
configuration at the carbon atom in the
7-position of naturally occurring cephamycins
(e.g., cephamycin C).
Also included within the scope of this
invention are racemic mixtures which contain
the above-described B-lactams~
1 0 ~I 1~5 1l
B-Lactams having a -C-N~-SO2-N - C-R6
substituent in the l-position of the G-lactam
nucleus and an acylamino substituent in the
3-position of the ~-lactam nucleus, and salts
thereof, have activity against a range of
gram-negative and gram-positive organisms.
The compounds of this invention can be
used as agents to combat bacterial infections
(including urinary tract infections and
respiratory infections) in rnammalian species,
such as domesticated animals (_ g., dogs,
~ cats, cows, horses, and the li~e) and humans.
- For combating bacterial inections in
mammals a compound of this invention can be
administered to a mammal in need thereof in
an amount of about 1.4 mg/kg/day to about
350 mg/kg/day, preferably about 14 mg/kg/day
to about 100 mg/kg/day. ~11 modes of adminis-
tration which have been used in the past to
3n deliver penicillins and cephalosporins -to the
site of the infection are also contemplated
1~ 7Z~Z~; GC].83a
-16-
for use with the novel family of ~-lactarns
of this invention. Such methods of administration
include oral, intravenous, intramuscular, and
as a suppos.itory
0 15 11
A -C-NH-SO2-N - C-R6 activatlng group can
be introduced onto the nitrogen atom of a
~-lactam by reacting a ~-lactam having the
formula
II
-NH R2 R4
C C-R3
//C NH
O
wherein Al is a nitrogen protecting group,
with the appropriate isocyanate having the
formula
III R5 o
O=c=N-so2 -N--C -R6
As the protective group Al ~or amino above,
: any of those used for this purpose in the field
of ~-lactam or peptide synthesis may conveniently
:: be employed. Examp~es of such amino protecting
group include aromatic acyl ~roups such as phthaloyl,
p-nitrobenzoyl, p-tert-butYlbenzoyl, p-tertbutyl-
benzenesulfonyl, henzenesulfonyl, toluenesulronyl,
~7~
GC183a
-17--
etc., aliphatic acyl groups such as Eormvl, acetyl,
propionyl, monochloroacetyl, dichloroacetyl, tri-
chloroacetyl, methanesulfonyl, ethanesulfonyl, ~ri-
fluoroacetyl, maleyl, succinyl, etc., and
esterifled carboxyl groups such as methoxycarbonyl,
ethoxycarbonyl, t-butoxycarbonyl, isopropoxy-
carbonyl, 2-cyanoethoxycarbonyl, ~ -trichloro-
ethoxycarbonyl, ~-trimethylsilylethoxycarbonyl,
~-methylsulfonylethoxycarbonyl, benzyloxycarbonyl,
p-nitrobenzyloxycarbonyl, p-methoxybenzyloxy-
carbonyl, di-phenylmethyloxycarbonyl, methoxymethyl-
oxycarbonyl, acetylmethyloxycarbonyl, isobornyloxy-
carbonyl, phenyloxycarbonyl, etc., as well as non-
acyl amino-protecting groups such as trityl,
2-nitrophenylthio, benzylidene, 4-nitrobenzylidene,
trialkylsilyl, benzyl, p-nitrobenzyl, proton, etc.
The choice of amino-protecting group is not critical
in the present invention.
The reaction is preferably run in an organic
solvent, e.g., an inert solvent such as tetrahydro-
furan or dimethoxyethane, in the presence of a base
such as triethylamine or alkyl lithium.
The activating group can also be inserted onto
the Formula II ~-lactam in sequential segments.
Thus ~or example, with reference to the activating
group as represented ~elow:
O I R ~ o
~ 5l 1!
~ , N ~ R6,
(a) (b) (c)
there are various methods known in the art by which
the sequential portions (a), (b) and (c) above can
be added to the Formula II ~-lactam in single or
double bond portions or all together.
~7v~ GC183a
--18-
One such method, which is ~referred
~omPrises first reactina a B-l~çt~ ~f
formula II with an isocyanate havina the formula
IV
o=C=N-S02- Z
wherein.Z is a leaving group, e.g., a halogen
such as chlorine. The reaction is preferably
run in an inert organic solvent, ~ , a
halocarbon such as dichloromethane, or in
: acetonitrile, and yields an intermediate
having the formula
A -NH R2 =R4
1 ~ C - C-R3
O O
Reaction of an intermediate of formula V
with a RsN~12 amine preferably in the form of a
silylated derivative such as with a silyl compound having
,!,' the fon~a ~ Si(CH3)3
R -N
\ Si(CH3j3
`~ yields an intermediate having the formula
VII
1 ~ C - C-R
; I 1 3 15
~C N-l-NH-so2-~-si(CH3)3
:' O
~ 2~ GC183a
-19-
which can be reacted wi.th an acyl halidehaving the formula
VIII R
R6-c-halogen
to yield a ~-lactam having the formula
1 ~`C - C-R3 l5
~C - N-CI-NH-So2 N ~1 6
Alternatively, compounds of formulas VI
and VIII can be first reacted to yield a
~ ~ compound having the formula
: X R~
2n (CH3)3-Si-N ICl R6
which can be reacted with a compound of formula
V to yield a compound of formula IX.
Sti.ll another procedure for preparing a
~ c~mpound of formula IX wherein R6 is -NR7R8,
: comprises reactin~ a compound of formula V
with a urea having the formula
XI l5 , R7
HN - C-N
11 ~ R
0 8
in the presence of triethylamine.
~ y~ ~ GCl83a
.
-20-
Deprotection of a compound of formula IXusing conventlonal techni~ues yields thecorrespondiny key intermediate having the
formula
XII
NH2 ~ -2 4~ R3 R
1 1 15
O~,C N-1_NH_SO2-N_C_R6 ,
or a salt thereof. The particular deprotection
reaction used will, of course, depend on the
protecting group ("Al") present. If, for
example, Al is a t-butoxycarbonyl protecting
group, deprotection can be accomplished by
treatment of a compound of formula IX with
acid (e.g., formic acid or trifluoroacetic
acid). If, for example, Al is a benzyloxy-
carbonyl protecting group, deprotection can
be accomplished by catalytic hydrogenation of-
a compound of formula IX.
Well known acylation techniques can be
used to convert an intermediate of formula XII
to a corresponding product of formula I.
It is also evident that the desired acyl group
in the Formula I product can also function as
the protecting group for the amino group (NH2-)
in the Formula XII compound above, or as the
Al-group in the Formula II reactant shown previously
where Al-is defined as a nitrogen protecting group.
In such cases, there will be no need for removing
the Al-protecting group after insertion of the
o R5~
-c-NH-so2-N-c R6 activating group.
~2 72 726 GC183a
-21-
Exemplary techniques include reacti.on of A
compound of Eormula XII with a carboxylic acid
(Rl-OH), or corresponding carboxylic acid
halide or carhoxylic acid anhydride. The
reaction with a carhoxylic acid proceeds
most readily in the presence of a carbodiimide
such as dicyclohexylcarbodiimide and a substance
capable of forming an active ester in situ such as
N-hydroxybenzotriazole. In those instances where
the acyl group (Rl) contains reactive functionality
(such as amino or carboxyl groups) it may be
necessary to first protect those functional groups,
then carry out the acylation reaction, and finally
deprotect the resulting product.
In general terms, the process or preparing
the desired compounds therefore comprises reacting
a ~-lactam having the formula
R2 R4
Ri - NH C C R3
I
~C ~ NH
O
wherein R2, R3 and R4 are as previously defined and
Ri is acyl (Rl) as previously defined or the group
Ri-NH- is a protected amino group with an isocyanate
having the formula
R O
~5 11
O= C- N -S02 -N C - R6
or sequential segments thereof wherein R5 and R6 are
as prevlously defined, and where Ri-NH- is a
`~ protected amino group removing said protecting group
and acylating with the appropriate acylating group
to form the desired product.
~L2'~72~;
GC18 3a
--22--
The azetidinQneS of formula II can be
prepared utilizing the procedures described in
United Kingdom patent application 2,071,650,
published September 23, 1981.
The preferred compounds of formula I
are those wherein R3 and R4 are each hydrogen.
Also preferred are those compounds of formula I
wherein R6 is a 5,6 or 7-membexed heterocycle,
especially a 4-alkyl-2,3-dioxo-1-piperazinyl group,
a 2-oxD-l-imidazolidinyl group, a 3-alkyl-2-o~o-1-imidazolidinyl
group or a 3-(substituted alkyl)-2-oxo-1-imidazolidinyl
group. Specific groups that are preferred are
the 4-ethyl-2,3-dioxo-1-piperazinyl, 3-ethyl-2-
oxo-1-imidazolidinyl, and 3-(2-aminoethyl)-2-
oxo-l-imidazolidinyl groups. Preferred
3-acylamino groups are those wherein the acyl
portion of the group is ~Z)-2-amino-a-(alkoxy-
imino)-4--thiazoleacetyl or (Z)-2-amino-~-
[I(substituted alkyl)oxy~imino~-4-thiazoleacetyl,
especially (Z)-2-amino-a-(methoxyimino)-4-
thiazoleacetyl and (Z)-2-amino-a-[(1-carboxy-1
methylethoxy)imino]-4-thiazoleacetyl.
The followiny examples are specific
embodiments of this invention.
:~ .
~ 7Z ~2~ GCl a 3a
-23-
Example 1
¦3S(Z)]-N~ [~[(Aee-t ~ sul ~ ~
carborlyl]-2-oxo-3-azetidinyl].-2-amino-cL-
(_ethoxyim ~ zoleacetamide, dipotassium
salt
A) (S)-[l-l[¦(Acetylamino)sulfonyl]amino~-
carbonyl3-2-oxo~3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt
(S)-3-~L(Phenylmethoxy)carbonyl~amino]-
2-azetidinone (4.4 g) wassuspended in dry
ethyl acetate. The mixture was cooled to
-5 C and 3.1 g of chlorosulfonyl isocyanate
was dropped in with stirring at such a rate
that the temperature did not exceed 0 C.
lS Stirring at 0 C was continued for an addi~ional
20 minutes. After this time 4 g of hexa-
methyldisilazane was added and the solution
was stirred at ambient temperature for 12
hours. Acetyl chloride (3.2 g) was added and
the solution was again stirred for 48 hours.
The ethyl acetate was then washed with water
and extracted twice with 50 ml portions of
saturated aqueous bicarbonate. The organic
layer was discarded and the aqueous phase
was treated with 25% hydrochlorie acid until
the pH waslØ Extraction with ethyl acetate,
drying and evaporation of the solvent yielded
4 g of the title compound in crude form.
~his was dissolved in acetone/water
GC183a
-~4-
the pH was adjusted to 6.5, the solvent was
removed ln vacuo and the crysta~l.ine title
compound was filtered off with ether. Further
purification achieved by HP-20 chromatography
(water/acetone 9:1 as eluent) yielded the title
compound, melting point 130-135C, dec.
B) [3S~Z)]-N- [l-[[~(Acetylamino)sulfonyl~-
amlnoJcarbonyl]-2-oxo-3-azetidinyl]-2-
amino-a-(methoxyimino)-4--thiazoleacetamide,
dipotassium salt
(s ) - [ 1- [ I ~(Acetylamino)sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl~carbamic acid,
phenylmethyl ester, potassium salt (940 mg)
was dissolved in 50 ml of dry dimethylformamide
and hydrogenated in the presence of 500 mg of
10% palladium on charcoal for 30 minutes,
after which the catalyst was filtered off.
: (Z)-2~Amino-~-(methoxyimino~-4-thiazoleacetic
acid ~450 mg), 1 g of dicyclohexylcarbodiimide
and 150 mg of ~-hydroxybenzotriazole were
added to the filtrate. The solution was
stirred overnight, the precipitated urea
was filtered off and the solvent was removed
in vacuo. The remaining solid was chroma-
tographed on HP-20*using water as eluent,
and yielded 400 mg of product, melting point
255-260C, dec.~
30 * The terms "HP-20" and "HP-20 resin" refer to
macroporcus styrene-divinylbenzene copolymer.
~ ; GC1~3a
-
-~5-
E mple 2
~ Z ~ y__m ~ yl~-
amino]carbonyl]-2-oxo-3 azet:idinyl]amino]-1-
( amino-4-thiazolyl)-2-oxoethylidene]amino]-
oxy]-2-methylpropanoic acid
A) 2-[~2-L _-~[~(Acetylamino)sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl]amino]-1-(2-amino-
4 thiazolyl)-2-oxoethylidene]amino]oxy]-2-
: 10 methylpropanoic acid, diphen~lmethyl ester,
monopotassium salt
(S) - Ll- [ I [ ~Acetylamino)sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinylJcarbamic acid,
phenylmethyl ester, potassium salt (1.38 g;
see example lA) was hydrogenated in dry
dimethylformamide in the presence of 700 m~
of 10% palladium on charcoal for 30 minutes
after which the catalyst was filtered off.
(Z)-2-Amino-~-[~2-(diphenylmethoxy~-1,1-
dimethyl-2-oxoethoxy]iminol-4-thiazoleacetic
acid (1.32 g), 1.2 g of dicyc.lohexylcarbodiimide
: and 150 mg of N-hydroxybenzotriazole were
; added and the solution was stirred Xor 12
~ hours. The precipitated urea was filtered
: 25 off and the solvent was removed in vacuo.
The residue was dissolved in 20 ml of acetone,
filtered and poured into 100 ml of ether.
The precipitated title compound was filtered
off and dried yielding 2.5 g of material.
GC183a
2~i
B) [3S(Z) ~ [2-[[1-[[~(Acetylamino)sulfo~l]-
amlno] _ r ~ _]-2-oxo-3-azetidirlyl]aminoJ-l-
(2-amino-4-thiazolyl)-2-oxoethylldene]amjno]-
oxy~-2-methylpropanoic acid
2-[[[2-~1- E ~ [ (Acetylamino)sulfonyl~aminol-
carbonyl~-2-oxo-3-azetidinyl~amino]-1-(2-amino-
4-thiazolyl)-2-oxoethylidene]amino]oxy]-2-
methylpropanoic acid, diphenylmethyl ester,
monopotassium salt (2.5 g) was suspended in
5 ml of anisole and cooled to 0C. Trifluoro-
acetic acid (12 ml) was slowly dropped in with
stirring, maintaining the temperature at 0C.
Af:ter 3.5 hours, the solution was poured into
100 ml of ether, precipitating the desired
product. The crude product was filtered off
and purified by HP-20 chromatography using
water/acetone;(9:1) as eluent and yielding
620 mg of product, melting point 225-230 C,dec.
.
Example 3
[l-[[~[(Chloroacetyl)amino]sulfonyl]amino]carbonyl]-
2-oxo-3-azetidinyl]carbamic acid, phenylmethyl ester
(S)-3-~[(Phenylmethoxy)carbonyl]amino~-2-
azetidinone (4.4 g) was su~pended in 150 ml of
dry ethyl acetate. The mixture was cooled to
-50 C and 3.3 g of chlorosulfonyl isocyanate
was added with stirring; stirring was continued
without cooling until the temperature reached
0 C. N ~Trimethylsilyl)chloroacetamide (6.0 g)
was added and the solution was stirred overnight.
GCla3a
--27-
The insoluble material was filtered off and
the filtrate washed with water. The organic
layer was extracted twice with saturated
aqueous sodium bicarbonate. The alkaline
aqueous layer was acidified to pH 1 with 20%
hydrochloric acid and extracted twice with
150 ml portions of ethyl acetate. The organic
layers were combined, dried and evaporated to
dryness~ The oily residue was dissolved in
50 ml of acetone and the pH was adjusted to 6.5
by addition of lN potassium hydroxide. The
solvent was removed ln vacuo and the crystalline
residue filtered with ether, yielding 3.9 g
of the title compound.
Example 4
E 3S (R)] N-[2-lll-t[[(Acetylamino)sulfonyl]-
amino]carbonyl]-2-oxo-3-azetidinyl]amino]-2-
~ oxo-l-phenylethyl]-4-ethyl-2,3-dioxo-1-
; 20 ~_perazinecarboxamide, potassium salt
Following the procedure of example lB,
but substituting (R)-~-[t(4-ethYl-2,3-dioxo-
l-piperazinyl)carbonyl]amino]benzeneacetic
acid for (Z)-2-amino-a (methoxyimino)-4-
thiazoleacetic acid, yie ded the title compound
meltlng point 160-165C, dec.
.. .. .
~. 2 ;~ GC 183a
-28-
Exarnple 5
~ chloroacetyl)amino]-
sulfonyl]amino~carbonyl]-2 oxo-3-azetidinyl]--
(methoxyimino)-4-thia2Oleacetarnide, potassium salt
[l-[~I(Chloroacetyl)amino]sulfonyl]amino~-
carbonylJ-2-oxo-3-azetidiny~]carbamic acia, phenyl-
methyl ester (1.0 g; see example 3) was slowly
added to 20 ml of 40~ HBr in acetic acid at
10C. When the addition was complete the
solution was stirred for 5 minutes. Dry
diethyl ether (100 ml) was added and the
precipitate was filtered off, washed with
ether and dried carefully. This compound
was dissolved in 50 ml of water, the pH adjusted
to 6.5 with lN KOH and the resulting solution
freeze-dried. The compound obtained was dissolved
in 50 ml of dry dimethylformamide and (Z)-2-
amino-~-(methoxyimino)-4-thiazoleacetic acid,
0.04 g of N-hydroxybenzotriazole and 0.76 g
dicyclohexylcarbodiimide are added and the
solution was stirred overnight at ambient
temperature. The precipitated urea was filtered
off and the solvent removed in vacuo. The
residue was chromatographed using HP-~0 resin
(eluent:water), yielding 0.19 g of product,
melting point 215-220C.
~7f2~6 GC183a
..
-29-
Example 6
~_ (Z)]~2~ l (2-Amino-4-thiazolyl)-2- ~
[ [ [ I ~chloroacetyl ? amino~5ulfonyl]amino]carbonyl]
_-oxo-3-azetidinyl~amino]-2-oxoe ~ylidene~amino]-
oxy]-2-methylpropanoic acid, dipotassium salt
I (Chloroacetyl ) amino ] sulfonyl ] amino ] -
carbonyl]-2-oxo-3-azetidinyl~carbamic acid,
phenylmethyl ester (2.0 g; see example 3) was
added at 5C with stirring to 40 ml of 40~
hydrogen bromide in acetic acid; after 5 minutes
a clear solution was obtained. Dry ether (200 ml)
was slowly dropped in and the precipitate was
filtered off, washed with ether and dried
carefully. The white powder was then dissolved
in 50 ml of water and the pH adjusted to 6.5
and the solution freeze-dried. The compound
obtained was dissolved in 100 ml of dry dimethyl-
formamide and 2.72 g of (Z)-2-amino-a-[~2-
(diphenylmethoxy)-l.l-dimethyl-2-oxoethoxy]-
imino~-4-thiazoleacetic acid, 0.08 g N-hydroxy-
benzotriazole and 1.52 g of dicyclohexylcarbodiimide
was added and the solution was stirred for 12
hours. The precipitate was filtered off and the
; solvent removed in ~cuo. The residue was stirred
with 100 ml of dry ether and filtered. The
compound was then suspended in 6 ml of anisole
and cooled to -15~C. At this point 12 ml of
trifluoroacetic acid was dropped in with stirring
at such a rate, that the temperature did not
exceed -10 C. After completion of the addition,
~ ~ 7~ ~-3~ GC183a
.
-30-
stirl-ing was continued at -10 C for 2 hours.
Cold ether (200 ml) was added and the precipitated
compound was filtered off, washed with ether,
dried and dissolved in 50 ml of acetone/water
(1:1). The pH was adjusted to 6.5 with lN KOH,
the acetone removed 1n vacuo and the remaining
aqueous solution freeze-dried, 2.25 g of crude
title compound ~as obtained. Purification
was achieved by HP-20 chromatography (water
eluent), yielding 0.5 g of product, melting
point 220-225C, dec.
Example 7
[3S(Z)]-2-[I[1-(2-Amino-4-thiazolyl)-2-~[1-
[Il[(methoxyacetyl)amino]sulfonyl]a no]carbonyl~-
2-oxo-3-azetidinyl]amino]-2-oxoethylidene]amino]-
oxy]-2-methylpropanoic acid, dipotassium salt
A) (S)-[l-[l~[(Methoxyacetyl)amino]sulfonyl]-
amino]carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl e ter, monopotassium salt
(S)-3-~[(Phenylmethoxy)carbonyl]amino~-2-
azetidinone (4.4 g) was suspended in 100 ml
of dry ethyl acetate. The mixture was cooled
to -30 C and 3.12 g of chlorosulfonylisocyanate
was added with stirring. ~he cooling was stopped
and when the temperature had reached 0C a clear
solution was obtained. After 30 minutes at 0 C
13.9 g of N-trimethylsilyl methoxyacetamide
were added and stirring was continued for 24
hours at am~ient temperature. After this time
GC183a
.
-31-
the ethyl acetate solution was washed with water,dried and the solvent removecl. The residue
was dissolved in acetone/water t~:l) and the
pH was adjusted to 6.5 with lN KOH. The acetone
was removed ln vacuo and the aqueous solution
was free~e dried.
B) [3S(Z)] 2-[[~1-(2-Amino-4-thiazolyl)-2-[rl-
[ [ [ r (methoxyacetyl)amino]sulfonyl]amino]carbonyl]-
2-oxo-3-azetidinyl]amino]- -oxo-ethylidene]amino]-
oxy~-2-methylpropanoic acid, dipotassium salt
(s) ~ [1- [ ~ I I (Methoxyacetyl)amino]sulfonyl]-
amino]carbonyl]-2-oxo-3-azetidinyl]carbamic
acid, phenylmethyl ester, monopotassium salt
(0.43 g) was dissolved in 50 ml of dry dirnethyl-
formamide. Palladium on charcoal (10%; 0.25 g)
was added and hydrogen was bubbled through
for 30 minutes with stirring. The catalyst
was filtered off and 0.42 g of (Z)-2-amino-a-
[[2-(diphenylmethoxy)-1,1-dimethyl-2-oxoethoxy]-
imino]-4-thiazoleacetic acid, 0.01 g N-hydroxy-
benzotriazole and 0.24 g of dicyclohexylcarbodiimide
was added; the solution was stirred at ambient
temperature for 12 hours. The precipitate was
filtered off and the solvent removed ln vacuo.
The residue was suspended in diethyi ether and
the precipitate filtered off (0.76 g~. This
compound was suspended in 3 ml o anisole and
cooled to -15C; 6 ml of trifluoroacetic acid
was slowly dropped in with stirring, so that
:
;
l~ 7~æ~ GC183a
-32-
the temperature did not exceed -10C. After
the addition was complete, the temperature
was snaintained for 2 hours. Cold diethyl ether
(100 ml) was added and the precipitate was
filtered off, dried and dissolved in water.
The pH was adjusted to 6.5 by addition of lN KOH
and the solution was chromatographed on HP-20
resin, usiny water as eluent, yielding 200 mg
of product, melting point 245-250 C, dec.
Example 8
13$(Z)]-2-Amino-N-Il-[l[I(methoxyacetyl)amino]-
sulfonyl]amino]carbonyl]-2-oxo-3-azetidinyl~-
-(methoxyimino)-4-thiazoleacetamide,potassium salt
(S)~ [¦[¦(Methoxyacetyl)amino~sulfonyl]-
amino]carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, monopotassium salt (O.S g;
see example 7A) was hydrogenated in 30 ml of
dry dimethylformamide in the presence of 0.25 g
10% palladium on charcoal for 30 minutes.
The catalyst was filtered off and 0.22 g of
(Z)-2-amino--(methoxyimino)-4-thiazoleacetic
~ acid, 0.01 g of N-hydroxybenzotriazole and
:~ 0.21 g of dicyclohexylcarbodiimide were added.
~5 The solution was stirred overnight, ~iltered
and evaporated to dryness. The residue was
chromatographed (HP-20, water as eluent),
yielding 0.12 g of product, melting point
170-175C,dec.-
GC1~3
-33-
Ex~ e 9
f3S~Z)-2~[[~1-(2-Amino-4-tlliazolyl)-2~f[1-
ff[(ben~oylamino)sulfonyl]amino]carbonyl]-2-
; oxo-3-azetidinyl]amino]-2-oxoethylidene]amino]-
oxo~-2-methylpropaI7oic acid, dipotassium salt
A) (S)-[l-~¦(Aminosulfonyl)amino]carbonyl]-2-
oxo-3-a2etidinyl~carbamic acid, phenylmethyl ester,
potassium salt
Method I
(S)-(2-Oxo-3-azetidinyl)carbamic acid,
phenylmethyl ester (11 g) was dissolved in a
mixture of 200 ml of acetonitrile and 50 ml
of dichloromethane. The mixture was cooled
to -50C and a solution of chlorosulfonyl
isocyanate (9 g) in 25 ml of dichloromethane
was added with stirring. After warming the
mixture to -30C a solution of 6 g of ammonia
in 60 ml of acetonitrile was added slowly.
The reaction temperature was raised to -10 C
and finally to 0-5C. The reaction time was
3 hours. The ammonium salt of the title
compound precipitated and was filtered by
suction (20 g). The crude product was purified
by HP-20 chromatography (100-200 mesh) eluting
with 2000 ml of water and water/acetone (8:2);
20 ml fractions were taken. The elution was
monitored by thin-layer chromatography. From
fractions 142-154, 9.3 g of product was obtained
by evaporation.
~ ~7v~ c 18 3a
-3~-
The ammonium salt of the title compound
was dissolved in 100 ml of water, layered with
200 ml of ethyl acetate and acidified. After
separation and washing of the aqueous layer
twice with ethyl acetate, the or~anic layer
was washed with saturated sodium chloride
solution, dried with anhydrous magnesium
sulfate and evaporated to yield 8.1 g of the
~ree acid of the title compound.
Method II
A ~ixture of (S) (2-oxo-3-azetidinyl)carbamic
acid, phenylmethyl ester (11 g) in 175 ml of
dichloromethane was cooled to -30C. While
stirring, 7.7 g of chlorosulfonyl isocyanate
in 75 ml dichloromethane was added dropwise
within 15 minutes. The temperature of the
solution was allowed to rise to 0C over 30
minutes. Subsequently the clear solution was
again cooled to -30C and 8.~ g of bis-(tri
methylsilyl)amine dissolved in 30 ml of
dichloromethane, was dropped in, while passing
dry nitrogen through the flask. After an hour
the reaction temperature was allowed to rise
to -15C and was maintained for an additional
30 minutes. The solvent was distilled off
in vacuo, and the residue was triturated with
400 ml of ether to give a solid (16.6 g) which
was washed with an additional 20 ml of ether.
From the ethereal motherliquor there was obtained
~ 7X~,~ GC183a
-35-
a second crop of 4.2 g of product.
The crude mater:ial (18.0 g) along with
about 20 g of ~P-20 resin was suspended in
30 ml of water and the mixture was chroma
tographed on an HP-20 column eluted with a)
3 L of water; b) 2.5 L of water/acetone (a 2~;
3) 4 L of water/acetone (7:3); d) 6 L of water/
acetone (6:4). Fraction d yielded 6.2 g of
the title compound,melting point 150-152 C.
B) (S)-[l-[[~(Benzoylamino)sulfonyl~amino]-
carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt
(S)-~ (Aminosulfonyl)amino]carbonyl]-
2-oxo-3-azetidinyl]carhamic acid, phenylmethyl
ester, potassium salt (2.3 g) and 2.2 g of
potassium carbonate were stirred in 50 ml of
dry dimethylformamide with 5 g of benzoyl chloride
and 0.6 g of dimethylaminopyriaine overnight.
The solvent was removed in vacuo and the residue
was extracted at pH 1 (aqueous solution) with
ethyl acetate. The organic layer was dried and
evaporated to dryness. The residue was dissolved
in water/acetone (1:9) and the pH adjusted to
6.5 with lN KOH. The acetone was removed in vacuo
and the remaining aqueous solution fxeeze-dried.
Purification of the resulting white powder was
achieved by HP-20 chromatography using water/
acetone (9:1) as eluent, and yielding 1.1 g
of product melting point 96-99C, dec.
~7X,7~; GC183a
_36_
C) [3S(2,)~-2-[~[1-~2-Amino~4-thiazolyl)-Z-~[1-
~[~(~enzoyl~mino)sulfonyl]amin _ arbonylJ-2-
oxo-3-azetidinyl~amino~-2-oxoethy]idene]amino]-
oxy]-2-methylpropanoic acid, dipotassium salt
(S)-[l-[II(Benzoylamino)sulonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl~carbamic acid,
phenylmethyl ester, potassium salt (0.5 g) was
hydrogenated in 100 ml of dry dimethylformamide
in the presence of 0.25 g of 10% palladium on
charcoal for 1 hour. The catalyst was filtered
off and 0.46 g of (Z)-2-amino-a-[12-(diphenyl-
methoxy)-l,l-dimethyl-2-oxoethoxy~imino]-4-
thiazoleacetic acid, 0.01 g N-hydroxyben~otriazole
and 0.24 g of dicyclohexylcarbodiimide were added
and the solution was stirred for 12 hours. The
solvent was distilled off in vacuo and the residue
was filtered of with 100 ml of ether and dried
(0.92 g). This compound was suspended in 2 ml
of anisole, cooled to -10 C and 4 ml trifluoroacetic
acid was added with stirring. The temperature
was main'cained for 5 hours. After this time 100 ml
o dry ether was added and the precipitated
compound was filtered off, dissolved in 5 ml of
water and the pH was adjusted to 6.5 with lN KOH.
2S The resulting aqueous solution was chromatographed
on HP~20 resin ~eluting with water) and yielded
0.24 g of product, melting point 225-230C, dec.
,
.
~ 7~ GCl83a
Example 1()
[3S(R)]-N-[2 ~ ~ (Benzoy~amino)Sulfony~la _no]-
carbonyl]-2-oxo-3-azetidlnyl]amino]-2-oxo-l-
phenylethyl~-4 ethyl-2, dioxo-l-piperazine
carboxamide, potassium salt
s ) - [ ~ I (Benzoylamino)sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt (0.25 g;
see example 9B) was hydrogenated as described
in example 9C. (R)-~ (4-Bthyl-2,3-dioxo-
l-piperazinyl)carbonyl]amino]benzeneacetic acid
(0.17 g), 0.01 g of N-hydroxybenzotriazole
and 0.13 g of dicyclohexylcarbodiimide were
added to the resulting solution which was
stirred overnight. The solvent was distilled
off in vacuo and there~due chromatographed
using HP-20 resin and water/acetone (l9:1)
as eluent, yielding 0.14 g of product, melting
point 180-185C, dec.
- E_ample ll
l3s (Z? ~-2-Amino-~-[l-~[(benzoylamino)sulfonyl]-
amino]carbonyl~-2-oxo-3-azetidinyl] -a- (methoxy-
imino)-4-thiazoleacetamide, potassium salt
(S)~ [(Benzoylamino)sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl~carbamic acid,
phenylmethyl ester, potassium salt (0.25 g;
see example 9B) was hydrogenated as described
in example 9C. To the resulting solution 0.01 g
of N-hydroxybenzotriazole, 0.11 g of (Z)-2-amino-
~-(methoxyimino)-4-thiazoleacetic acid and 0.13 g
of dicyclohexylcarbodiimide were added. After
GC183a
7~7~;
-38-
stirring overnight at room temperature~ the
solvent was removed ln vacuo and -the residue
chromatographed using HP-20 resin and water/acetone
(19:1) as eluent, yielding 0.05 y o product,
melting point 205 210C.
Example 12
S) - [ 1- [ I [ I (Acetyl)methylamino3sulfonyl~amino]-
_arbonyl]-2-oxo-3--azetidinyl]carbamic acid,
phenylmethyl ester
(S)-3-[~(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (2.2 g) was suspended in 100 ml
of anhydrous tetrahydrofuxan and cooled to
-50 C with stirring. Chlorosulfonylisocyanate
(1.56 g) was added and the temperature was
maintained at 0 C for 30 minutes. After this time
`~ 3.51 g of heptamethyl disilazane was added and
stirring at room temperature was continued over-
night. Acetyl chloxide (3.14 g) was added and
the solution was stirred for an additional 48
hours. The solvent was removed in vacuo, and the
residue dissolved in ethyl acetate and extracted
twice with 50 ml portions of water. The aqueous
layer was discarded and the ethyl acetate
solution was dried ~ith Na2SO4 and evaporated
to dryness. The residue was dissolved in
acetone~water (9:1), the pH adjusted to 6.5 with
1i~ KOH and the acetone removed ln vacuo. The
remaining aqueous solution was free~e-dried.
The resulting crude compound (1.9 g) was purified
by HP-20 chromatography, yielding 1.03 g of
product.
GCI83a
-39-
13
_
_s(z)~-2-Amino~N~ 1 I ( 2 -me t~yl.~rop~oyl-) a
sulfonyl]amino]carbonYl]-2-oxo-3-azet~dinyl]-
__ __ _~
~-(me _oxyimino)-4-thiazoleacetam1de potassium salt
(S)-3-ll(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (2.2 g~ was suspended in 100 ml of
dry ethyl acetate, cooled to -50C and 1.56 g
chlorosulfonylisocyanate was added. After 30
minutes at 0C a clear solution was obtained and
4~78 g o~ N-trimethylsilyl 2-methylpropionamide
was added and stirring at ambient temperature
was continued for 12 hours. The solution was
washed with 100 ml of water, dried and evaporated
to dryness. The residue was dissolved in
water/acetone (1^9) and the pH adjusted to 6.5
with lN KOH. Acetone was removed in vacuo and
the remaining aqueous solution freeze-dried,
ylelding 0.28 g of compound~ This compound was
dissolved in 20 ml of dry dimethylformamide and
hydrogenated with 0.1 g of 10% palladium on
charcoal for 30 minutes. The catalyst was - -
filtered off and 0.1 g of (Z~-2-amino--(me1hoxy-
imino)-4-thiazoleacetic acid, 0.01 g of N-hydroxy-
benzotriazole and 0.13 g of dicyclohexylcarbodiimide
; 25 were added and the mixture was stirred overnight
at room temperature. The solvent was removed
in vacuo and the residue chromatographed on
HP-20 resin eluting with H2O/acetone (19:1)
and yielding 50 mg of product, melting point
; 30 ~ 185-190C, dec.
~ GC183a
- ~ O ~
Example 14
l3S~Z)]-2-~nino-N~ (aminocarbon~l)amino]-
sulfonyl]amino]carbonyl]-2-oxo-3-azetidinyL]-~-
(me ~ mid~, potassium salt
A) S)~ r ~ I I (Aminocarbonyl)amino]sulfonyl]-
amino]carbonyl~-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt
(S) -3- [ L (Phenylmethoxy)carbonyl~amlno]-2-
azetidinone (13.2 g) was suspended in 300 ml of
dry tetrahydrofuran at 0C. Chlorosulfonyl-
isocyanate (11.1 g) dissolved in 80 ml of dry
dichloromethane was dropped in with stirring.
The temperature was maintained with cooling at
0 C for 30 minutes. Trimethylsilyl
urea (10.5 g) was added and stirring was
continued at room temperature overnight. The
solvent was removed in vacuo and 200 ml of
methanol was added. The solution was stirred
for 30 minutes, the solvent evaporated and the
residue treated with 200 ml of ethyl acetate
and 100 ml of water. The organic layer was
separated, dried and filtered. After removal
of the solvent,the oily residue was dissolved
in 100 ml of acetone and 10 ml of water, and
the pH was adjusted to 6.5 by addition of lN
potassium hydroxide. After evaporation of the
solvent, the title compound remaine~. Yield
11.0 g; melting point 120-125 C,dec.
~Z ~ 2~ GC183a
B) 13S(Z)]-2-~mino-N~ [[l~(aminocarbonyl)amino]-
s_lfonyl]amino~carbonyl~-2-~oxo-3-azetidi.nyl~-u-
(methoxyimino)-4-thiazoleace~clnlide~_~_tassiunl sa:Lt
(s) - [ 1- ~ [ I ~ ( Aminocarbonyl)amino3sulfonylJ-
amino~carbonyl~-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt (1.4 g) was
hydrogenated in 100 ml of dry dimethylformamide
with 0.7 g of 10% palladium on charcoal as a
catalyst. Ater 30 minutes the hydrogenation
was completed, the catalyst filtered off and
0.66 g of (Z)-2-amino--(methoxyimino)-4-~hiazole- . i.
acetic acid and 0.2 g of N-hydroxybenzotriazole
were added. Dicyclohexylcarbodiimide (720 mg),
dissolved in 100 ml of dry dimethylformamide
was slowly dropped in with stirring during a
period of 10 hours. Stirring was continued for
an additional 12 hours. The mixture was cooled
to 0C, the precipitated urea filtered off, the
solvent removed in vacuo and the residue chroma-
tographed on HP-20 resin using water as eluent.
Yield 900 mg; melting point 205-210C, dec.
Example 15
[3s(z)]-2 ~ 2-[~ (Aminocarbonyl)amino]-`
sulfonyllamino3carbonyl]-2-oxo-3-a~etidinyl]amino3-
1-(2-amino-4-thiazolyl)-2-oxoethylidene]amino]oxy~-
2-methylpropanoic acid, dipotassium salt
~ S) - El- ~ L ~ ~ (Aminocarbonyl)amino~sulfonyl]-
amino~carbonyll-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt tl.93 g; see
example 14A) was hydro~enated in the presence of
1 g of 10% palladium on charcoal in 200 ml of
X ~ ~ GCI83a
-~2-
dry dimethylformamide or 30 mlnutes. The catalystwas filtered off and 30~ mg of N-~vdrGxy~enzo-
triazole alld 2.5 g of (Z)-2-amino-~-LL2~(diphenyl-
me-thoxy)-l,l-climethyl-2-oxoethoxy~lmino]-4-
thiazoleacetic acid was added. Dicyclohexyl-
carbodiimide (1.1 g~, dissolved in 100 ml of
dimethylformamide was slo~ly dropped in (10 hours).
When the addition is complete, stirring was
contirlued for an additional 10 hours. The solvent
was removed in vacuo and the solid residue was
suspended in 10 ml of anisole and cooled to 0 C.
At this temperature 20 ml of trifluoroacetic
acid was dropped in with stirring. The temperature
was maintained at 0 C with cooling. After 3.5
hours, the reaction was complete and the solution
was poured in-to 200 ml of ether. The precipitate
was filtered off, dried and dissolved in 10 ml
of water/acetone (1:1), and the pH was adjusted
to 6.5 by the addition of 1 N potassium hydroxide.
The acetone was removed in vacuo and the aqueous
phase chromatographedon HP-20 resin using water
as eluent. Yield 1.5 g; melting point 258C, dec.
Example 16
13S~R)~-N-I2-[~1-[l[[(Aminocarbonyl)amino]sulfonyl]-
amino]carbo~ -2-oxo-3-azetidinyl~amino]-2-oxo-1-
phenylethyl]-4-ethyl-2,3-dioxo-1-piperazine-
carboxamide,_potassium salt
(s) -Il- [ I I I (Aminocarbonyl)amino]sulfonyl~-
amino]carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt (1.34 g; see
example14A) was hydrogenated with 0.6 g of
10% palladium on charcoal as catalyst in 100 ml
3~
GCI83a
-~3~
of dry dirnethylformarnide for 30 minutes. The
catalyst was filtered of~ and 1.23 g o (R)-a-
I[(4-ethyl-2,3-diQ~o-l-piperazinyl)carbonyl]-
amino]benzeneacetic acid and 100 mg of N-hydroxy-
benzGtriazole were added. Dicyclohexyl-
carbodiimide (0.8 g),in 100 ml of dimethyl-
formarnide was dropped in with stirring over a
period of 10 hours. Stirring was continued for
an additional 10 hours. The solvent was removed
ln vacuo and the residue chromatographed on
HP-20 resin, using water as eluent. Yield
590 mg; melting point 198C, dec.
Example 17
~3S(Z)]-2-Amino-a-(methoYyimino)-N-[l-~f¦(methyl-
amino)carbonyl]amino]sulfonyl]amino3carbonyl3-2-
oxo-3-azetidinyl]-4-thiazoleacetamide, potassium salt
A) (S)~[l-~[[~[(Methylamino)carbonyl]amino]sulfonyl]-
amino]carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, ~otassium salt
( s) - :f 1~ (~minosulfonyl)amino~carbonyl~-
2-o~o-3-azetidinyl~car~amic acid, phenyl-
methyl ester, potassium salt (1 g; see
example 9A) was dissolved in 20 ml of dry dimethyl-
formamide. Methylisocyanate (500 m~,was added
and the solution was stirred overnight at ambient
temperature. The solvent was removed in vacuo
and the residue was treated with acetone/ether
(1:1) and filtered. Yield 0.9 g; melting point
153-160C, dec.
GC1~3a
-44-~
B) ~ Z)~ 2-Amino-u-(methoxy ~
I [ I [ ~ methylam o)carbonyl] amino] sulfonylJamino]-
carbonyl]-'2-'oxo-3-azetidinyl']-4'-'thiazoleacetamide,
potassium salt
(S) -Il- L [ I I I (Methylamino)carbonyl]amino]-
sulfonyl]amino]carbonyl]-2-oxo-3-azetidinyl]-
carbamic acid, phenylmethyl ester, potassium salt
(437 mg) was hydrogenated in 50 ml of dry dimethyl-
formamide in the presence of 200 mg of 10%
palladium on charcoal. After 45 minutes the
reaction was complete and the catalyst was
filtered off. (5)-2-Amino-~-(methoxyimino)-4-
thiazoleacetic acid (220 mg), 50 mg of N-hydroxy-
ben~otriazole and 300 mg of dicyclohexylcarbodiimide
were added with stirring at ambient temperature.
Stirring was continued for 12 hours, the solution
cooled to 0C and the precipitated urea was
filtered off. The solvent of the mother liquor
; was removed _ vacuo and the residue was chroma-
tographed on HP-20 resin, using water. Yield
305 mg; melting point 220~225C, dec.
~ 7~7~
-~5~
Example 18
¦3S(R)]-4_E,t~y__N-12~ [ I llmet~ l[(methy~amino)-
~ ino~sul'fonyl]'amino3carbonyl] -2-oxo-3-
azetidinyl~amino~ ~ 2,3-dioxo-
l-pipera~inecarboxamide, potassium salt
A) (S)~ Methyl~methylamino~carbonyl]amino3-
sulfonyl] mino]carbonyl]-2-oxo-3-azetidinyl]-
carbamic acid, phenylme-thyl ester, potassium salt
10(s) -3- [ I tPhenylmethoxy)carbonyl~amino]-2-
azetidinone (4.4 g) was suspended in 100 ml of
dry tetrahydrofuran and the mixture was cooled
to -10C. At this temperature, 1.6 g of chloro-
sulfonylisocyanate was added and the reaction
mixture was allowed to reach room temperature.
Trimethylsilyl N,N'-dimethylurea (5~2 g) were added
'~ and stirring at ambient temperature was continued
for 12 hours. The solvent was removed in vacuo
and the residue was dissolved in 100 ml of
0 ethyl acetate. The organic solution was washed
twice with 50 ml portions of water, with 50 ml
of 2 N phosphoric ac~d and with brine. The
organic layer wasevaporated to dryness, the~
residue dissolved in water/acetone (1:1) (50 ml).
The pH was adjusted to 6.5 with l N potassium
hydroxide. The acetone was removed in vacuo
and the aqueous solution ~reeze dried to yield
8 g of crude title compound pure enough for
further reactions.
~7~7~6 GC183a
_ -~6-
~ 3S(R)]-4-Eth l-N-~2-[~1-[[[~methyl[(methyl-
Y_ ___ _
amino)carbonyl~aminoJsulfonyllamino _arb~ny1 -2-
ox - _ azetidinyl]amino]-2-oxo-1-phenylethyl]-2,3-
dioxo-1-piperaz;necarboxamide, potassium salt
(s)-~ I [MethylI(methylamino)carbonyl~-
amino]sulfonyl]amino]carbonyl]-2-oxo-3-azetidinyl~-
carbamic acid, phenylmethyl ester, potassium salt
(900 mg) was hydrogenated in 50 ml of dry dimethyl-
formamide in the presence of 400 mg of 10% palladium
on charcoal. The hydrogen uptake ceased after
about 30 minutes. The catalyst was filtered off
and 700 mg (R)-~-~l(4-ethyl-2,3-dioxo-1-piperazinyl)-
carbonyl]amino]benzeneacetic acid, 100 mg of
N-hydroxybenzotriazole and 600 mg of dicyclo-
hexylcarbodiimide were added with stirring. After
12 hours at ambient temperature, the precipitated
urea was filtered of, and the solvent removed
in vacuo. The residue was chromatographed on
HP-20 resin, using water/acetone (9:1) as eluent.
Yield 700 mg; melting point 177 C, dec.
Example 19
3S~Z)]-2-l[ Il- (2-Amino-4-thiazolyl)-2-[ll-[~[lmethyl-
~(methylamino)carbonyl]amino]sulfonyllamino]carbonyl]-
2-oxo-3-azetidinyl]amino]-2-oxoethylidene~amino~-
oxy]-2-methylpropanoic acid, dipotassium salt
(s) - ~ Methyl~(methylamino)carbonyl]-
amino]sulfonyl]amino]carbonyl]-2-oxo-3-azetidinyl]-
carbamic acid, phenylmethyl ester, potassium salt
(900 mg; see example18A) was hyarogenated in
50 ml of dry dimethylformamide in the presence
of 450 mg of 10~ palladium on charcoal for 30
minutes. The catalyst was filtered off and 1 g
CCl~a
-~7--
of (Z)-2-amino-a-~I2-(dipheny]methoxy)-1,1-
dimethyl-~-oxoethoxy3imino]~ thiazoleacetic acid,
100 mg of N-hydroxybenzotriazole and 600 my o~
dicyclohexylcarbodiimide were added with stirring
at ambient temperature. After 12 hours, the
solution was cooled to 0C and the precipitated
urea was filtered off. The solution was evaporated
to dryness and the residue was filtered off with
50 ml of ethyl acetate. The crude compound was
suspended in 2 ml of anisole, cooled to -5C
and 6 ml of trifluoroacetic acid was dropped
in at such a rate that the temperature could be
maintained at 0C. After 2 hours, 100 ml of dry
ether was added and the precipitate was filtered
off, washed with ether and dried. The crude
compound was dissolved in 20 ml of water and
the pH adjusted to 6.5 with lN potassium hydroxide.
After freeze drying, the crude potassium salt
was chromatographed using HP-20 re~in and water
as eluent and then freeze-dried. Yield 400 mg;
melting point 220C, dec.
Example 20
[3S(Z))-2-Amino-a-(methoxy _ino~-N-[l-[¦~methyl-
~(methylamino)carbonyl]amino]sulfonyl]amino~-
carbonyl]-2-oxo-3-azetidinyl3-4-thiazoleacetamide,
potassium salt
: ( s ) - I 1- ~ L ~ ~ethyl L ( methylamino)carbonyl~
amino]sulfonyl~amino3carbonyl~-2-oxo-3-azetidinyl3-
carbamic acid, phenylmethyl ester, potassium salt
(440 mg; see example18A) was hydrogenated as
described in example 6. (Z)-2-A~ino-a-(methoxyimino)-
4-thiazoleacetic acid (220 mg), 50 mg of N-hydroxy-
benzotriazole and 250 ml of dicyclohexylcarbodiimide
.
~7~7~ GCl8~a
..
-48-
were added after removal of the catalyst~ Stirringat ambient tempera-ture was continuecl for 12 hours.
The solvent was removed in vacuo and t:he resldue
~hromatographed on HP-20 resin, using water/acetone
(9:1) as eluent. Yield 350 mg (isolated by
free~e drying); melting point 192C, dec.
Example 21
__.
[3S (æ) ]-2-[l[1-(2-Amino-4-thiazolyl)-2-~
~[~(dimethylamino)carbonyl]amino~sulfonyl~-
amino]carbonyl]-2-oxo-3-azetidinyl]amino]-2-
oxoethylidene]amino]oxy~-2-methylpropanoic acid,
potassium salt
lS A) (S)-~ [(Dimethylamino)carbonyllamino]-
sulfonyl]amino]carbonyl~-2-oxo-3-azetidinyl~-
carbamic acid, phenylmethyl ester, potassium salt
(S)-3-[[(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (4.4 g) was suspended in 150 ml of
ethyl acetate and cooled to -15 C. Chloro-
sulfonylisocyanate (3.0 g) was added and the
mixture was stirred at 0C until a clear solution
was obtained. N,N-Dimethylurea (2 g) and 2 g of
triethylamine were added and stirring was continued
overnight. The precipitate was filtered off and
the mother liquor washed with two 50 ml portions
of water, 50 ml of 2N phosphoric acid and brine.
The solvent was removed in vacuo and the residue
was dissolved in 50 ml of water/acetone (1:1).
The pH was adjusted to 6.5 with lN potassium
hydroxide. The acetone was removed in vacuo
and the aqueous solution was free~e dried. Crude
title compound (7 g) was obtained, which was pure
enough for further manipulation.
GC183a
-49-
B) ~3S(Z)]-2-[I¦1 [2-Amino-4-khiazolyl)-2-[¦1-
bonyl]amino]sul~onyl~-
amlno~carbonyl~-2-oxo-3-azetidinyl~amino]-2-
oxoethylidene~amino]oxy]-2-methylpropanoic acid,
potassium salt
(S)-[l-~ (Dimethylamino)carbonyl]amino]-
sulfonyl]amino]carbonyl~-2-oxo-3-azetidinyl]-
carbamic acid, phenylmethyl ester, potassi~ salt
(451 mg) was dissolved in 50 ml of anhydrous
dimethylformamide, 200 ml of 10% palladium on
charcoal was added and hydrogen was bubbled
throuyh the solution for 45 minutes. The catalyst
was filtered off and 450 mg of (Z)-2-amino-~-
[I2-(diphenylmethoxy)-1,1-dimethyl-2-oxoethoxy]-
imino]-4-thiazoleacetic acid, 100 mg of N-hydroxy-
benzotriazole and 300 mg of dicyclohexylcarbQdiimide
were added and the solution was stirred at
ambient temperature for 12 hours. The precipitated
urea was filtered off and the solvent removed
in vacuo. The residue was chromatographed using
HP-20 resin and water/acetone (7:3) as eluent.
The benzhydryl ester of the title compound (600 mg)
was isolated by freeze dryingO This compound was
suspended in 2 ml of anisole, the mixture cooled
to -10 C and 4 ml of trifluoroacetic acid was
slowly dropped in, s~ that the temperature did
not exceed -5C. When the addition was complete,
stirring at -5C was continued for 2 to 5 hours.
The title compound was precipitated by the addition
f 100 ml of ether, filtered off, dissolved in 5 ml
of water and the p~I adjusted to 6.5 with 2N
potassium hydroxide. The solution was chroma-
tographed on HP-20 resin using water as eluent.
Yield 280 mg; melting point 191C, dec.
~ 7~,~; GC183a
-50
Example 22
_S(Z)~-2-[[Il-(2-Amino-4-thiaz lyl)-2-1~1-
~ I [ I 1 ( 3-ethyl-2-oxo-l-imidazolidinyl)carbonyl]-
amino]sulfonyl]amino]carbonyl]-2-o~o-3-azetidinyl~-
amino]-2-oxoethylidene]amino]oxy]-2-methyl-
propanoic acid, dipotassium salt
A~ (S)-~ [ I (3_E yl-2-oxo-1-imidazolidinyl)-
carbonyl]amino]sulfonyl]amino]carbonyl]-2-oxc-3-
10 _ ~
otassium salt
P
(S)-3-ll(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (6.6 g) was suspended in 200 ml of
anhydrous ethyl acetate and the mixture was
cooled to -15C. At this temperature, 5 g of
chlorosulfonylisocyanate was added with stirring
at 0C until a clear solution was obtained
(10 minutes). Triethylamine (4 g) and 5.2 g
of l-aminocarbonyl-3-ethylimidazolidin-2-one
were added and stirring was continued at room
temperature for 12 hours-. The reaction mixture
was washed with two 50 ml portions o~ water,
50 ml of 2 N phosphoric acid and brine. The
organic phase was evaporated to dryness, the
residue dissolvea in lS0 ml oE water/acetone
~ and the pH was adjusted to 6.5 with 1 N
potassium hydroxide. The acetone was distilled
off in vacuo and the remaining aqueous solution
was free~e dried. The crude title compound --
(12.2 g) was pure enough for further reactions.
GCL83a
~ D
-51-
) _3S(Z)~-2-~[¦1-(2-Amino-4--thiazolyl)-2-
[ Il- f I ~I~(3-ethyl-2-oxo-1-imlda_olidinyl)carbony~3~
amino]sulfonyl]aminoJc_rbonyl]-2-oxo-3-az_t inyll-
a o~-2-oxoethylidene]amino]oxy]-2- ethyl-
propanoic acid, dipotassium salt
(S) ~ [l [l I (3-Ethyl-2-oxo-l-imidazolidinyl) -
carbonyl]amino]sulfonyl]amino]carbonyl~-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,
; potassium salt (520 mg) was hydrogenated in S0 ml
of anhydrous dimethylformamide in the presence
of 200 mg of lO~ palladium on charcoal for 40
minutes. The catalyst was filtered off, 460 mg
of (Z~-2-amino~ 2-(diphenylmethoxy)-l,l-
dimethyl-2-oxoethoxy]imino]-4-thiazoleacetic
acid, lO0 ml of N-hydroxybenzotriazole and 300 mg
of dicyclohexylcarbodiimide were added and the
solution was stirred overnight. The sol~-ent
was distilled off in vacuo and the residue was
filtered off with ethyl acetate, suspended in
2 ml of anisole and cooled to -lO C. Trifluoro-
acetic acid (4 ml) were dropped in at such a rate
that the temperature did not e~ceed -5C. Stirring
at this temperature was continued for 2 hours.
After addition of lO0 ml ether, the precipitate
~.~s filtered off, dissolved in lO ml of water,
the pH adjusted to 6.5 with 1 N potassium hydroxide
and the aqueous solution was chromatographed on
HP-20 using water as eluent, and the title compound
isolated by freeze drying. Yield 250 mg; melting
point 245C, dec.
7,~ GC1~3
-~2-
Example 23
_35(Z)]-2-Amino-~-(ethoxylmlno)~[1~[[l[[(3-
ethyl-2-oxo-1-imidazolidinyl)carbonyl]amino]-
sulfonyl]amino]carbonyl3-2-oxo-3-azetidinyl]-4-
thia~oleacetamide, potassium salt
(s) - Il- ~ L L I [ ~3-Ethyl-2-oxo-l-imidazolidinyl)-
carbonyl]amino]sulfonyl]amino~carbonyl]-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt (520 mg; see example 22~) was
hydrogenated in 50 ml of dimethylformamide
in the presence of 200 mg of 10% palladium
on charcoal for 40 minutes. The catalyst was
filtered off and 230 mg of (Z)-2-amino-a-
(ethoxyimino)-4-thiazoleacetic acid, lO0 mg of
N-hydroxybenzotriazole and 300 mg of dicyclo-
hexylcarbodiimide were added. The solution was
agitated at room temperature for 12 hours. After
this time the solvent was removed ln vacuo and
the residue filtered with ethyl acetate. Purifi-
cation of the crude compound was accomplishedby HP-20 chromatography using water/acetone (9:1)
as eluent, and the title compound isolated by
freeze drying. Yield 420 mg; melting point 125 C.
,
GC1~3a
-53-
Example
. _ .
~3s (z) ] -~-Amino-N~ l l;I [ L (3-ethyl~2-oxo-1
imidazolidinyl)carbonyl]amino]sulfonyl]amin
_
carbonyl]-2-oxo-3-azetidinyl]--(methoxyimino)-
4-thiazoleacetamide, potassium salt
-
Following the procedure of example 23,
but replacing (z)-2-amino-~-tethoxyimino)-4-
thiazoleaceticiacid by (Z)-2-amino-~-(methoxy-
imino)-4-thiazoleacetic acid, yielded 400 mg of
the title compound; melting point 176C, dec.
Example 25
[3S(R)]-4-Ethyl-N-I2-~ ( 3-ethyl-2-oxo-1-
imidazolidinyl)carbonyl]amino]sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl]amino]-2-oxo-1-
phenylethyl]-2,3~dioxo-1-piperazinecarboxamide
potassium salt
(S) -Il- [ [ [L [ (3-Ethyl-2-oxo-1-imidazolidinyl)-
carbonyl]amino]sulfonyl~amino]carbonyl]-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,potassium salt (520 mg; see example22~ was
hydrogenated in 50 ml of dimethylformamide in
the presence of 200 mg of 10% palladium on
charcoal for 40 minutes. After filtration,
330 mg of ~R)-~ (4-ethyl-2,3-dioxo-1-piperazinyl)-
carkonyl]amino]benzeneacetic acid, 300 mg of
dicyclohexylcarbodiimide and 100 mg of N-hydroxy
benzotriazole were added and the solution was
stirred for 12 hours at room temperature. The
solution was evaporated in vacuo to dryness
and the residue filtered with ethyl acetate.
' '' ''
GC183a
5~-
Purification of the crude compound was achievedby chromatography on HP-20 resin using water/
acetone (9:1) as eluent. Yleld 480 mg; meltiny
point 181C, dec.
Example 26
-
~3S(Z)]-2-Amino--(metho~yiminO)-N-[l~~ILI(4~
ethyl-2,3-dioxo-1-piperazinyl)carbonyl~amino]
; sulfonyl]amino]carbonyl] 2--_xo-3-azetidinyl]-4-
thiazoleacetamide, potassium salt
A) (S)-[l-~[ I [I(4-Ethyl-2,3-dioxo-l-piperazinyl)
carbonyl]amino]sulfonyl]amino]carbonyl]-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt
(S)-3-~[(Phenylmethoxy)carbonyl]amino]-2-
; azetidinone (4.4 g) was suspended in 200 ml of
ethyl acetate and at -15C, 3 g of chloro-
sulfonylisocyanate was added with stirring. After
15 minutes at 0C a clear solution was obtained.
2.5 g Triethylamine and 4 g of 1-aminocarbonyl-4-
ethyl-2,3-dioxopiperazine was added and stirring
was continued for 16 houxs. The mixture was
washed with two 50 ml portions of water, 50 ml
of 2 N phosphoric acid and brine. After evaporation
of the solvent, the residue was dissolved in
100 ml of acetone/water (1~ he pH adjusted
to 6.5, the acetone removed and 8 g of crude
compound gsolated ~y freeze drying.
.~7~6 GC183a
-55--
B) L3S(Z)]-2--Amino~ (methoxyi.mino)-N-[l_
I I I I I ( 4-ethy'1-'2,3-'dioxo-1 piperazinyl)carbonylJ-
amino~su_fonyl]amino]carbonyll--2~oxo-3-azetidinyl]-
4-th:iazoleacetamide, potassium salt
(s) - Il- [ I [ I I (4-Ethyl-2,3-dioxo-1-piperazinyl)-
carbonyl~amino~sulfonyl~amino3carbonyl~-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt (S48 mg) was hydrogenated in
50 ml of dimethylformamide in the presence o
270 mg of 10% palladium on charcoal for 45 minutes.
To the hydrogenation solution, 210 mg o~ (Z)-2-
amino-~-(methoxyimino)-4-thiazoleacetic acid,
50 mg of N-hydrox~benzotriazole and 300 mg of
dicyclohexylcarbodiimide were added and the
solution was stirred at 20C for 16 hours. The
solverlt was removed in vauco, the residue filtered
with ethyl acetate and the crude compound was
chromatographed on HP-20 resin using water/aceLone
(9:1) as eluent. Yield 280 mg.
~ Example ~7
[3S(Z')]-2-[~12-[[1-[[1[(4-Ethyl-2,3-dioxo-1-
piperazinyl)carbonyl~amino]sulfonyl]amino]carbonyl]-
2-oxo-3-azetidinyl]-1-(2-amino-4-thiazolyl)-2-
oxoethylidene)amino~oxy]-2-methylpropanoic acid,
dipotassium salt
(s) - [1- [ I I [ ~ ( 4-Ethyl-2,3-dioxo-1-piperazinyl)-
carbonyl]amino]sulfonyl]amino]carbonyl]-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,
3~ potassium salt (548 mg; see example 2~) was
hydrogenated in 50 ml of dimethylformamide in
the presence of 270 mg of 10~ palladium on charcoal
GC183a
'7~
56~
for 45 minutes. After removaL of the catalyst,
~50 mg of ~Z)-2-amino--[l2-(diphenylmetho~y)-
1,1-dimethyl~2-oxoethoxy]lmino] 4~thiazoleacetic
acid, 100 mg of ~-hydroxybenzotriazole and 300 mg
of dicyclohexylcarbodiimide were added. The
solution was stirred for 16 hours. The solvent
was removed in vacuo and the residue was filtered
with ethyl acetate. The crude benzhydryl ester
of the title compound was suspended in 2 ml of
anisole, cooled to -10C and 4 ml of trifluoro-
acetic acid was drGpped in with stirring. The
temperature was maintained at -5C for 2 hours.
Ether (100 ml) was added and the precipitate
was filtered off, dried and dissolved in 5 ml
of water, the pH adjusted to 6.5 and the solution
chromatographed on HP-20 resin using water as
eluent. The title compound (180 mg) was isolated
by freeze drying.
Example 28
~3S(R)]-4-Ethyl-N-[2-[~ [~[tmethylamino)-
carbonyllamino]sulfonyl]amino]carbonyl~-2-oxo-3-
azetidinyl]amino]-2-oxo-1-phenylethyl-2,3-dioxo-
l-piperazlnecarboxamide,_potassium salt
(S)-ll-[ll[~(Methylamino)carbonyl~amino]~
sulfonyl]amino]carbonyl~-2-oxo-3-azetldinyll-
carbamic acid, phenylmethyl ester, potassium salt
(437 mg; see example17A) was hydrogenated in
50 ml of dry dimethylformamide in the presence
of 200 mg of 10% palladium on charcoal for 45
minutes. The catalyst was filtered off, 350 mg
GC183a
-57-
of ~R) -a- ~ I (4-ethyl~2,3-dioxo-1-pi~erazinyl)-
carbonylJamino~benzeneacetic acid, 50 m~ of
N-hydroxybenzotriazole and 300 mg of dicyclo-
hexylcarbodiimide were added; the solution was
S stirred for 12 hours. After removal of the
solvent in vacuo, the residue was chromatographed
on HP-20 resin using water/acetone (9:1) as
eluent. Yie]d 380 m5; melting point 178-183C,dec.
Example 29
[3S(Z)]-2-[I~1-(2-~mino--4-thiazolyl)-2-~
~ [ I ~ ~ (methylamino)carbonyl]amino]sulfonyl3amino]-
carbonyl]-2-oxo-3-azetidinyl]amino]-2-oxo-
ethylidene]amino]oxy~-2^methylpropanoic acid,
dipotassium salt
~ S) - Il- L [ E ~ [ (Methylamino)carbonyl~amino]-
sulfonyl]amino]carbonyl~-2 oxo-3-azetidinyl]-
carbamic acid, phenylmethyl ester, potassium salt
(437 mg; see example17A) was hydrogenated in
50 ml of dimethylformamide in the presence of
200 mg of 10% palladium on charcoal for 45
minutes. The catalyst was filtered off and 450 mg
of (Z~-2-amino-~-~[2-(diphenylmethoxy)~
dimethyl-2-oxoethoxy~imino3-4-thiazoleacetic
acid, 50 mg o~ N-hydrocvben~otria ole and 300 mg
of dicyclohexylcarbodiimide were added. The
solution was stirred overnight, evaporated to
dryness, the residue suspended in 2 ml of
anisole, cooled to -10C and 4 ml of trifluoro-
acetic acid was dropped in. Stirring was
~ ; GC1~3a
-~8-
continued for 2 hours at -5 C. After addition
of 100 ol oE ether, the precipitate was filtered
of, dissolved in 5 ml of water, the pH adjusted
to 6.5 with 1 N potassium hydroxide and the
aqueous solution chromatographed on HP-20 resin
using water as eluent. The title compound
(280 mg) was isolated by freeze drying, melting
point 215-220C, dec
Example 30
I3S(Z)]-2-Amino-N-~l-[I~II(dimethylamino)carbonyl]-
amino]sulfonyl]amino]carbonyl~-2-oxo-3-azetidinyl~-
a-(methoxyimino)--4-`thiazoleacetamide, potassium salt
(s) -~1- [ ~ (Dimethylamino)carbonyl]amino]-
sulfonyl]amino]carbonyl]-2-oxo-3-aæetidinyl]-
carbamic acid, phenylmethyl ester, potassium salt
(451 mg; see example 21A) was hydrogenated in
50 ml of dimethylformamide in the presence of
200 mg of 10~ palladium on charcoal for 45
minutes. The catalyst was filtered off and 201 mg
of (Z)-2-amino-~-(methoxyimino)-4-thiazoleacetic
acid, 50 mg of N-hydroxybenzotriazole and 300 mg
of dicyclohexylcarbodiimide were added~ The
solution was stirred for 12 hours at ambient
temperature. Tne solvent was removed in vacuo
and the crystalline residue chromatographed on
HP-20 resin using water/acetone (9.5:0~5) as
eluent. Yield 2~5 mg; melting-point 201C, dec.
30
1~7~7~ GC~3a
-59-
~ 31
3S(Z)]-2-Amino-~-(methoxyimino)-N-[2-oxo~l-
[[[[[(2-oxo-l-pyrrolidinyl)carbonyl]amino]
sulfonyl]amino]carbonyl]-3-azetidinyl]-4-
thiazoleacetamide, potassium salt
A) (S)-[2-Oxo-1-~ L [[(2-oxo-1-pyrrolldinyl)carbonyl]-
amino]sulfonyl]amino]carbonyl]-3- ~
carbamic acid, phenylmethyl ester, potassium salt
(S)-3-~[(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (3g) was suspended in 100 ml of dry
tetrahydrofuran at 0C. Chlorosulfonylisocyanate
(2.1 g) was slowly dropped in with stirring
while the temperature was maintained at 0C;
after 30 minutes, a clear solution was obtained.
At 0C, 1.7 g of triethylamine and 2.05 g of
N-carbamoylpyrrolidone were added. Stirring
was continued at ambient temperature for 15 hours.
After this time the precipitate was filtered
off and the mother liquor evaporated to dryness.
~- The residue was dissolved in 50 ml of water/acetone
(1:1), the pH adjusted to 6.5 with 1 N potassium
hydroxide, the acetone removed in vacuo and the
xemaining aqueous solution freeze dried yielding
6 g of the title compound.
.
:
~7~ 7~; GC183a
..
-6~-
B) 3S(Z)]--2-~nino- -(methoxyimino)-N-~2-oxo_l~
[[[[[(2-oxo~ pyrrolldinyl)carbonyl]arnino~-
sulfonyl]amino]carbonyl]-3-azetidinyl]-4-
thiazoleacetamide, potassium salt
_
~S)-[2-Oxo~ [[[I(2-oxo-1-pyrrolidinyl)-
carbonyl]amino~sulfonyl]amino]carbonyl]-3-
azetidinyl~carbamic acid, phenylmethyl ester,
potassium salt (1 g) was hydrogenated in dimethyl-
formamide using a 10~ palladium on charcoal
catalyst. The catalyst was filtered off, and
0.44 g of (Z)-2-amino-~-(methoxyimino)-4-
thiazoleacetic aaid, 30 mg N-hydroxybenzotriazole
and 0.82 g dicyclohexylcarbodiimide were added
to the mother liquor. The solution was stirred
overnight, the solvent removed ln vacuo and
the residue chromatographed on HP-20 resin
(eluting with water) yielding 300 mg of the
title compound.
Example 32
[3S(Z)]-2-[¦[1-(2-Amino-4-thiazolyl)-2-oxo-2-
L~2-oxo-1-I[~I~(2-oxo=1-pyrroldinyl)carbonyl3-
amino]sulfonyl~aminojcarbonyl]-3-azetidlnyl~-
amino~ethylidene~amino]oxy~-2-methylpropanoic
acid, dipotassium salt
(S)-I2-Oxo-1-[Ill[(2-oxo-l-pyrrolidinyl)-
carbonyl]amino~sulfonyl]amino]carbonyl]-3-
azetidinyl]carbamie acid, phenylmethyl ester,
potassium salt (1.5 g; see example 31A) was
hydrogenated in dimethylformamide using a 10~
palladium on eharcoal catalyst. The catalyst
2 ~ 3 GC1~3a
-61-
was filtered off, and 1.47 ~ of (Z)-2-amino-~-
~[2{diphenylmethoxyj-1,1-dimethyl-2-oxoethoxy]-
lmino]-4-thiazoleacetic acid, 40 mg of N-hyAroxy-
benzotriazole and 1.26 g of dicyclohexylcarbodiimide
were added to the mother li~uor~ The solution was
stirred overnight, the solvent removed ln vacuo
and the residue filtered with ether. The residue
was suspended in 5 ml of anisole and cooled to
-5 C. Trifluoroacetic acid (13 ml) was slowly
dropped in with stirring, and after 30 minutes
.the crude product was precipitated by the
addition of 100 ml of ether and filtered off.
The product was dissolved in 5 ml of water and
the pH adjusted to 6.5 with 1 N potassium hydroxide.
Chromatography on HP-20 resin (eluting with
water) yielded 500 mg of the title compound,
melting point 233C, dec.
:
Example 33
~3S(R)]-4-Ethyl-2,3-dioxo-N-¦2-oxo-2-[~2-oxo-1-
[l[(2-oxo-1-pyrrolidinyl)carbonyl]amino3sulfonyl]
amino]carbonyl~-3-azetidinyl3amino3-1-phenylethyl~-1-
: piperazinecarboxamide, potass:ium salt
(S)-[2-Oxo-l-l[lCI(2-oxo-l-pyrroldinyl)-
carbonyl]amino3sulfonyl3aminolcarbonyl~-3-
azetidinyl3carbamic acid, phenylmethyl ester,
potassium salt (1 g; see example 31A) was hydrogenated
in dimethylformamide using a 10% palladium on
charcoal catalyst. The catalyst was filtered
off, 0.7 g of (R)-~-[I(4-ethyl-2,3-dioxo-1-
piperazinyl)carbonyllamino]benzeneacetic acid,
Cl~3a
~62-
30 mg of N-hydroxyben~otriazole and 0.82 y of
dicyclohexylcarbodiimide were added to the mother
liquor, and ~e mixture was s-tirxed overnight at
ambient temperature. The solvent was removed
ln vacuo and the residue chromatoyraphed on
HP--20 resin, eluting with water, to yield 300 mg of
the title compound, melting point 186C.
Example 34
~3S(Z)~-2-Amino-~-(methoxyimino)-N-[2-o o-l-
I ~[~[(2-oxo-3-oxazolidinyl)carbonyl]amino]sulfonyl]-
amino]carbonyl]-3-azetidinyl] 4-thiazoleacetamide,
- potassium salt
-
A) (S~-[2-Oxo-1-[[[[[(2-oxo-3-oxazolidinyl)-
carbonyl~amino~sulfonyl]amino]c rbonyl]-3-
azetidinyl~carbamic acid, phenylmethyl ester,
potassium salt
` (S)-3-[[(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (3.0 g) was suspended in 300 ml of
dry tetrahydrofuran and cooled to -70C. Chloro-
sulfonylisocyanate (2.3 g) dissolved in 10 ml
of dry tetrahydrofuran was dropped in with
stirring. The temperature was allowed to rise
to 0C and this temperature was maintained for
10 minutes. Afterwards it was cooled to -40C.
Triethylamine (1.65 g) and 1.95 g of N-carbamoyl-
2-oxooxazolidine were added. The mixture was
stirred at room temperature for 14 hours and
the solvent was removed in vacuo. After dis~
solution in ethyl acetate, 100 ml of 2N phosphoric
acid was added and extracted three times with
' ~
~ r~ ~ GC183a
..
-63-
etllyl acetate. The combined oryanic layers were
evaporated in vacuo, the residue was dissolved
in 100 ml of acetone/water (1:1) and ~e pH
was adjusted to 6.5 with lN potassium hydroxide.
After removal o~ -the acetone ln vacuo, the
aqueous solution was freeze dried yielding
5.2 g of crude product.
B) [3S(Z)]-2- mino-~-(methoxyimino)-N-12-oxo-1-
-
E ~ ~ I [ ( 2-oxo-3-oxazolidinyl)carbonyl]amino]
sulfonyl]amino]carbonyl]~3-azetidinyl]-4-
thiazoleacetamide, potassium salt
(S) [2-Oxo-1-[~[~[(2-oxo-3-oxazolidinyl)-
carbonyl]amino]sulfonyl]amino]carbonyl]-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt (1 g) was hydrogenated in 80 ml
; of dry dimethylformamide using 0 . 5 g of 10
palladium on charcoal as catalyst. After
20 minutes, the hydrogenationwas completed
and the catalyst~as filtered off. (Z)-2-
~; Amino-~-(methoxyimino)-4-thiazoleacetic acid
(0.44 g),30 mg of ~-hydroxyben~otriazole and
O.91 g of dicyclohexylcarbodiimide were added
to the mother liquor. The solution was stirred
2~ for 12 hours at room temperature, the solvent
removed in vacuo and the 1.7 g residue was
chromatographed using HP-20 resin and water as
eluent, yielding 0.3 g of the title compound,
melting point 208c, dec.
Analysis for C14H15KN8OgS2: C: 30.99; H: 2.79;
N: 20.65; S: 11.82; K: 7.21;
Found: C: 30.08; H: 3.13; N: 19.52; S: 10~34;
K: 7.41
1 ~ 7~ i GC183a
-64-
Exam~ S
_
[3S(R)]~4-Ethyl-2,3-dioxo N-~2-o o-2~[[2-oxo-
1-~[~[~(2-oxo-3-oxazolidinyl)carbonyl]amino]
_ _
sulfonyl]amino]carbonyl~-3-azetidinyl]amino]-1-
phenylethyl]-l-piperazinecarboxamide, potassium salt
(S)-~2-Oxo-l-I~L[~2-oxo-3-oxazolidinyl)-
carbonyl]amino]sulfonyl]amino]carbonyl]-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt (1 g; see example 34A) was
hydrogenated in 80 ml of dry dimethylformamide
using 0.5 g of a 10% palladium on charcoal
catalyst. The catalyst was filtered off, 0.7 g
of (R)--[~(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]-
amino]benzeneacetic acid, 30 mg of N-hydroxybenzo-
1~ triazole and 0.91 g of dicyclohexylcarbodiimide
- were added to the mother liquor, and the mixture
was stirred ~or 14 hours at ambient temperature.
The solvent was removed ln vacuo and the residue
chromatographed on HP-20 resin, eluting with
water, to yield 0.36 g of the title compound,
melting point 187C, dec. `
Analysis for C23H25K~8O11S: C: 41.81; H: 3.81;
N: 16.96; S: 4.85, K: 5.92
Found: C: 40.04; H: 3.93; N: 16.33; S: 4.45; K: 5.59
~5
7~ GC183a
~65-
E ~[3S(Z)]-2-[[[1-(2-Amino-4-thiazolyl)-2-oxo-2-
I[2-oxo-1-~[[[~(2-oxo-3-oxazoli ~ _bonyl]-
amino]sulfonyl]amlno]carbonyl]-3-azetidinyl]-
amino]ethylidene]amino]oxy]-2-methylpropanoic
acid, dipotassium salt
(S)-[2-Oxo-l-~[~[ L ( 2-oxo-3-oxazolidinyl3-
carbonyl]amino~sulfonyl]amino]carbonyl~-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt (1 y; see example 34A) was
hydrogenated in 80 ml of dimethylformamide
using 0.5 g of a~10% palladium on charcoal
catalyst. The catalyst was filtered off, and
0.97 g of (Z)-2-amino-~-L~2-(diphenylmethoxy)-
1,1-dimethyl-2-oxoethoxy]imino]-4-thiazoleacetic
acid, 30 mg of N hydroxybenzotriazole and 0.91 g
of dicyclohexylcarbodiimide were added to the
mother liquor. The solution was stirred for
14 hours and the solvent removed ln vacuo. The
residue was suspended in 4 ml of anisole and
cooled to -5C. Trifluoroacetic acid (12 ml)
was 510wly drapped in with stixring, and after
20 minutes the crude product was precipitated
by the addition of 100 ml of ether and filtered
off. The product was dissolved in 20 ml of
water/acetone (1:1) and the pH adjusted to 6.5
with 1 N potassium hydroxide. Acetone was
evaporated, the residue was freeze-dried and
the crude product was chxomatography on HP-20
resin to yield 0.28 g of the title compound,
melting point 250C, dec.
AnalysiS for Cl7Hl8K2N8olls2
N: 17.17; S: 9.83; K: 11.98
Found: C: 29.54; H: 3.23; N: 16.93; S: 8.97; K: 11.86
~-7~D~ GC183a
-66-
Example 37_S(Z)3-2-A_ino-N-[1-[~[[[~3-~2-amlnoethyl)-2_
oxo-l-imidazo ~
amlno]carbonyl] 2-oxo-3-a~etidinyl~ (methoxy-
imino)-4-thiazoleaceta _ de, potassium salt
(S)-3-~[(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (2 g) was suspended in 200 ml of dry
tetrahydrofuran and cooled to -70C. Chloro-
sulfonylisocyanate ~1.4 g) dissolved in 7 ml
of dry tetrahydrofuran was added with stirring.
The temperature was allowed to rise to 0 C
and this temperature was maintained for 10 minutes.
Afterwards it was cooled to -40C, 1.2 g of
triethylamine was dropped in and 2.9 g of
1-l2-[(t-butoxycarbonyl)amino3ethyl]-3-carbamoyl-2-
oxoimidazolidine was added. This mixture was
stirred at room temperature for 14 hours. Following
the work-up procedure described in example 34A
yielded 5.4 g of (S)-[1-[[[[~[3-f2-(t-butoxy-
carbonylamino)ethyl]-2-oxo-1-imidazolidinyl]-
carbonyl]amino]sulfonyl]amino]carbonyl]-2-oxo-3-
a~etidinyl)carbamic acid, phenylmethyl ester,
potassium salt.
The above-prepared compound (2.7 g) was
; 2~ hydrogenated in 250 ml of dry dimethylformamide
using 1.4 g of 10~ palladium on charcoal catalyst.
After 30 minutes, the hydrogenation was completed
and the catalyst was filtered off. (Z)-2-Amino-~-
tmethoxyimino)-4-thiazoleacetic acid (0.95 g),
54 mg of N-hydroxybenzotria~ole and 1.75 g of
dicyclohexylcarbodiimide were added to the mother
liquor. The solution was stirred for 12 hours at
~ G~1~3a
..
-67-
room temperature, the solvent removed ln vacuoand the residue dissolved in 6 ml of anisole and
cooled to -5C. Trifluoroacetic acid ~15 ml)
was careflllly dropped in with stirring. The
temperature was maintained for an additional
3 hours, and the title compound precipitated by
the addition of 150 ml of dry ether. The product
was filtered and dissolved in 30 ml of water/acetone
(1:1), and the pH was adjusted to 6.5 by the
addition of lN potassium hydroxide. Acetone was
evaporated, the residue free2e-dried and 2.7 g
of crude product was chromatographed on HP-20
resin eluting with water, to yield 0.25 g of
the title compound, melting point 193C, dec.
Example 38
[3S(Z)]-2-[[[2-[[1-[ I [ ~ ~ [3-(2-Aminoethyl)-2-
oxo-l-imidazolidinyl]carbonyl]amino~sulfonyl]-
amino]carbonyl]-2-oxo-3-azetidinyl]amino]-1-
(2-amino-4-thiazolyl)-2-oxoethylidene]amino]-
; oxy]-2-methylpropanoic acid, dipotassium salt
( s ) ~ [1- I [ I [ I I 3~ I 2-(t-Butoxycarbonylamino)-
ethyl]-2-oxo-1-imidazolidinyl]carbonyl]amino]-
suifonyl]amino]carbonyl]-2-oxo-3-azetidinyl]
carbamic acid, phenylmethyl ester, potassium
salt (2.7 g; see example 37) was hydrogenated
in dry dimethylformamide using 10% palladium
on charcoal as a catalyst. The catalyst was
filtered off, and (Z)-2-amino-~ 2-(diphenyl-
methoxy)-1,1-dimethyl-2-oxoethoxy]imino]-4-
thiazoleacetic acid (2.1 g), 54 mg of N-hydroxy-
benzotriazole and 1.75 g o dicyclohexylcarbodiimide
were added to the mother liquor. Following the
work-up procedure described in example 37 and
GC183a
-6~-
chromatography on HP-20 resin eluting with water
yielded 0.3 g of ~e title compound, melting
point 221C, dec.
~xample 39
[3S(Z)]-2-Amino-N-~ [1~[3-(2-hydroxyethyl)-2-
oxo-l-imidazolidinyl]carbonyl]amino]sulfonyl]-
___
amlno~carbonyl]-2-oxo-3-azetidinyl]-~-(methoxy-
-- _ ____
imino)-4-thiaæoleacetamide, potassium salt
(S)-3-[[(Phenylmethoxy)carbonyl]amino]-2-
aæetidinone (1.5 g) was suspended in 100 ml of
dry tetrahydrofuran and cooled to -70C. Chloro-
sulfonylisocyanate (1.1 g) dissolved in 10 ml
of dry tetrahydrofuran was dropped in with stirring.
The temperature was allowed to rise to 0 C
and maintained there for 20 minutes, followed
by cooling to -40C. Trie~hylamine (0.9 g) and
2.8 g of 1-carbamoyl-2-oxo-3-[2-[(triphenylmethyl)oxy]-
ethyl]imidazolidine were added and the mixture
was stirred at room temperature for 14 hours.
The solvent was removed in vacuo and the work-up~
procedure of example 34~ was followed yielding
4.3 g of (S)-~ I [ I ~ ~ 3-(2-hydroxyethyl)-2-oxo-1-
imidazolidinyl]carbonyllamino]sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt.
The above-prepared compound was hydrogenated
in 80 ml of dry dimethylformamide using 0.3 g
of 10% palladium on charcoal catalyst. After
30 minutes the hydrogenation was completed and
the catalyst was filtered off. (Z)-2-Amino-~-
.
GC183a
-69-
(methoxyimino)-4-thlazoleacetic acid (195 mg),
13 mg of N-hydroxybenzotriazole and 360 my of
dicyclohexylcarbodiimide were added to the mother
liquor. The solution was stirred for 12 hours
at room temperature, the solvent removed ln vacuo
and the residue precipitated with ether. Crude
product (0.5 g) was purified on HP-20 resin
(eluent water/acetone (9.5:0.5)) yielding 30 mg
of the title compound,melting point 193C, dec.
Analysis for C16HlgKNgOgS2: C: 32.87; H: 3.28;
N: 21.56; S: 10.97; K: 6.69
Found: C: 31.26;! H: 3.44; N: 18.56; S: 8.86; K: 6.56
Example 40
L3S (Z) ] -2- ¦ [ ~1- (2-A_ ino-4-thiazolyl)-2-~
[ I ~ 3-(2-hydroxyethyl?-2-oxo-1-imidazolidinyl]-
carbonyl]amino]sulfonyl]amino]carbonyl]-2-oxo-3-
azetidinyl]amino~-2-oxoethylidene]amino]oxy] 2-
methylpropanoic acid, dipotassium salt
(S)-~1-[1[[~3-(2-Hydroxyethyl)-2-oxo-1-
imidazolidinyl]carbonyl]amino]sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl~carbamic acid,
phenylmethyl ester, potassium salt (470 mg; see
example 39) was hydrogenated ln 80 ml of dry
dimethylformaide using 0.3 g of 10~ palladium on
charcoal as a catalyst. After 30 minutes the
catalyst was filtered off, and (Z)-2-amino-~-~12-
(diphenylmethoxy)-l,l-dimethyl-2-oxoethoxy]imino]-4-
thiazoleacetic acid, 13 mgof N-hydroxybenzotriazole
and 360 mg of dicyclohexylcarbodiimide were added
to the mother liquor. Following the procedure
~ GC183a
--70
clescrlbed in example 36 yielded the title compound,
melting point 219C, dec.
Example 41
[3s(z~]-2-~~L2-[[~ [l(3-Amino-2-oxo-l-lmidazolidinyl)
carbonyl]aminolsulfonyl]amino]car~onyl~-2-oxo-3-
azetidinyl]amino]-1-(2-amino-4-thiazo]yl?-2-
oxoethylidene]amino]oxy]-2-methylpropanoic acid,
dipotassium salt
A) (S)-~l-[~ 13- L (t-Butoxycarbonyl)~mino]-2-oxo-1-
imidazolidinyl]carbonyl]amino]sulfonyl]amino]-
carbon~l~-2-oxo-3-azetidinYl]carbamic acid,
~ . _
phenylmethyl ester, potassium salt
15(S)-3-~[(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (1.5 g) were suspended in 150 ml of
dry tetrahydrofuran and cooled to -60C. Chloro-
sulfonylisocyanate (1.1 g) dissolved in 20 ml
of dry tetrahydro~uran was dropped in with stirring.
The temperature was allowed to rise to 0 C and
this temperatuxe was maintained for 15 minutes
and then cooled to -60C. Triethylamine(0.9 g)
- and 1.7 g of 1-carbamoyl-3-l(t-butoxycarbonyl)-
amino]-2-oxoimidazolidine were added. The
mixture was stirred at room temperature for
14 hours and then worked-up usinq the procedure
described in example 34, part A, yielding 1.5 g
of the title compound.
~'7~ j GCl83a
-71~
B) [3S(Z)]-2-[[[2-[~l-[[[[[(3-Amino-2-oxo-1
_ ___ _ _ _ _.__
~ ~amlno]sulfon~l]amino]-
carbonyl]-2-oxo-3-azetidinyl]amino]-l-(2-amino-4
thi.aæolyl)-2-oxoethylidene~amino3Oxy]-2-methyl-
propanoic_acid, dipotassium salt
(s) - [l-I [ I [ [ [3-I (t-Butoxycarbonyl)amino]-2-
oxo-l-imidazolidinyl]carbonyl]amino]sulfonyl~-
amino]carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt (0.79 g)
was hydrogenated in 60 ml of dry dimethylformamide
using 0.4 g of 10% palladium on charcoal catalyst..
After 20 minutes,the hydrogenation was completed
and the catalyst was filtered off. (Z)-2-Amino-a-
1~2-(diphenylmethoxy)-l~l-dimethyl-2-oxoethoxy]
imino]-4-thiazoleacetic acid (0.63 g), l9 mg of
N-hydroxybenzotriazole and 540 mg of dicyclo-
hexylcarbodiimide were added to the mother liquor.
Following the procedure described in example 34B,
yielded 48 mg of the title compound, melting point
208C, dec.
Example 42
~3S(Z)]-2-Amino-~-(methoxyimino)-N-[1-[[[[[[3-
I(l-methylethylidene)amino]-2-ox -l-imidazolidinyl]-
carbon l~amino]sulfonvl]amino]carbonyl~-2-oxo-3-
Y
azetidinyl]-4-thiazoleacetamide, potassium salt
(S)-11~[~[~[3-[(t-Butoxycarbonyl)amino]-2-
oxo-l-imidazolidinyl]carbonyl]amino]sulfonyl]-
amino]carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester~ potassium salt (0.7 g; see
example 41A) was hydrogenated and then reacted
with 0.26 g of (Z)-2-amino-a-(methoxyimino)-4-
thiazoleacetic acid following the procedures
~ r~ GC18~a
..
72
described in example 37B, yieldlng 65 my oE thc
title compound, ~elting point 207C, dec.
Analysis for C17~21KN108 2
N: 23.47; S: 10.75; K: 6.55
Found: C: 32.56; H: 3.88; N: 21.37; S: 9.10; K: 7.03
Example 43
~3S(Z)]-2-Amino-N-~l-f[~[I(3-methyl-2-oxo-l-
imidazolidinyl)carbonyl]amino~sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl]-a-tmethoxyimino)-4-
thiazoleacetamide, potassium salt
A) (S)-Ll-[~[~(3-Methyl-2-oxo-1-imidazolidinyl)-
carbonyl]amino]sulfonyl3amino]carbonyl]-2~oxo-3-
_zetidinyl]carbamic acid, phenylmethyl ester,
potassium salt
(S)~3-[[(Phenylmethoxy)carbonyl~amino~-2-
azetidinone (3.0 g) was suspended in 300 ml of
dry tetrahydrofuran and cooled to -70C. Chloro-
sulfonylisocyanate (2.1 g) dissolved in 10 ml
of dry tetrahydrofuran was dropped in with
stirring~ The termperature was allowed to rise
to 0C and this temperature was maintained for
10 minutes, the~ cooled to -40C. Triethylamine
(1.7 g) and 2.3 g of 1-carbamoyl-3-methyl-2-
oxoimida~olidine were added. Following the
procedure described in example 34A yielded 4.3 g
of the title compound.
~ 7~,~ GC1~3a
.
-73-
B) [3S(b)]-2-Amino-N-[1-[~[~[(3-methyl-2-oxo 1
imidazolidinyl)_c~ amlno _ulfonyl]amlno]-
carbonyl]-2-oxo-3-azetidinyl]-a~(me ~oxyimino ? -4 -
t~iazoleacetamide, potassium salt
(S)-~1-[~[~[(3-Methyl-2-oxo-1-imidazolidinyl)-
carbonyl]amino]sulfonyl]amino]carbonyl~-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt (1.2 g) was hydrogenated in 80 ml
of dry dimethylformamide using 0.6 g of 10%
palladium on charcoal as catalyst. After 30 minutes
the catalyst was filtered off, and (Z)-2-amino-~-
(methoxyimino)-4~thiazoleacetic acid (0.52 g),
32 mg of N-hydroxybenzotriazole and 1.0 g of
dicyclohexylcarbodiimidë were added to the mother
liquor. The solution was stirred for 13 hours
at room temperature and the solvent was then
removed in vacuo. The residue was chromatographed
on HP-20 resin, eluting with water, to yield
0.120 g of the title compound, melting point 205C,dec.
Analysis for C15H18KNgO8S2: C: 32.43; H: 3.27;
N: 22.69; S: 11.54; K: 7.04
; Found: C: 30.83; H: 3.44; N: 21.34; S: 10.20; K: 7.30
.
GC183a
_7a _
Example 44
~3s(z)]-2-[[l2-[ll-[~[l[(3-Methyl-2-oxo-l-
imidazolidinyl)carbon~l]amino]sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl]amino~-1-(2-amino 4-
thiazolyl)-2-oxoethylidene]amino]oxy]-2-methyl-
propanoic acid,_dipotassium salt
~ S)-~1-[~ (3-Methyl-2-oxo-l-imidazolidinyl)-
carbonyl]amino]sulfonyl]amino]carbonyl]-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt (1.5 g; see example 43A) was
hydrogenated in 80 ml of dry dimethylformamide
using 0.8 g of 10~ palladium on charcoal as
catalyst. After 30 minutes the catalyst was
filtered off and 1.4 g of (Z)-2-amino-~ 2-
(diphenylmethoxy)-1,1-dimethyl-2 oxoethoxy]-
imino]-4-thiazoleacetic acid, 40 mg of N-hydroxy-
benzotriazole and 1.3 g of dicyclohexylcarbodiimide
were added to the mother liquor. Following the
procedure of ecample 36 yielded 0.5 g of the
title compound, melting point 211C, dec.
Analysis for ClgH21K2NgOloS2-
N: 18.94; S: 9.63; K: 11.75Found: C: 30.55; H: 3.53; N: 18.12; S: 8.65; K: 10.97
j GC183a
-75-
E~ le 45
[3S(Y.)]-2-[¦[2-[[1-[[1[[(2 Oxo-l~imidazolidinyl)-
carbonyl]amino~sulfonyl]amino]carbon 1]-2-oxo-3-
azetidinyl]amino]-1-(2-amino_4-thiazol_1)-2-
_oethylidene}amino3Oxy]-2-methylpropanoic
acid, dipotassium salt
-
A) (S)-ll-[l[l[r2-Oxo-3-(triphenylmethyl)-1-
imidazolidinvl]carbonvl]amino]sulfonyl]amino]-
.. . .
carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt
(S)-3-~[(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (1.3 g) was suspended in 130 ml of
dry tetrahydrofuran and cooled to -70C.- --
Chlorosulfonylisocyanate (0.9 g) dissolved in5 ml of dry tetrahydrofuran was dropped in with
stirring. The temperature was allowed to rise
to 0C and maintained there for 10 minutes,
followed by cooling to -40C. Triethylamine
(0.8 g) and 0.65 g of 1-carbamoyl-2-oxo-3-
(triphenylmethyl)imidazolidine were added
and the mixture was worked-up following the
procedure described in example 34A ~o yield
3.2 g of the title compound.
7~ C;C183a
-76-
B) _S(Z)1-2-[l[2-Lll-[[[[~(2-Oxo--l-imidazolldlnyl)-
carbony:L]amino~sulfonyl]amino]carbonyl]-2-oxo-3-
azetidinyl]amino]-1-(2-amino-'l-thiaæolyl)~Z-oxo-
.
ethylidene]amino]oxy]-2-methylpropanoic acld,
dipotassium salt
(S) - ~ [ 12-Oxo-3- (triphenylmethyl) -1-
imidazolidinyl]carbonyl]amino]sulfonyl]amino]-
carbonyl]-~-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt (1.5 g) was
hydrogenated in 80 ml of dry dimethylformamide
using 0.9 g of 10% palladium on charcoal as
catalyst. After 30 minutes the catalyst was
filtered off and 0.98 g of (Z)-2-amino-a-~[2-
(diphenylmethoxy)-l,l-dimethyl-2-oxoethoxy~-
imino]-4-thiazoleacetic acid, 27 mg of N-hydroxy-
benzotriazole and 0.84 g of dicyclohexylcarbodiimide
were added to the mother liquor. Following the
procedure of example 36 yielded 0.28 g of the
title compound, melting point 228 C, dec.
- Example 46 - -
: ~3S(Z)]-2-Amino-N-ll~ I (2-oxo-1-imidazolidinyl)-
carbonyl]amino~sulfonyl]amino]carbonyl]-2-oxo-3-
azetidinyl]-~ ~methoxyimino)-4-thiazoleacetamide,
pot~ssium salt
A) (S)-[1-1[~[(2-Oxo-1-imidazolidinyl)carbonyl~-
amino~sulfonyl]amino]carbonyl~-2-oxo-3-azetidinyl]-
carbamic acid, phenylmethyl ester, potassium salt
(S)-~ [l[~2-Oxo-3-(triphenylmethyl)-1-
imidazolidinyl]carbonyl]amino]sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl estert potassium salt (1.7 g; see
example 45A) was treated with 2N phosphoric acid
~ 7~ GC 18 3a
-77
and :LN potassium hydroxide following the proceduxe
described in exarnple 34A to yield 1.73 g of the
title compound.
B) [3S(Z)]-2-Amino-N~ (2-oxo-1-imidazolidinyl)-
.
carbonylJamino]sulfonyl~amino]carbonyl]-2-oxo-3-
azetidinyl]--(methoxyimino)-4~thiazoleacetamide,
potassium salt
(s) - [1- L [ ~ ~ I (2-Oxo-l-imidazolidinyl)carbonyl]-
amino]sulfonyl]amino]carbonyl]-2-oxo-3-azetidinyl]-
carbamic acid, phenylmethyl ester, potassium salt
(0.8 g) was hydrogenated in 70 ml of dry
dimethylformamide using 0.5 g of 10% palladium
on charcoal as catalyst. After 20 minutes the
catalyst was filtered off, and (Z)-2-amino--
(methoxyimino)-4-thiazoleacetic acid (0.38 g),
27 mg of N-hydroxybenzotriazole and 0.78 g of
dicyclohexylcarbodiimide were added to the
mother liquor. The solution was stirred for
14 hours at room temperature and the solvent
was then ~emoved in vacuo. The residue was
chromatographed on ~P-20 resin, to yield 200 mg
of the title compound, melting point 216C, dec.
GC183a
-78-
Example 47~3S(Z)~-2-Amino-N-~l- I [~[[3-(methylsulfonyl)-2-
oxo-l-imidazolidinyl]carbonyl]aminoJsulfon~l]-
amino]carbonyl]-2-oxo-3-azetidinyl]-~-(methoxy-
.
imino)-4-thiazoleacetamide, potassium salt
A) (S)-[l~ 3-(Methylsulfonyl-2-oxo-1-
imidazolidinyl]carbonyl]amino]sulfonyl]amino]-
carbonyl]-2~oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt
(S)-3-11(Phenylmethoxy)carbonyl]amino]-2-
aæetidinone (3.0!g) was suspended in 300 ml
of dry tetrahydrofuran and cooled to -70C.
Chlorosulfonylisocyanate (2.1 g) dissolved in
10 ml of dry tetrahydrofuran was dropped in
with stirring. The temperature was allowed
to rise to 0C and this temperature was maintained
for 10 minutes, then cooled to -40C. Triethyl-
amine (1.7 g) and 2.3 g of 1-carbamoyl-3-(methyl-
sulfonyl)-2-oxoimidazolidine were added.
Following the procedure described in example 34A
yielded 2.8 g of the title compound.
B) ¦3S(Z)~2-Amino-N-~1-[~ 3-(methylsulfonyl?-2-
oxo-l-imidazolidin l]carbonyl]amino]sulfonyl]-
- Y
amino]carbonyl-2-oxo-3-azetidinyl~-~-(methoxy-
imino)-4-thiazoleacetamide, potassium salt
(S)-Il-[~ 3-(Methylsulfonyl)-2-oxo-l-
imidazolidinyl~carbonyl]amino~sulfonyl]amino]-
carbonyl~ 2-oxo-3-azetidinyl]carbamic acid,
phenylmethyl ester, potassium salt (1.8 g) was
hydrogenated in 300 ml of dry dimethylformamide
using 2.0 g of 10% palladium on charcoal as catalyst.
7~; GC183a
_79_
After 20 minutes the catalyst was filtered off,
and (Z)-2-amino-~ (methoxyimino)-4-thiazoleaceti.c
acid (1.1 g), 70 mg of N-hydroxybenzotriazole and
2.0 g of dicyclohexylcarbodiimide were added to
the mother liquor. The solution was stirred for
12 hours at room temperature and the solvent was
then removed in vacuo. The residue was chromatographed
on HP-20 resin, eluting with water/acetone (9:1),
to yield 200 mg of the title compound, melting
point 204C, dec.
! Example 48
[3S(z)]-2-[I [2-L[l-I ~[[~[3-(Methylsulfonyl)-2-
oxo-l-imidazolidinyl]carbonyl]amino]sulfonyl]-
_ino]carbonyl]-2-oxo 3-azetidinyl]amino]-1-
(2-amino-4-thiazolyl)-2-oxoethylidene~aminol-
oxy]-2-methylpropanoic acid, dipotassium salt
(S)-~1-[[[¦[~3-(Methylsulfonyl)-2-oxo-1-
imidazolidinyl]carbonyl]amino]sulfonyl]aminol-
carbonyl~-2-oxo-3-azetidinyllcarbamic acid,
phenylmethyl ester, potassium salt (1.5 g;
see example 47A) was hydrogenated in 150 ml
of dry dimethylformamide using 0.9 g of 10%
palladium o~ charcoal as catalyst. After 20 minutes
the catalyst was filtered off and 1.23~g of
(Z)-2-amino~ 2-(diphenylmethoxy)-1,1-dimethyl-2-
oxoethoxy3imino]-4-thiazoleacetic acid, 40 mg of
N-hydroxybenzotriazole and 1.1 g of dicyclo-
hexylcarbodiimide were added to the mother liquor.
3~ Following the procedure of example 36 yielded 180 mg
of the title compound, melting point 236 C, dec.
,.
GC183a
-80-
Exam~le 49
[3S(Z)]-2-Amino N~ I [ I E I ( 3- isopropyl 2-oxo-L-
imidazolidinyl)carbonyl]amino]sulfonyl]amino]-
carbonyl]-2-oxo-3-azetidinyl]-~-(methoxyimino)-4-
thiazoleacetamide, potassium salt
A) ( ~ l I 1 I [(3~ propyl-2-oxo-1-imidazolidinyl)-
carbonyl]amino]sulfonyl]amino]carbonyl]-2-oxo-3-
azetidinyl]carbamic acid, ~henylmethyl ester,
potassium salt
(S)-3-~[(Phenylmethoxy)carbonyl]amino]-2-
azetidinone (3.0~g) was suspended in 300 ml of
; dry tetrahydrofuran and cooled to -70C. Chloro-
sulfonylisocyanate (2.1 g) dissolved in 10 ml
f dry tetrahydrofuran was dropped in with stirring.
The temperature was allowed to rise to 0C
and tllis temperature was maintained for 10 minutes,
then cooled to -40C. Triethylamine (1.7 g) and
2.74 g of 1-carbamoyl-3-isopropyl-2-oxo-
imidazolidine were added. Following the procedure
described in example 34A yielded 5.9 g of the
title compound.
.
B) [3S(Z)3-2-Amino-N-~ [[~L(3-isopropyl-2-
; 25 oxo-l-imidazolidinyl)carbonyl~amino~sulfonyl~-
amino]carbonyl3-2-oxo-3-azetidinyl]-u-(methoxyimino)-4-
thiazoleacetamide, potassium salt
(S) - [1- ~ ~ [ [ E (3-Isopropyl-2-oxo-1-imidazolidinyl)-
carbonyl]amino]sulfonyl~amino]carbonyl]-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt (1.5 g) was hydrogenated in 150 ml
of dry dimethylformamide using 0.8 g of 10%
; palladium on charcoal as catalyst. After 20 minutes
GC183a
-81-
the catalyst was filtered off, and 0~6 g of
(Z)--2-amino--(methoxyimino)-4--thiazoleacetic
acid, 40 mg of N-hydroxyben7.0triazole and 1.2 y
of dicyclohexylcarbodiimide were added to the
mother liquor. The solution was stirred for
14 hours at room temperature and the solvent
was then removed ln vacuo. The residue was
chromatographed on HP-20 resin, eluting with
water/acetone (9.25:0.75) to yield 30~ my of~
the title compound, melting point 189C, dec.
~ Example 50
[3S(Z)]-2-[~2-~[1- r [ [ [ [ (3-isopropyl-2-oxo-1-
imida~olidinyl)carbonyl]amino]sulfonyl]amino]-
.
carbonyl]-2-oxo-3-azetidinyl]amino]-1-(2-amino-4-
._ _
thiazoly~-2-oxoethylidene]amino]oxy]-2=methyl-
propanoic acid, dipotassium salt
(s~ - [1- [ [ [ [ r (3-Methyl-2-oxo-1 imidazolidinyl)-
carbonyl]amino]sulonyl]amino]carbonyl~-2-oxo-3-
azetidinyl]carbamic acid, phenylmethyl ester,
potassium salt (1.5 g; see example 43A) was
hydrogenated in 150 ml of dry dimethylformamide
using 0.8 g of 10% palladium on charcoal as
catalyst. After 20 minutes the catalyst was
filtered off and 1.3 g of (Z)-2-amino--~[2-
(diphenylmethoxy)-1,1-dimethyl-2-oxoethoxy]-
imino]-4-thiazoleacetic acid, 40 mg of N-hydroxy-
benzotriazole and l.2 g of dicyclohexylcarbodiimide
were added to the mother liquor. Following the
procedure of example 36 yielded 0.4 g of the
title compound, melting point 234C, dec.