Note: Descriptions are shown in the official language in which they were submitted.
1~:'7~
1 61109-7287D
29,348
(2-IMIDAzoLlIN-2-y-L)T~lIENo- AND _UROL2,3~hl AND
~3,2-hlPYR:[DINES AND INTERME,DIATES FOR_TIIE PREPARATION
_IEREOF~ AND USE OF SAID COMPOUNDS AS llERBICIDAL AGENTS
The present divisional invention relates to novel (2-
imidazolin-2-yl)thieno-and Europyridine compounds, and
intermediates for the preparation oE said pyridine compounds and a
method for controlling undesirable annual and perennial plant
species therewith. It is a divisional application divided out oE
parent application no. 455,178 iled on the 1st of June, 1984.
The parent application relates to 6-(2-imidazolin-2-
yl)thieno- and furo[2,3-b] and 5-(2-imidazolin-2-yl)thieno- and
furo[3,2-b]pyridine compounds and the corresponding 2, 3-
dihydrothieno and 2,3-dihydroEuro compounds having the structures
(Ia) and (Ib):
Y ~ X ~N ~ ~ W ~ R2
B B
[2,3-b] [3,2-b]
(Ia) (Ib)
~ ~7Z~
wllcrci~ rcprcscllts a s:lngle or a ~loub:lc bond; Rl :is Cl~C4 alkyl;
R2 is Cl-C4 al~yl or C3-C6 cycloalkyl; and whell Rl and R~ aro takcn
together with carbon to which they are attaclled they may represent C3-
C6 cycloalkyl optionally substituted with methyl; A is COOR~, CiiO,
011, COC~120H, CONIIC1-l2Cll20~l, CON~10}1, or
--<
RD
K3 iS hydrogen, Cl-Cl~ alkyl, ~ ich may be interrupted by one or more
O or S and is optionally substituted with one of the following groups:
Cl-C3 alkoxy, halogen, hydroxy, C3-~6 cycloalKyl ~ benzyioxy, furyl,
p~lenyl, ~urfuryl, halophenyl, lo~eralkylphenyl, loweralkoxyphenyl, nitro-
phenyl, carboxyl, loweralkoxycarbonyl, cyano, Cl-C~ alkylthio or tri-
loweralkylammonium, C3-C6 alkenyi oplionally substituted with one of
the following groups: Cl-C3 alkoxyJ phenyl, halogen or witn two Cl-C3
alkoxy groups or two halogen groups: C3-C6 cycloalkyl optionally sub-
stituted with one or two Cl-C~ alkyl groups; C~-~ alkynyl optionally
substituted witn phenyl, halogen or C1120~1; or a cation for example a
cation of al~ali metals, alkaline earth metals, (Ca, Ba) manganese,
copper, iron, ammonium or organic ammonium; RC and RD are H or ~`H3; B
is H, COR4 or SO2R5, provided that when B is COK4 or SO2R5, and A is
ZO COOR3, R3 cannot be hydrogen or a salt-~orming cation; R4 is Cl-Cll
alkyl~ chloromethyl or phenyl optionally substituted with one chloro,
one nitro, one methyl or one methoxy group; R5 is Cl-C~ alkyl or phenyl
optionally substituted with one methyl group, chloro or nitro;
~L~7~
l~ is O or S; X is ~, S, or, wllere -_ is a singlc bond, ~5-0; Y nnd
Y', Z and Z' are hydrogen, )lalogcn, Cl-C6 "lkyl, Cl-C~ hydroxylower^
alkyl, Cl-C6 alkoxy, Cl-C6 acyloxy, benzoyloxy optionally substituted
Wi~h one or ~wo Cl-C4 alkyl, Cl-C~ alkoxy, halogen; Cl-C4 alkylthio,
phenoxy, Cl-C4 haloalkyl, Cl-C4 haloalkoxy, ni.tro, cyanO, Cl-C4 alkyl~
amino, Cl~C4 dialkylamino, Cl~C4 alkylsulfonyl or phenyl optionally
substituted with one or two Cl-C4 alkyl, Cl-C4 alkoxy, halogen, or any
combination of two of these groups and wherein Y and Z are the same
group provided that Y and Z are H, hologen, alkyl or alkoxy, and when
Y and Y' or Z and Z' are the same group they are hydrogen or alkyl;
and, when taken together, ~ and Z may form a ring in which YZ are rep-
sented by the structure -(CH~)n-, where n is an integer selected from
3 or 4; or IL 1l IQ l7
-C - C - C - C-, where L, M, Q and R7 each represent hydrogen,
halogen, nitro, Cl-C4 loweralkyl, Cl-~4 lowcralkoxy, methoxy, phcnyl
and phenoxy, with the proviso that only one of L, M, ~ or R7, may rep-
resent a substituent other than hydrogen, halogen, Cl-C4 alkyl or Cl-
C4 alkoxy; or the pyridine N-oxides thereof, when W is O or S and A
is COOR3; and when Rl and R2 are not the same, the optical isomers
thereof, and, except when R3 is cation, the acid addition salts there-
of.
~ 7~7~ ~
A preferred group of 6 (2-imidazolin 2-yl)-
thieno- and furo[2,3-b]pyridine and 5~ imidazolin
2-yl)thieno- and furo[3,2 b]pyridine compounds have the
formula shown as (Ia) and (Ib) above, wherein Rl is
methyl; R2 is methyl, ethyl, propyl, isopropyl, or when
taken togethPr form a cyclohexyl or methylcyclohexyl
ring; W is oxygen or sulfur; B is hydrogen; A is COOR3,
where R3 is as described above; Y and Z are hydrogen,
Cl-C3 alkyl, Cl-C3 alkoxy, alkylthio, halo, nitro,
cyano, trifluoromethyl, monofluoromethyl, difluoro-
methyl, monofluoromethoxy, difluoromethoxy, trifluoro-
methoxy, methylsulfonyl, methylsulfonamido, methyl-
amino, dimethylamino, or isopropylamino and when Y and
Z are taken together, YZ is -(CH2)3~ -(CH2)4-~ or
-CH=CH-CH=CH-.
The most preferred formula (Ia) and (Ib)
(2-imidazolin-2-yl)thieno and furo compounds are those
wherein B is H, Y and Z are hydrogen, chloro, methyl or
methoxy provided ~hat one of either Y or Z is hydrogen;
W is oxy~en; A is COOR3 where R3 is hydrogen, furfuryl,
propynyl, C3-haloalkenyl, Na~ or isopropylammonium and
Rl and R2 are as defined for the preferred compounds.
It should also be understood that when B is
H the imidazolinyl thieno- and furo[2,3-b] and [3,2-b]-
pyri~dines represented by formula (Ia~ and (Ib) above
may be ~automeric. While, for convenience, they are
depicted by single structures identified as formula
(Ia) and ~Ib), they may exist in either of the tauto-
meric forms illustrated as follows:
~"l~R2 ~ R~
W HN W ,'
H
or or
~;R 2 ~R ~ ~
N W N W .,
,,
As such, both tautomeric forms of ~aid imidazolinyl
pyridlnes are meant to be included under the formula
(Ia) and (Ib) d~finiti.on.
The divisional invention relates to novel
substituted thieno- and furoimidazopyrrolopyridinedione
co~pound~ of structure (Ila) and (IIb) below and a
method for con~rolling undesirable annual and perennial
plant species therewith in soybeans and certain cereal
crop~:
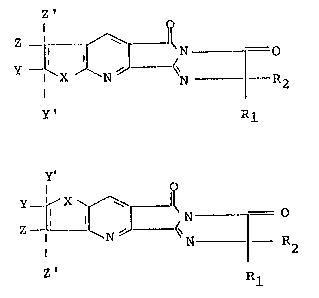
~ Y X~ '`".
2 5 Z ~ l l N ---= ~ ~ II N ~o ...
X N N --- R 2 1 ~--<~N~R 2
Y' . ~1 7' Rl ~.
~IIa~ (lIb)
;,.
. . .
":, : .
: .
~ ~t7Z~
wherein X, Y, Y', ~, Z', W, Rl and R2 are as described
for (Ia) and (Ib) above and wherein X, Y, Z, W, Rl and
R2 in the preferred and most pre~erred formula (IIa)
and (IIb) compounds are as described for the preferred
and most preferred formula (Ia) and formula (Ib) com
pounds.
Herbicidal substituted pyridine and quino-
line2-imida~olin-2-yl acids~ esters and salts are
disclosed in the Canadian Patent 1187498, which issued
May 2, 1385. The present inven-
tion relates to novel thieno- and furo~2~3~~pyridines
and thieno- and furo~3,2-b]pyridines which when
substituted in the 6 or 5 position with an imidazo-
linone ring and in the 5 or 6 position with a group A
as previously defined, provide potent herbicidal
lS agents. The finding that imidazolinyl thieno- and
-furopyridines provide potent herbicides is unexpected
as there is no prior indication tha~ such [2,3-b] or
[3,2-b] ring systems may be employed for any agronomic
or herbicidal utility. This new class of herbicidal
agents is highly effective when applied as a pre- or
postemergence treatment, and individual members of this
class exhibit unusual selectivity in soybean and cereal
crops such as wheat, barley, rice, rye and oats. Further,
it has been found that selectivity in cereals may be
enhanced when A is C02H, by the preparation of esters,
particularly furfuryl, alkynyl and haloalkenyl esters.
Additionally some members of this class
exhibit unexpected plant growth regula~ing effects such
as reduced plant height and antilodging activity and
increased tillering in cereal crops.
7~
~:L 1.09-7~87n
29,34
-7
The compounds oE the parent invention may
conveniently be prepared from the appropriately sub-
stituted thieno- and furol2,3-b~ and l3,~-b]pyridine-
dicarboxylic acids and esters oE Eormula (IIIa) and
(IIIb):
S
y~ ~r 0 R and ~ ~
(I3Ia~ tIIIb)
wherein X, Y and Z are as previously described and R is
methyl or ethyl.
Methods suitable for preparing novel ~ormula
(Ia) and formula (Ib) unsaturated compounds wherein - -
is a double bond from the novel ~ormula (IIIa) and
(IIIb) pyridinedicarboxylic acid esters are illustrated
in Flow Diagram I below.
Thus formula (IIIa) an~ (IIIb) diesters may
be hydrolyzed to the corresponding thieno- and ~uro-
2,3-pyridinedicarboxylic acids of ormula ~IVa) and
(IVb) by reaction thereof with a strong base such as
potassium hydroxide or sodium hydroxide. Acid anhy-
drides oE formula (Va) and (Vb) may then be prepared by
treatment oF the formula (IVa) and (IVb) pyridine-
dicarboxylic acids with, for example, acetic anhydride.
~ ~'72 ~ ~
Reaction of formula (Va) and (Vb) anhydrides with an
appropriately substituted aminocarboxamide or amino-
thiocarboxamide depicted by formula (VI) yields car
bamoyl nicotinic acids of formula (VIIa) and (VIIb).
Treatment of the thus-formed formula (VIIa) and (VIIb)
carbamoyl nicotinic acids with about 2 to lO molar
equivalents oÇ aqueous or aqueous alcoholic sodium or
potassium hydroxide, preferably under a blanket of
inert gas such as nitrogen, cooling and acidifying tO
pH 2 to 4 with a strong mineral aoid such as hydro-
chloric acid or sulfuric acid gives herbicidally
effective 6-(4,4-disubstituted-5-oxo-(or thioxo)-2-
imidazolin-2-yl)thieno- and furo[2,3-b]pyridine-5
carboxylic acids, and 5-(4,4-disubstituted-5-oxo-(or
thioxo)-2-imidazolin-2-yl)thieno- and furo[3,2-b]-
pyridine-6-carboxylic acids encompassed by formulas
(Ia) and (Ib).
Formula (Ia) and (Ib) 5 or 6-(2-imidazolin-2-
yl)thieno- and furopyridine esters, wherein A is COOR3
and R3 represents a substituent other than hydrogen or
a salt-forming cation, and Rl, R2, Xt Y and Z are as
described above can be prepared by reacting a novel
thieno- or furoimidazopyrrolopyridinedione, represented
by formulas (IIa) and (IIb), hereinbelow, in Flow
Diagram (II), with an appropriate alcohol and corres-
ponding alkali metal alkoxide at a temperature ranging
between about 20C and about 50C.
Formula (IIa) and (IIb) novel ~hieno- and
furoimidazopyrrolopyridinediones may conveniently be
prepared from formula (Ia) and (Ib) acids, where B is H
by treatment with one equivalent of dicyclohexylcarbo-
diimide in an inert solvent such as methylene chloride
as illustrated in Flow Diagram (II) below.
~c
S y~CO~R Z~N~
(IIIb)
( IIIa)
~ 1. Aqueous ethanolic NoOlt
l 2. HCl
Z~COOH Y~/ ~ ~OOH
OOH
Y~XlN _COOH N
( IVb)
( IVa )
.~ I
lAc20
R
zYr~ O Yz~O
0
(Vb)
(Va)
7~
-10-
FLOW DIAGRAM (I) (Continued)
(Vo) (Vb)
~1
NH 2~W~NH 2
R2
(VI )
Z~COOH ~1 Y~ ~OOH ~1
Y~ ,~ LCONH~j:~W--NH~ R2
(VIIa) (VIIb)
,~
NaOH
~: 25 ~/~R2 Z~ ~ R2
11 _W ~ WH H
(Ia) (Ib)
'7~7'~3
_LO~
z_ ~COOH Rl y__f X ~COOH Rl
y~ ~/N~R2 z.~l~ /~L</ =~w2
H H
( Ia) ( Ib)
DCC
t
~3R2 N ~2
Rl R
(IIa~ (IIb)
R30-Ml+
.
z~CO~R3 Rl y~ ~l~cOOR3 Rl
2 5 y~ ~1~ ~k~N~R 2 Z I ~ /) </N~R 2
X N 1~--W N 111 W
H H
( la) ( Ib)
~7~
-12-
where Ml ls an alkali metal, and X, Y, Z, Rl, R2 and R3
are as above defined.
Many formula (IlIa) thieno[2,3-b]pyridinedi-
carboxylic acids and (IIIb~ thieno[3,2-b]pyridinedi-
carboxylic acids may conveniently be prepared by
reacting the appropriately substituted 2 or 3-amino-
thiophene of Eormula (VIlIa) or ~VIIIb), where R is
hydrogen or chloro, with a Cl-C4 alkyl ester of
acetylenedicarboxylic acid of formula (IX) as described
by Blecker~ et al. Chem. Ber. 1978, 106S 368. The
thus-formed ~-aminothieno-a,~-unsaturated ester of
formula (X) is then reacted with an immonium salt de-
picted by the formula Cl-CH=N-(R''')2 C~ wherein R'''
is Cl-C6 alkyl or Cl-CH= ~(CH2)n' Cl where n' is 4 or
5, in the presence o a low boiling chlorinated hydro-
carbon solvent such as methylene chloride or dichloro-
ethane at a temperature between about 40C and 90C,
for a period of time sufficient to essentially complete
the reaction and yield the formula (IIIa) [2,3-b]-
thieno- or (IIIb) [3,2-b]thieno 2,3-pyridinedicarbo-
; 20 xylic acid as the dialkyl ester as illustrated in Flow
Diagram (III) below.
Formula (Illb) furo[3,2-b]pyridinedicarbo-
xylic acids may be prepared by reacting 3-amino-2-
formylfuran of formula (XI) prepared by the method of
S. Gronowitz et al., Acta Chemica Scand B29 224(1975)
with ethyl oxalacetate to give formula (IIIb) furo-
pyridine compounds directly, as illustrated in Flow
Diagram (IV) below while formula (IIIa) furo[2,3-b]-
pyridine compounds where Y and Z are H are obtained by
bromination of the reaction product (XII) of acetoace
tamide with the diethyl ester of ethoxymethyleneoxal-
acetic acid followed by treatment with sodium boro-
hydride and ~ toluene sulfonic acid in refluxing
xylene as illustrated in Flow Diagram (V~ below.
2'~2'
-l3-
Formula ~IIIb) 2,3-dihydrouro[3,2-b] and
~hieno[3,2-b~pyridines may be prepared by the reaction
of diethyletlloxymethylene oxalacetate with a mixture of
enamines derived from 3-keto-tetrahydrouran or 3-keto-
tetrahydrothiophene, followed by ~reatment with ammonia
or ammonium, as illustrated in Flow Diagram (VI).
. ~ ,
~l`
-14-
o_n ~
Z I I Z~NH2
I I or I I .
Y--~ ' - NH 2 Y--~ ~--R
S S
(VIIIa) (VIIIb)
R " O~C--C_C~OOR "
( I X )
~: 15 ~1 ~ Z I rN~--COOR "
Y--~ S /~N~j;--COOR " or YJ~ ~R HC--COOR "
HC--COOR "
(X~) (Xb)
¦C1 CH=N (R1l1)2 .Cle
¦CI--CH=N3 CH2 ) n ~ Cl ~
Z~OOR " Y~OOR "
COOR " OOR "
S N N
(IIla) (IIIb)
-15-
FLOW DIAGRAM ( IV)
Y~H O
NH2
( X I )
C2H502C~j~H2~02C2H5
- y~ ~C02C2~5
Z I ~ N/kO~C2H5
~ IIIb)
'`"' v ~
7~
-16-
FLOW DIAGRAM (V)
.~
CH3~ CH2~--NH2 + C2H50--~C~C02C2H5 C~H50H/NaOAc
H
C~i3--C~C02C2H5 48%H~r/Br2
o~ ~L 2C2HS
H
: (XII)
BrCH2 ~ 2C2~5 NaBH4
15 o2C2H5
~H
~H
20BrCH2-CH ~ 2C2~i5 (C2H5)3N
O ~ ~ 02C2H5
H
~ 02C2H5 p-Toluenesulfonic acid
o N 02C2H5 ~ - -
~C02C2H5
~ ~ / ~ O~C2H~
(IIIa~
3S
~. ,. J
.,.,~
7~
(VI )
lHNRlR2
~;jRlR2 C~NRlR2
~ C2H50--C--C--CO--C2C2~15
2. NH3 or NH4~X
x ~C02C2H5
ll~ /~LCO2C2H5
N
(IIIb)
~.~
~ 7~
wherein Rl and R2 each represents Cl C6 alkyl or taken
together with the nitrogen atom to which they are
attached form a 5 or 6 membered saturated heterocyclic
ring, optionally containing at most 2 hetero atoms.
Formula (IIIa) furo[2,3-b]pyridine compounds
where Z is H and Y is alkyl or optionally substituted
phenyl are prepared by reaction o an acetylene com-
pound with the iodopyridine diester (VII) [preparation
of this compound VII is described in J. Prakt. Chem.,
148; 72(1537?3, in the presence of cuprous salts, an
amine base, and a palladium (II) catalyst as shown in
Flow Diagram (VII).
i~
-19
FLOW DIAGRAM (VI I )
r~o2cH3
Y--C_CH + ol~ ,LCOiCH3
H
( V I I )
CuI
Et3N
( Ph3 P ) 2 PdCl 2
.~ 15
r~ 02CH3
Y~ 02CH3
O N
( IIIa)
~L~7
-20-
Substituents represented by Y and Z in
formula ~Ia), (Ib), (IIa) and (IIb) compounds of ~he
present invention may be prepared either by using the
appropriately substituted starting material for the
preparat.ion of formula (IIIa) and (IIIb) thieno- and
furopyridine-5,6-d;carboxylic acid esters or by elec-
trophilic substitution (halogenation, nitration, sul-
fonation, etc.) directly upon Formula (IIIa) or (IIIb)
diesters or Formula (Ia) or (Ib) final products, where-
in at least one of Y or Z is hydrogen. These substituted
Formula (IIIa), ~IIIb), (Ia) and (Ib) compounds then
may be used as starting materials for additional Y and
Z substitution by displacemen~, reduction, oxidation,
etc. Representative substitu~ed (IIIa~ and (IIIb)
compounds which may be prepared by these procedures are
as illustrated below.
ZT~co2R y ~ X ~I~C02R
20Y ~ X ~ N/ k o2R ~ 1 ~ / ~ o2R
~IIIa) (IIIb)
X Y
S H H CH3
S H Br CH3
S C~3 H CH3
S H Cl CH3
S Cl Cl CH3
S H I CH3
S H No2 CH3
S Br Br CH3
~ f~r,~'~g'rD~
-21-
X Y Z R
S CH3 Cl CH3
S H C~13 CH3
S Cl H CH3
S CH3 C~3 CH3
S H CN CH3
S H OCH3 CH3
S H N(CH3)2 CH3
S H SCH3 CH3
S H OCF2H CH3
0 H H C2H5
O H Br CH3
O H Br C2H5
; 0 H Cl CH3
: O H Cl C2H5
CH3 H CH3
: 0 CH3 H C2H5
O H CH3 CH3
: O C2Hs H CH3
O H C2H5 CH3
CH3 CH3 CH3
S -(CH2)3- CH3
S -(CH2)4- CH3
S -(CH)4- CH3
S C6H5 H CH3
C6H5 H CH3
S H S02N/C 3 CH3
CH3
.
~r
.~
X Y Z R
S H 0C6H5 CH3
0 H OC6H5 CH3
O CF3 H CH3
0 H No2 C2H5
0 Br Br C2~15
0 H C6HsS C2H5
0 H CF3 C2H5
i5
. ~
~3v~ r~
Additionally, novel herbicidal 2,3-dihydro-
thieno[2,3-b] and [3,2-bJpyridine compounds may be
obtained by starting the sequence in Flow Diagram (III)
above with a dihydrothiophenimin hydrochloride. Novel
herbicidal 2,3-dihydro furo[2,3-b] and [3,2-b]pyridines
may be prepared by catalytic reduction of the formula
(Ia) or (Ib) (2-imidazolin-2-yl~ product, or (IIIa) and
(XIIb) furo~2,3-b] and ~3,2-b]pyridine~5,6-diesters as
for example with hydrogen and palladium on carbon,
provided that Y and Z are substituents which are not
reduced by such a procedure. Other 2,3-dihydrofuro-
[2,3-b]pyridines are prepared by the reduction-re-
arrangement of a bromo ketone with sodium borohydride
followed by treatment with triethylamine, and p-toluene-
sulfonic acid as shown in Flow Diagram (VIII). This
then provides novel 2,3-dihydro herbicidal compounds
illustrated below.
Y'
z_ ~ C02R3 Rl Y- ~X ~ C02R3 R
~ ~ yN ~ R2 z_ ~ ~ /N - ~ R2
Y- X ~ ; !' ~ ~=W
B B
. :
: 25
wherein X, Y, Y', Z, Z', W, B, Rl and R3 are as
described for (Ia) and (Ib).
-24 -
FLOW DIAGRAM (VI I I )
__. __
R fo2C2H5
(CH3)2CH C CH2~N + C~H50 ~0~02C2H5
H
¦ C2 H5OH/Na OAC
(CH3 ) 2CH C~/ ~CO2C2H5
od~ ~C02C~H5
H
l48 /0 HBr/Br2
;
~r ~
(CH3 ) 2C~Q2C2H5
o=~ J C02C2H5
t
. ~
INC BH4
-25 -
FLOW DIAGRAM (VIII) (Continued)
S ~CH3)2C CHf ~02C2H5
O 02c2~l5
HOCH2~( CH3 ) 2~02C2H5
~ X 2C2 H5
¦OH
o2c2H5 HOCH2--C(cH3)2 f 2~2H5
CH3~ )\\N 02C2H5 0 02C2H5
CH3 H
~-Toluenesulfonic acid
CH3~CC02C2H5
co2C2Hs
0 N
3S
-26-
The formula (Ia) and formula (Ib) 6-(2~
imidazolin-2 yl)thieno and furo~2,3-b]pyrid;nes and
5~ imidazolin-2-yl)thieno- and furo[3,2-b]pyridines
and the formula (IIa) and formula (IIb) imidazopyrrolo-
pyridinediones of the present invention are exceedingly
effec~ive herbicidal agents useful for the control of
an exceptionally wide variety of herbaceous and woody
annual and perennial monocotyledonous and dicotyledo-
nous plants. Moreover, these compounds are herbici-
dally effective for controlling weeds indigenous to
both dry land and wet land areas. They are also useful
as aquatic herbicides and are unique in their effec-
tiveness in controlling the above-said plants when
applied ~o the foliage thereof or to soil or water
containing seeds or other propagating organs of said
plants such as tubers, rhizomes or stolons, at rates of
from about 0.016 to 4.0 kg/ha, and preferably at rates
from about 0.032 to 2.0 kg/ha.
It is, of course, obvious that rates of
application above the 4.0 kg/ha level can also be used
to effectively kill undesirable plant species; ~owever,
rates of application of toxicant above t~e level neces-
sary to kill the undesirable plants should be avoided
since application of excessive amounts of toxicant is
cos~ly and serves no useful function in the environment.
~;~72'7~'~
-27
Among the plants which may be controlled with
the compounds of this invention are: Elatine triandra9
pygmaea, Scirpus hotarui, Cyperus serotinus,
Ecli~ta alba, Cyperus _fformis, Rotala indica,
Lindernia pyridoria, Echinochloa ~ ~8~ Digitaria
~ , Setaria viridis, Cyperus rotundus, Convol-
vulus arvensis, A~ropyron repens, Datura stramonium,
Alopercurus myosuroides, Ipomoea spp., Sida spinosa,
Ambrosia artemisiifolia9 Eichhornia crassie~s, Xanthium
pensylvanicum, Sesbania exaltata, Avena fatua, Abutilon
theophrasti, Bromu_ tectorum, Sorghum halepense, Lolium
, Panicum dichotomiflorum, Matricaria ~ , Amaran-
thus retroflexus, Cirsium arvense and Rumex iaponicus.
It has been found that the formula (Ia) and
- 15 (Ib) t2-imidazolin-2-yl)thieno- and furopyridines are
generally selective herbicides, particularly effective
for controlling undesirable weeds in the presence of
leguminous crops such as soybean5, and cereal crops
such as wheat, barley, oats and rye. However9 certain
of the formula (Ia) and formula (Ib) compounds are less
selective than others in this series.
It has also been found that several of the
formula (Ia) and formula (Ib) (2-imidazolin-2-yl)-
pyridines are effective as antilodging agents in cereal
crops when applied at rates of application between
about 0.016 to 4.0 kg hectare. At rates of application
not exceeding about 0.010 kg per hectare, it has also
been found that certain of the formula (Ia~ and formula
(Ib) thieno- and furopyridines are effective for in-
creasing branching of leguminous crops and tillering of
cereal crops.
~ ~';72~
Since the formula (Ia) and formula (Ib)
imidazolinylthieno- and furopyridines and derivatives,
wherein R3 is a salt-forming cation, are water solublet
these compounds can simply be dispersed in water and
applied as a dilute aqueous spray to the foliage of
plants or to soil containing propagating organs there-
of. These salts also lend themselves to formulation as
flowable concentrates.
The formula (Ia) and formula (Ib) (2-imidazo-
lin-2-yl)thieno- and furopyridines and the formula
(IIa) and formula ~IIb) imidazopyrrolopyridinediones
can also be formulated as wettable powders, flow con-
centrates, emulsifiable concentrates, granular formu-
lations and the like.
Wettable powders can be prepared by grinding
together about 20% to 45% by weight of a finely divided
carrier such as kaolin, bentonite, diatomaceous earth,
attapulgite, or the like, 45% to 80% by weight of the
active compound, 2% to 5% by weight of a dispersing
agent such as sodium lignosulfonate, and 2% to 5% by
weight of a nonionic surfactant, such as octylphenoxy
polyethoxy ethanol, nonylphenoxy polyethoxy ethanol or
the like.
A typical flowable liquid can be prepared by
admixing about 40% by weight of the active ingredient
with about 2% by weight of a gelling agent such as
bentonite, 3% by weight of a dispersing agent such as
sodium lignosulfonate, 1% by weight of polyethylene
glycol and 54% by weight of water.
~ 7~ 6l109-7237D
A typical ~mulslfiable concentrate can be preparc~ by
dissolvin~ abou~ 5~ to 25~ by welcJht o~ ~he activ~ ln~Jre~lel-t ln
about G5~ to 90% hy weight of N-metllylpyrrolidone, lsophorone,
butyl cellosolve, methylacetate or the like and dispersiny therei
about 5~ to 10% by weight of a nonionic surfactant such as an
alkylphenoxy polyetlloxy alcohol. This concen~rate is dispersed in
water for application as a liquid spray.
When the compounds of the inven~ion are to be used as
herbicides where soil treatments are involved, the compounds may
be prepared and applied as granular products. Preparation of the
granular product can be achieved by dissolving the active compound
in a solvent such as methylene chloride, N-methylpyrrolidone or
the llke and spraying ~he thus prepared solution on a granular
carrier such as corncob grlts, sand, attapulgite, kaolin or the
like.
The granular product thus prepared generally comprises
about 3~ to 20% by weight of the active ingredient and about 97
~o 80~ by weight of the granular carrier.
In order to facilltate a further understanding of the
invention of the parent and divisional applica~ion, the following
examples are presented primarily for the purpose of illustrating
certain more specific detalls thereof. The invention is not to be
deemed 11mited thereby excapt as defined in the claims. Unless
otherwise noted, all parts are by weight.
I hl. '
-30-
EXAMPLE 1
Preparation oE dimethyl thieno~ pyridine-5,6-
dicarbox~late
NHCO 2 P r i L~ ~L NH 2 DMAD
SPOC13/DMF ~ S ~C02CH3
~ ~ IrC02CH31~ co2CH3
~ ~ L-C02CH3 N
H
A mixture of isopropyl-3-thiophenecarbamate
(177 g; 0.975 mol) in methanol (1.2 1) and water (2.8 1)
containing sodium hydroxide (200 g) is heated at reflux
for our hours. Methanol is removed under reduced
~ pressure and the cooled reaction extracted with ether
; (S 1), and these extracts are washed with water, aqueous
sodium chloride and dried. Evaporation under reduced
pressure afEords 3-aminothiophene as an oil in 57%
crude yield.
3-Aminothiophene is redissolved in methanol
(500 mL) cooled in an ice bath and dimethylacetylene-
~icarboxylate (80 g; 0.50 mol) is added dropwise. The
mixture is stirred at room temperature for 15 hours and
30 minutes, the me~hanol removed under reduced pressure
and 1,2-dichloroethane is added. This solvent is also
evaporated off to give dimethyl 3-thienylaminobutene-
dioate as an oil.
~, .. -, ...... . ..
-31-
A Vilsmeier reagent is prepared by adding
dropwise, with stirring phosphorus oxychloride (86 g,
0.56 mol) to a cooled (5C) solution of DMF (41 g,
0.56 mol) in 1,2-dichloroethane (200 mL). This reagent
is stirred at room temperature for one hour and 40
minutes, diluted with 1,2-dichloroethane (100 mL),
cooled to 5C and then the above dimethyl ester dis-
solved in 1,2-dichloroethane (400 mL) is added to the
Vilsmeier reagent at 5C dropwise over a 25 minute
period. The reaction temperature is raised to room
temperature for 15 minutes, then to reflux for a
further two hours and 25 minutes. The cooled reaction
mixture is chromatographed directly on a silica gel
column a~fording 3~.7 g (15%) oE dimethyl thieno[3,2-b
]pyridine-5,6-dicarboxylate mp 124-125.5C after
crystallization from hexane-ethylacetate. A second crop
10.3 g with mp 121-124C is obtained giving an overall
yield from isopropyl 3-thiophenecarbamate of 19%.
Utilizing the above procedure and substituting
the appropriate substituted aminothiophene for isopropyl
3-aminothiophenecarbamate yields the compounds illus-
trated below.
y,~ s ~COOR
Z I ~ ~ N/ ~ OOR
Y Z R mpC
-
H H CH3 12~-127
CH3 H CH3
Cl H CH3 149-151
^w ~
EXAMPLE 2
Preparation of dime~yl thienoL3,2-bl pyridine-5,6-
dicarb_xylate
1. H2S4 ~ S~CO2CH3
NHCOCH3 3 POC13/DMF Ll~ /~02CH3
To concentrated sulfuric acid (170 mL),
stirred at room temperature is added in portions 3-
acetylamino-2-formylthiophene (17~5 g, 0.103 ~ol). The
mixture is heated at 50C for 30 minutes, cooled and
poured into an ice-water mixture. After neutralizing
with an excess of sodium acetate, the mixture is ether
(1 x 2 mL) extracted. The organic layer was dried over
anhydrous Na2S04 and stripped to a dark red gum con-
sisting of 3-amino-2-formylthiophene. Dimethylacetyl-
enedicarboxylate (DMAD) (13 mL) in acetic acid (5 mL),
piperidine (5 mL), methylene chloride (100 mL) and
toluene (100 mL) is added to the 3-amino-2-formylthio-
phene and the mixture stirred overnight. Methylene
chloride is removed by distillation and then the mixture
heated at reflux for 24 hours. After cooling an addi-
tional 13 mL of DMAD is added and the reaction heated
to reflux again for seven and one-half hours. After
standing for 60 hours at room temperature, the solvents
are removed and the dimethyl thieno[3,2-b]pyridine-5,6-
dicarboxylate product is obtained by chromatography,
after eluting with hexane-ethyl acetate, mp 124 125C.
~ r
EXAMPLE 3
Pre~aration of dimethyl 3-chloro[3,2-kL~ ne-5,6-
dicarboxylate and dimethyl 2,3-dichlorothieno[3,2-~]-
pyrid_ne-5,6-dicarboxylate
/ ~ C02CH3 C12
02CH3
Cl S ~ 02CH3 ~ 02CH3
Cl ~ N/ ~ 02C~I3Cl ~ / ~ ~2CH3
A solution of dimethyl thienol3,2-b]pyridine-
5,6-dicarboxylate (15 g 0.0525 mol) in acetic acid
(680 mL) and sodium acetate (86 g, 0.093 mol) is main-
tained at 58C while chlorine is slowly introduced
during five hours and 45 minutes. After reaction is
complete, the mixture is flushed with nitrogen, ethyl
acetate ~200 mL) is added and solid sodium chloride
filtered off and washed with ethyl acetate. The mother
liquors and washes are combined and the solvents removed
under reduced pressure. The residue is dissolved in
methylene chloride and the solution washed with water,
back extracted with methylene chloride and the combined
methylene chloride layers washed with aqueous sodium
bicarbonate, dried and stripped to give 18 g of solid.
Chromatography on silica gel with 15% ethyl acetate-
hexane, then 20% ethyl acetate-hexane gives the 2,3-
dichloro compound, mp 173-178C, 1.3 g, followed by the
3-chlorothieno compound mp 166-173C after crystalliza-
tion from ethyl acetate-hexane.
~7~
-34-
EXAMPLE 4
~paration of dimethyl 3-bromothieno~3,2-b]pyridine-
5,6-dicarboxylate
S ~ 02CH3 Br~ ~ S ~ 02CH3
02cH3 Br I ~ f~ ~02CH3
N
A solution of bromine t20 g, 0.125 mol) in
acetic acid (50 ml) ;s added dropwise over three hours
to a solution of dimethyl thieno[3,2-b]pyridine~5,6-di-
carboxylate, (26.3 g, 0.104 mol), containing sodium
acetate (17.2 g, 0.2 mol) in acetic acid (300 mL) at
85C. Additional sodium acetate (18 g) and bromine
(20 g) in acetic acid (50 mL) is added over an hour and
the mixture stirred at 85C overnight. Bromine (10 g)
is added in one portion then left at 85C for four
hours. The mixture is cooled and treated with aqueous
sodium bisulfite, diluted with ethyl acetate and con-
centrated. The reaction product is partitioned between
water and methylene chloride and the organic layer
washed with aqueous sodium chloride and the solvent
removed. The residue is washed with ether to give 25 g
of crude product, mp 165-168C. Recrystallization from
methanol gave needles of dimethyl 3-bromothieno[3,2-b]-
pyridine-5,6-dicarboxylate, mp 168-16gC.
3~
-35-
EXAMPLE 5
Preparation of ~hicno[3,2-~]pyridine-5,6-di~ y~
acid
_ .
02CH3 N OH~ 5\ ~ ~ 02H
O~CH3 H20 02H
N N
10Dimethyl thieno[3,2-b]pyridine-5,6-dicarboxy-
late (3~75 g, 0.0149 mol) is added to a solution of
sodium hydroxide (1.8 g, 0.045 mol) in water (~0 mL)
and the mixture is warmed at 60C for 20 hours. The
reaction mixture is diluted with water, cooled in an
15ice bath, and acidified by the addition of concentrated
hydrochloric acid. A precipitate of thieno[3,2-b]-
pyridine-5,6-dicarboxylic acid is filtered off and
dried overnight to give 3.1 g (93%) mp >380C.
Utilizing the above procedure and substi-
tuting the appropriate substituted thieno[3,2-b]-
pyridine-5,6-dicarboxylic acid diester yields the
compounds illustrated below.
.
; 25 y ~ a2H
02H
N
.1~
~'7~';i'~8
~36-
Y Z mpC
H H >380
H Cl None taken
H Br >380
H
H F
H CN
H OCH3
H NO~ -
H N(CH3) 2
CH3 H
H CH3
CH3 CH3
H OCHF2
H SCH3
H SO2N (CH3) 2
C~,Hs H
- (CH2) 3-
- (CH2) 4~
- (CH) 4~
Cl Cl
H C6Hs
C6H5 H
H OC6H5
CF3 H
C2H5 H
: H C2H5
H SC6Hs
H CF3
H CHO
H CH2Cl
~72 ~ ~
EXAMPL~ 6
aration of 3-chlorothieno~3,2-~]pyridine 5,6-
dicarboxylic acid anhydride
cl~J_ COOH Cl ~
iO 3-Chlorothieno[3,2-b]pyridine-5,6-dicarboxylic
acid (1.45 g) is heated at 85 to 90C for 30 minutes
then 90 to 102C for 30 ~inutes in acetic anhydride
~7 mL). The reaction is cooled, the solids filtered
off and washed with ether to give 1.2 g of 3-chloro-
thieno[3,2-b]pyridine~5,6-dicarboxylic acid anhydride.
The proton magnetic resonance spectrum is consistant
with the structure.
Utilizing the above procedure and substitu-
ting the appropriate pyridine-5,6-dicarboxylic acid for
~ 3-chlorothieno[3,2-b]pyridine-5,6-dicarboxylic acid
yields the compounds illustrated below.
z ~ O
N
1;~7~
- 3 ~ -
Y Z ~pC
___
H H 266-267
H Cl Sol id no mp
obta ined
H Br >380
Cl H
Cl Cl
H N02
CH3 H
H N (CH3) 2
H SCH3
H OCH3
0 H CH3
H F
. H
CH3 C~3
H CN
H OCHF2
H S02N(CH3) 2
- ( CH2) 3 -
- (CH2) 4- _
- (CH) 4 -
H C6Hs
C6Hs H
:~ H OC6Hs
30 . CF3 H
C2H5 H
H C2Hs
H SC6H5
H CF3
3 5 H CHO
H CH2Cl
.. ..
t~ 7,~
-39-
EXAMPLE 7
Preparation of 5-[(1 carbamoyl-~ lpro~ 3-
chlorothieno ~
o + NH2~j:--CONH2
N o CH(CH3)2
~ OOH fH3
Cl N
CH(CH3)2
2-Amino-2t3-dimethylbutyramide tO.71 g) all in
one portion is added to a stirred solution of 3-chloro-
~hieno[3,2-b]pyridine-5,6-dicarboxylic acid anhydride,
(1.2 g) in THF (1~0 mL). After s~anding for five minutes,
the ice bath is removed and the reaction stirred at
room temperature for 28 hours. THF (5 mL) is added and
the mixture hea~ed at reflux for two hours and then set
aside overnight. The cooled mixture is filtered and
the collected solid washed with ether to give 1.4 g of
the desired 5-[(l-carbamoyl-1,2-dimethylpropyl~carbamoyl]-
3-chlorothieno[3,2-b]pyridine-6-carboxylic acid.
Utilizing the above procedure and substituting
the appropriate pyridine-5,6-dicarboxylic acid anhydride
for 3-chlorothieno[3,2-b]pyridine-5,6 dicarboxylic acid
anhydride and the appropriate aminoamide yields the
compounds illustrated below.
~'~ 7~2
-40-
y ~ S ~ O~H ~1
Z I 1~ CONH ~ f~CONH2
N R2
_ Z Rl R2 mpC
H H CH3 i-C3~17
H Cl CH3 i_C3H7 not pure
Cl H CH3 i_C3H7
Cl Cl CH3 i_C3H7
H Br C~13 i_C3H7
H CH3 CH3 i-C3H7
: H No2 CH3 i-C3H7
H N(CH3)2 CH3 i-C3H7
H SCH3 CH3 i_C3H7
: H OCH3 CH3 i_C3H7
CH3 H CH3 i-C3H7
H H CH3 C3H7
H H CH3 C2~5
H OCHF2 CH3 i-C3H7
CH3 CH3 CH3 i_C3H7
H CN CH3 i_C3H7
H F CH3 i_C3H7
H I CH3 i_C3H7
H S02N(CH3)2 CH3 i_C3H7
C6H5 H CH3 i-C3H7
-(CH2)3- CH3 1-C3H7
-(CH2)4- CH3 1-C3H7
-~CH34- CH3 i-C3H7
H C6H5 CH3 1-C3H7
~2'~ 2
-41-
y Z Rl R2 I~.pc_
C2H5 H CH3 i-C3~17
: H 0C6H5 CH3 1_C3H7
H CH2Cl CH3 1_C3H7
CF3 H CH3 i_C3H7
H C2H5 CH3 1_C3H7
H CH0 CH3 1_C3~17
H CF3 CH3 1_C3H7
H SC6H5 CH3 i_C3H7
.
`:
~;.
.
:
-~2-
EXAMPLE 8
lin-2-yl)thieno[3,2 ~]pyridine-6-carboxylic acid
5 S 2H S~
~C Ac~O ~ ~l O
--~ ~J CO2H N O
fH3
10NH2--f~CONH2 ~ S ~02H fH3
CH ( CH3 ) 2 1 ~ /~CONH--Cl -CONH2
CH ( CH 3 ) 2
¦ 1. NaOH
12. H+
~CH~ ) 2
11 0
Thieno[3,2-b]pyridine-5,6-dicarboxylic acid
(2.5 g, 0.011 mol) is heated slowly to 85C for one
hour with acetic anhydride (25 mL), then cooled, fil-
tered and washed with diethyl ether to give the anhy-
dride as a solid, mp 266-267C. A mixture of the an-
hydride and 2-amino-2,3-dimethylbutyramide (2.6 g,
0.02 mol) in THF (70 mL) is stirred at room temperature
for 15 hours.
, !
~7~ 7
-43-
After heating at reElux for two hours, the mixture is
cooled and diluted with THF (50 mL). Solid 5-[(1-car-
bamoyl-1,2-dimethylpropyl)carbamoyl]thieno[3,2-b]-
pyridine-6-carboxylic acid is filtered off, washed with
ether and dried. The above solid is mixed with an
aqueous 60 mL) solution of sodium hydrox;de (6 g 0.05 mol)
and heated at 85C for two hours and 30 minutes, then
set aside at room temperature overnight. Af~er cooling
in an ice bath, the mixture is acidified to pH 3 with
concentrated hydrochloric acid. A solid (3 g) is
filtered off and dried. Crystallization from ethyl
acetate affords (5-(5-isopropyl-5-methyl-4-oxo 2-imidazo-
lin-2-yl)thieno~3,2-b]pyridine-6-carboxylic acid, mp
242-244C in 46% yield.
Utilizing the above procedure and substituting
the appropriate pyridine-5,6-dicarboxylic acid for
thieno[3,2-b]pyridine-5,6-dicarboxylic acid yields the
compounds illustrated below.
Y~02H H3
/J~/~CH ( CH 3 ) 2
1' o
H
y z mpC
H H 242-244
H Cl 238-239
H Br 226-227
Cl H 247-248
3L2~'7~3
Y Z mpC
Cl Cl
C~13
CH3 H
H NO2
H N(CH3) 2
H SC6H5
H SCH3
H OCH3
H OCHF 2
CH3 CH3
}~ CH
H So2N(cH3) 2
C6H5 H
- (CH2) 3-
(CH2)4- _
- (CH2) 4-
H C6Hs
H OC6Hs
CF3 H
C2H5 H
H C2Hs
: H
H F
H CHO
H CH2Cl
' H CF3
-45-
EXAMPLE 9
_reparation of diethyl furo[3,2~ ridine-5,6-dicarbo-
xylate
~ ~10 ~`~ 02C2Hs
N~12 co2C2H5
3-Amino-2 formylfuran, prepared from 3-azido-
2-formylfuran (8.9 g 0.065 mol) is dissolved in ethanol
and to this solution diethyl oxalacetate (12.23 g,
0.065 mol) and ten drops of piperidine are added. In
addition pulverized 3A molecular sieve is added and
the reaction stirred at 65-60C for three hours, then
additional diethyl oxalacetate (2.2 g) is added. The
reaction is essentially complete ater 12 hours at
55 60C. On cooling the reaction is filtered, and the
filtrate concentrated and then dissolved in ethyl ace-
tate, water washed, then brine washed, dried over an
hydrous magnesium sulfate and stripped to dryness. The
residue is dissolved in 3:1 hexane:ethyl acetate and
passed through a flash chromatographic column in two
stages. First it is filtered by vacuum through a four
to Eive inch pad of silica from which ~he last three
fractions containing the required product are collected
and combined. These combined fractions are then passed
through a six inch column eluting under pressure with
ethyl acetate:hexane 3:1 and 2.1. Diethyl furo[3,2~b]-
pyridine-5,6-dicarboxylate 4.15 g (24%) is obtained
after crystallization from hexane-ether, of mp 60-64C,
and with a mass spectrum m/e of 264.
~'7~
-46-
Utilizing the above procedure and substituting
the appropriate furan for 3-am:ino-2-formylfuran ylelds
the compounds illustrated below.
Y- ~ ~ CO~R
z L ~ ",L C02R
N
y z R mpC
_
H H C2H5 60-64
H Cl C2Hs
CH3 H C2H5
H CH3 C2H5
C2H5 H C2H5
H C2H5 C2H5
CH3 c~3 C2H5
, ~:
~7~
-~7-
EXAMPLE 10
Preparation_of iUIO 3 Z hlpYridi -5~6_dicarboxylic
acid
~ 2C2H5 , ~ ~ C2
2C2H5 N
Furo[3,2-b]pyridine-5,6-dicarboxylic acid,
diethyl ester (1.1 g, 0.0042 mol) is dissolved in 95%
ethanol (20 mL) containing 10% aqueous sodium hydroxide
(20 mL) and set aside at 0C for two days. The mixture
is cooled, acidified and the solvent removed under
reduced pressure. Wa~er 5 mL is added and the hydrated
product diacid obtained as a brown solid by filtration,
; 3.31 g (99%), mp 183C (dec). Anal calcd. as
CgHsNOs.2 1/2 H2O C, 42.86; H,3.99; N,5.55 found:
C,42.63; H, 2.63; N,5.46.
. Utilizing the above procedure and substituting
the appropriate furo[3,2-b]pyridine-5,6-dicarboxylic
ester yields the compounds illustrated below.
Y Z R mpC
H H H 183 (dec)
H Cl C2Hs
CH3 H C2H5
: H CH3 C2Hs
C2H5 H C2H5
H C2~5 C2Hs
CH3 CH3 C2~5
-48-
EXAMPLE 11
_r~e~ration of furo[3,2-b]pyridine-5,6--dicarboxylic
acid anhydride
~ ~ o H ~ 0
FuroE3,2-b]pyridine-5,6-dicarboxylic acid
(3.3 g, 0.0159 mol) in acetic anhydride (100 mL) is
heated to 70-30C for six hours. The reaction mixture
is cooled, Eiltered and the solid is washed with ether
to give 3.01 (100%) of crude furo[3,2-b]pyridine-5,6-
dicarboxylic acid anhydride.
Utilizing the above procedure and substituting
the appropriate furo[3,2-b]pyridine-5,6-dicarboxylic
acid yields ~he compounds illustrated below.
Y Z
H H
H Cl
CH3 H
; H CH3
C2H5 H
H ~2H5
CH3 CH3
-49-
EXAMPLE 12
Preparation of 5-[(1-carbamoyl--1,2-dimethylpropyl)-
carbamoyl]furo[3,2-b~pyridine-6-carboxylic acid and
5-(5-isopropyl-5-methyl-4-oxo-2-imidazolin-2-yl)furo-
[3,2-b]pyrldine-6-carboxylic _c d
~ fH (CH3 )2
L~ ,1 / H2N--f~CONH2
N ~ CH3
~ o ~ C02H fH3
CONH--C--CONH 2
CH ( CH3 ) 2
1. NaOH
2 . Ac id
2 5~ O ~COOH ~;H ( CH3 )2
~ //L</N~H3
H
~7~2
-50-
Furo[3,2-b]pyridine-5,6-dicarboxylic acid
anhydride (3.01 g, 0.015 mol) is suspended in THF
(lQ0 mL) to which 2-amino-2,3-dime~hylbutyramide (2.3 g,
0.018 mol) is added. After stirring for 20 hours, the
solution is stripped to an oily solid which dissolves
in a water/dilute sodium hydroxide solut;on. The
alkaline solution is extracted with methylene chloride,
and then acidified and reextracted with methylene
chloride but on stirring only minute traces of material
is isolated. The water layer is concentrated to an
oily solid which is dissolved in ethanol, filtered and
concentrated to a purple gum which is predominantly the
crude product, 5-[(1-carbamoyl-1,2-dimethylpropyl)-
carbamoyl]furo[3,2-b]pyridine-6-carboxylic acid and is
used without further purification to prepare the final
2-imidazolin-2-yl product by dissolving it in 10%
sodium hydroxide solution (40 mL) and warming at 80C
for three hours. On cooling the reaction is acidified
and a small am~unt of solid precipitated out and was
filtered off. Concentration of mother liquors gives a
second crop, which is collected and combined with the
first crop. Purification is e~fected by taking half of
the material and separating on silica gel preparative
glass plates as bands. The slower running band using
methylene chloride:ethyl acetate:chl~roform: methanol
l:l:l:l as eluant, affords the desired 2-imidazolin-2-
yl product9 mp 214-223C(dec), Esters may then be pre-
pared by the procedures described in Example 20.
-51-
Utili~ing the above procedure and substituting
the appropria~e furo[3,2-b~pyridine-5,6-dicarboxylic
anhydride yields the compounds illustrated below.
~H ( CH3 ) 2
N O
y z mpC
_ _
H ~ 214-223 (dec)
H Cl
~ CH3 H
: H CH3
C2H5 H
H C2H5
: CH3 CH3
'
,
~'7~
-52-
EXAMPLE 13
__.
Prepara~ion of dime~hyl th _ o[2,3-b]pyridine-5L6-dicar-
e
-
_ rC02CH3 DMF~,[~02CH3
~ 5 1 N ~LCO2CH3 POC1302CH3
I
H
A Vilsmeier reagent is prepared by adding
dropwise, with stirring, phosphorus oxychloride
(40.29 g, 0.26 mol) to a cooled (10C) solution of DMF
(19.0 g, 0.26 mol) in 1,2-dichloroethane (40 mL) in an
N2 atmosphere. This reagent is stirred at room tempera-
: ture for one hour and 45 minutes. Dimethyl-2-thienyl-
aminobutenedioate (63.4 g, 0.26 mol) dissolved in 1,2-
dichloroethane (300 mL) is added dropwise to ~he
Vilsmeier reagent at 7-10C. The reaction temperature
is raised to room temperature for 15 minutes, then to
reflux for 12 hours. The cooled reaction mixture is
` concentrated and the residue chromatographed on a
silica gel column with ethyl acetate-hexane, affording
dimethylthienol2,3-b3pyridine-5,6-dicarboxylate (29 g,
2S 45%) as a solid.
~0
~,V~'~2'7
-~3-
Utili~ing the above procedure and substituting
the appropriate dimethyl-2-thienylaminobutenedioate
yields the compounds illustrated below.
~ ~ 02CH3
S N O~CH3
y z mpC
CH3 H 80-82
H H 80-81
H CH3
CH3 CH3
H C6H5
6~l5 H
~F3 H
-(CH2)4- 118-121.5
,~
. 20
'7~ 8
54 -
EXAMPLE 14
Preparation of dim_t~y~ othieno[2,3-bley_~ __e_
5,6-dicarboxylate
, ~ C02C~I3 Br2 Br- r ~ ~ 02CH3
C02CH3 NaOAc 02CH3
Bromine (0.33, 0.00206 mol) in acetic acid
(8 mL) is added to a stirred solution of dimethyl-
thieno[2,3-b]pyridine-5,6-dicarboxylate (0.5 g,
0.00187 mol) in acetic acid containing sodium acetate
(0.31 g, 0.00377 mol) at 40C. The reaction mixture is
heated at 75C for 18 hours. Evaluation of the mixture
by tlc (sil;ca gel~ indicated incomplete reaction.
Additional bromine (0.33 g) in acetic acid and sodium
acetate (0.31 g) is added and heating at 75C continued
for six hours. The reaction mixture is diluted with
water and extracted into ethyl acetate. The separated
organic layer is dried over anhydrous MgS04, filtered,
and the filtrate concentrated to an oil which solidifies
~; on standing. Crystallization of the crude product from
ethyl acetate-hexanes yields the dimethyl 3-bromothieno-
[2,3-b~pyridine-5,6-dicarboxylate as white needles mp
86-~7.5C.
This compound may be readily converted to a
variety of substituted-thieno[2,3-b]pyridine compounds
as illustrated below, while electrophilic substi~ution
sueh as nitration or halogenation yields additional
compounds listed below.
; 7
-55-
Z ~02CH3
S N O~CH3
Y Z mpC
H H
H Cl 104-110
H Br 86-87. 5
H
H F
H CN
H SCH3
H OCH3
H N(CH3) 2
H OCHF2
H N02
H CHO
H CH2Cl
CH3 H 80-82
H CH3 oil
Cl
Cl ~1 84-~9
CH3 CH3
H S02N(CH3) 2
-(CH2)4- 118.5 - 121.5
- (CH) 4- _
- ( CH2 ) 3 -
G6H5 H
H C6H5
H . OC6Hs
CF3 H
H SC6H5
H CF3
C2H5 H
T~ r~
-56-
EXAMPLE 15
Preparation oE thieno[2,3-~]pyridine-5,6-dica
acid
-
r ~ _ 02CH3 KOH . ~ 2~J
A solution containing dimethyl th;eno~2,3-b]-
pyridine-5,6-dicarboxylate (27.75 g, 0.11 mol) and
potassium hydroxide (30.98 g, 0.55 mol) in methanol
(200 mL) under a N2 atmosphere is heated at reflux for
two hours. The reaction mixture is cooled and suffi-
cient water added to dissolve any solids present before
evaporating the mix~ure to dryness. The resulting
solid is dissolved in a minimum volume of water, cooled
in an ice bath and acidified with concentrated H2S04 to
pH~l. Thieno[2,3-b~pyridine-5,6-dicarboxylic acid is
filtered off and dried overnight to give 23.36 g mp
272-275~.
Utilizing the above procedure and substituting
the appropriate substituted dialkylthienol2,3-b]-
pyridine-5,6-dicarboxylate yields the compounds illus-
~rated below.
~;~;p~,t~
- 57 - 61109~7287D
z ~ ~J c02H
Y -- S - ~-~CO2H
Y Z ~?C
H H 272-275
H Cl > 300
H Br > 315
H
H F
H C~ -
H SCH3
H OOEI3
H N(CH3)2
H OCHF2
H N02
H CH0
H CH2Cl
H CH3 180-183 (dec)
CH3 H
Cl H
Cl Cl
CH3 CH3
. C6H5 H
H S02N ( CH3 ) 2
-(CH2)3-
- ( C~H2 ~ 4- 280-290
-(CH)4-
H C6H5
H C6H5
CF3 H
H CF3
H SC6H5
C2H5 H
H C2H5
'7
-S8-
EXAMPLE: 16
Preparation of thieno[2,3-~]pyridlne-5,6-dicarboxylic
anhydride
rsf~o2H Ac20 ~\~0
Acetic anhydride (37.4 g, 0.366 mol) is added
to a stirred suspension of thieno[2,3-b]pyridine-5,6-
dicarboxylic acid (21.52 g, 0.096 mol) in dimethoxy-
ethane (175 mL) in an inert N2 atmosphere. Upon addi-
tion of pyridine (16.78 g, 0.21 mol) at room tempera-
ture an exotherm to 45C is observed and a homogeneous
solution results. The reaction mixture is then stirred
at room temperature and the resulting solid Eiltered
off, washed with ether and air dried to give 14.8 g
(75%) of thieno~2,3-b]pyridine-5,6-dicarboxylic acid
anhydride.
Utilizing the above procedure and substituting
the appropriate substituted thieno[2,3-b]pyridine-5,6-
dicarboxylic acid yields the compounds illustrated
below.
727~B
~ 59 - 61 l09 -7287D
z~
Y S N~
Z mp C
CH3 H 176-180
H Br 228.5-231
H Cl 230-300
( slow dec )
H H 210-213
H
H F
H CN
H SCH3
N(CH3)2
~ H N2
i H CHO
H CH2Cl
H CH3
Cl H
Cl Cl
CH3 CH3
C6H5 H -
H S2~ (CH3) 2
-(cH2)3-
- (CH2) 4- 220-222
-(C~1)4
H C5H5
H OC6H5
CF3 H
H CF3
H OCHF2
H SC6H5
H OOEI3
C2H5 H
H C2Hs
''! ,~
~`'`.1
~'7~'7
-60-
EXAMPLE 17
Preparation of 6-[(1-carbamoyl-1,2-dime~hylpropyl)-
carbamoyl]thieno~2,3-b]p~__dine-5-carboxylic_ cid
~ 5 R
~ ~H3
"l +H2N~f--CONH2
S N ~I~ CH(CH3)2
~ ~ C02H fH3
/~ CONH--f ~ ONH2
S N
CHlCH3)2
2-Amino-2,3-dimethylbutyramide (9.84 g,
0.076 mol) is added to a stirred suspension of thieno-
[2,3-b]pyridine-5,6-dicarboxylic acid anhydride (14.8 g,
0.072 mol) in THF under an inert atmosphere of N2 at
room temperature. The dark solution is stirred at room
temperature overnight and the resulting solid filtered
off, washed with THF and air dried to give 17.35 g
(72%) of 6~ carbamoyl-1,2-dimethylpropyl)carbamoyl]-
thieno[2,3-b]pyridine-5-carboxylic acid.
Utilizing the above procedure and substituting
the appropriate substituted thieno[2,3-b]pyridine-5,6-
dicarboxylic acid ar.hydride yields the compounds illus-
trated below.
3S
- 6 1 -
z~CO~H fH3
Y CONH~f~CONH~
S N CH(CH3)2
y Z mD C_
CH3 H 207-208
H Br 176-178
H Cl 156-158
H H
H
H F
H CN
H SCH3
H OCH3
H N(CH3) 2
H NO2
H CHO
H CH2Cl
H CH3
Cl H
Cl Cl
CH3 CH3
C6H5 ~ _
H SO2N(CH3) 2
-(CH2)3-
- (CH2) 4- sol id
- ( CH) 4 -
H C6H5
H OC6Hs
CF3 H
H OCHF2
H CF3
H S C6H5
C2H5 H
H C2H5
?~
1~7~7
-62-
EXAMPLE 18
Preparation of 6-(5-isopropyl-5-methyl-4-oxo-2-imidazo-
lin-2-yl)~hieno[2,~-b~pyrldine-5-carboxylic acid
S ~
l~ \ ~ C02H fH3
CH(CH3)2
~ C02H H3
~ S ~ / ~ /N ~ H(CH3)2
H
6-[(l~Carbamoyl-1,2-dimethylpropyl)carbamoyl]-
: thieno~2,3-b~pyridine-5-carboxylic acid (17.35 g,
: 20 0.052 mol) i5 added to water (225 mL) containing sodium
hydroxide (10.35 g, 0.26 mol). The resulting basic
: solution is heated at 80C for two hours and 45 minutes,
cooled in an ice-water bath ancl acidified with 6N H2S04.
The product 6-(5-isopropyl-S-methyl-4-oxo-2-imidazolin-
2-yl)thieno[2,3-b]pyridine-S-carboxylic acid is filtered
off, washed with water and air dried yielding 1.54 g,
70.3%, mp 221-223C.
'7~8
-63-
EXAMPLE 19
-
Pre~ara _ n of_5_______zo[1',2':1,2~pyrrolo[3,4-b]-
thienol3,2-elpyridine-3~2~)_,5-dione, 2-iso~ro~ 2-
methyl
~1~
N CH(C~3)2
CH3
IDCC
~ H(C~3~2
CH3
Dicyclohexylcarbodiimide (1.07 g, 0.005 mol)
in methylene chloride (20 mL) is added dropwise to a
stirred methylene chloride (30 mL) suspension o~ 6-
(5-isopropyl-5-methyl-4-oxo-2-imidazolin-2-yl)thieno-
[2,3-b]-5-carboxylic acid (1.5 g, 0.0047 mol) under an
N2 atmosphere. After stirring the reaction mixture for
16 hours, it was clarified by filtration, concentrated
to dryness and the resulting ma~erial purified by column
chromatography on silica gel eluting with acetonitrile/
methylene chloride (1/2). The solid product was crystal-
lized from toluene to give the pure 3,5-dione as white
crystals mp 214.5-216.5C.
-64-
EXAMPLE 20
5-methyl 4-oxo-
2-imidazolin-2-yl)thieno[2,3-glpyridine-5-carboxylate
l- ~ N 0 HOH2CC--CH
S N N=CH(CH3)2
CH3
~ CH2C-CH
N ~ H(CH3)2
CH3
Sodium hydride (2.4 g, 60%, 0.126 mol) is
added to the 3,5-dione tO.9 g, 0.003 mol) in propargyl
alcohol (25 mL) at 10C under an inert N2 atmosphere.
The reaction mixture is stirred at room temperature for
60 hours and then neutralized with a saturated ammonium
chloride solution. The resultin~ mixture is concentrated
on a rotary evaporator, diluted with water and extracted
with ethyl ace~ate. The organic layer is separated,
dried over anhydrous MgS04 and concentrated to dryness.
Purification of the product by column chroma-
tography on silica gel with methylene chloride/acetoni-
trile (85/15) yields 2-propynyl 6-(5-isopropyl-5-methyl-
4-oxo-2-imidazolin-2-yl)pyridine 5-carboxylate7 which
after crystallization from toluene has a mp 188-189.5C.
~A
~72'7Z~
-65-
Utilizing the procedures of Examples 18, 19
and 20 and substituting the appropriate thieno [3,2-b]-
pyridine or thieno [2,3-b]pyridine compounds, yields
the compounds illustrated below~
Z ~ 02R3 ÇH(CH3)2
Y ~ S ~ N~
Y Z R~ mpC
H H CH3 215-217
H H H 220-223.5
(dec)
H H -CH2C-CH 188-189.5
H H ~ 140-142
-. --CH2~ o J
:~ ICH3
29 H H CH2~=CH2 108-110
CH3 H H 225.5-227.5
H Br H 274-276
H Cl H 266-267
H NO2 -CH3 201-202.5C
H N02 H 260 (dec)
Cl H H
Cl Cl H
H CH3 H
H N(CH3)2 H
Z~
- 6 6 -
Y Z
H SCH3 H
H OCH3 H
H OCHF2 H
CH3 CH3 H
H CN H
H S02N(CH3) 2 H
C6H5 H H
H C6H5 H
- (CH2) 3- H
- (CH2) 4 H
- (CH2) 4- H
H oC6H 5 H
CF3 H H
C2H5 H H
H C2H5 H
H I H
H F H
~: H CH0 H
~ H CH2Cl H
-~ 20 H CF3 H
: H SC5H6 H
7'~
-67-
: y ~ S ~ C02R3 H(CH3)2
Z I I / k yN ~ H3
y z R3 mpC
H H H 242-244
H Cl H 238-239
H Br H 226-227
H H ~ 156-157
~H2~ J
Cl H H 266-267
Cl I H
H CH3 H
; 15 H F H
CH3 H ~ _
Cl Cl H
: H NO2
:~ H N(CH3)2 H
H SCH3 H
; H OCH3 H
CH3 CH3 H
H CHO H
H OCHF2 H
H CN H
H SO2N(CH3)2H
C6Hs H H
C6H5 H
iJ ~ 8
-6~ -
Y Z R3 mpC
- (CH2) 3~ H
-(CH2)4- H
- (CH2) 4~ H
H C6~5 H
GF3 H H
H CF3 H
~2H5 H ~ _
H C2H5 H
H CH2Cl H
H SC6Hs H
:,
~'
.
-69-
EXAMPLE 21
Prepara~ion of methyl 6-~4-isopropyl-4-methyl-5-oxo-2-
imidazolin-2-yl)-3-nitrothieno[2,3-b]pyridine-5-carboxy-
1_
~CO2CH3 ~H ( CH3 ) 2
~5 ~ N/) </N~ 3 ¦HNO3
H
02N~CO2CH3 H ( CH3 ) 2
/~</~cH3
S N N 0
Methyl 6 (4-isopropyl-4-methyl-5-oxo-2-
imidazolin-2-yl)thieno[2,3-b]pyridine-5-carboxylate
(3.94 g, 0.0119 mol) is dissolved in 200 mL concen-
trated H2S04 at room temperature. While cooling to 3C
in an ice bath, 1.5 mL (0.024 mol) concentrated HN03 is
added, then the mixture is allowed to warm to room
temperature. AEter three hours, the reaction mixture is
poured onto ice, neutralized with solid NaHC03 to pH 6
and is extracted with methylene chloride. The extract
is filtered, then dried over sodium sulfate, refiltered
and concentrated to a yellow solid weighing 4.33 g
(97%~, which upon crystallization from methanol water
has mp 201-202.5G.
:
~70-
EXAMPLE 22
reparation of 6-(4-isopropyl-4-methyl-5-oxo-2-imi azo-
lin 2-yl)-3-nitrothienoL2~3-~]pyridine-5-carboxylic
acid
o2N I ~CO2CH3 ~H(C~3)2
S N ~H3 MeOH
H20
02NT~C02H H(CH3 ) 2
<~ N=j~CH 3
S N N --O
H
The ester (1.0 g, .00266 mol) from Example 21
is stirred in lO0 mL methanol and lO mL 10% sodium
;~ hydroxide solution for 24 hours. Water (25 mL) is
added and the methanol removed in vacuo. Acidification
of the aqueous layer ~ives a brown precipitate which
upon filtration and crystallization from methanol-water
has mp 260C.
1~ 7~7
EXAMPL~ 23
Preparation oE diethyl 5-acetyl-1,6-dihydro-6-oxo-2,3-
f~ (j)C2115 t;OOC2H5
5CH3--C--CH2~ NH2 ~ O~C~ C=O
CH--OC2115
jSodium acet~te
CH3 Rf ~2C2~15
o 02C2H5
1'
H
Sodium acetaee ~30 g, 0.37 mol~ is added to
a stirred mixture of diethyl(ethoxymethylene)oxal-
acetate (87 g, 0.36 mol) and acetoacetamide (36 g,
0.36 mol~ in absolute ethanol ~300 mL). After stirring
the reaction mixture for 30 minutes, the ethanol is
distilled off under reduced pressure9 the residue
acidified to pH 2 with dilu~e aqueous hydrochloric acid
and the resulting solid filtered off. Crystallization
from an ethanol-water mixture affords diethyl 5-acetyl-
1,6-dihydro-6-oxo-2,3-pyridinedicarboxylate as crystals
mp 101-110C.
'~1~ ~,
L~7;~7~1 3
-72~
EXAMPLE 24
__
yl acetonitrile
(~H3)2CHC02CH3 + CH3CN
1. NaH
2. HCl
( CH3 ) 2CHCCH 2CN
NaH (61.55 g of 60% dispersion, 1.54 mol) is
added to 650 mL anhydrous TH~ under N2. The suspension
is heated to reflux. Methyl isobutyrate (lO0 g, 98 mol)
and acetonitrile (63.16 g, 1.54 mol) are mixed in 140 mLs
anhydrous THF and added dropwise over one hour to the
refluxing suspension. The resulting solution is refluxed
for 16 hours. Enough water is added to the reaction
mixture to dissolve the salt that is formed. The THF
is removed in vacu_, and the basic aqueous solution is
extracted with ether, then acidified to pH 4 with con-
centrated HCl. The solution is extracted with ether.
The extracts are wasned with brine and dried over anhy-
drous MgS04, filtered and the solvent is removed
in vacuo, giving 97~25 g, (89.4%) of the title product
___
an orange oil.
1~7;~7~8
EXAMPLE 25
Preparation of diethyl-5 (2-methylpropionyl)-1,6-di-
hvdro-6-oxo-2,3-pvridinedicarboxylate
`: R =~C2H5 COOC2H5
(CH3)2CHCCH2CN ~ O ~ C=O
CH--OC2 Hs
l~laOAc
lo R
( CH 3 ) CH/ \ ~CO 2C2 H5
o ~C02c2H5
N
H
Isobutyl acetonitrile (50 g, 0.45) 24 and
diethyl(ethoxymethylene)oxalacetate (110 g, 0.45 mol)
are dissolved in absolute ethanol and then is added
sodium acetate (36.9 g, 0.45 mol) and one drop of
piperidine. After 12 hours, he mixture is concen-
trated, acidified with dilute hydrochloric acid then
extracted with methylene chloride. The extracts are
concentrated and recrystallized to give the title
product as a white solid 21.7 g (19.5%) mp 116-118C.
13
~'7~'7
74~
EXAMPL.E 26
Preearati_n of diethyl 5-(bromoacetyl)-1,6-dihydro-
6~oxo-2,3 ~yridinedicarboxylate
CH3-C // ~ 02C2H5 Br2BrCH2--C~ o2c2Hs
0 02C2H5 HBr 0 02C2H5
'
H H
Bromine (8.0 g, 0.050 mol) in 48% HBr is
added dropwise to a stirred solution of diethyl-5-
acetyl-1,6-dihydro-6-oxo-2,3-pyridinedicarboxylate
(14.05 g, 0.05 mol) in 48% HBr (200 mL). Upon com-
pletion of this bromine addition the reaction mixture
is poured onto ice (200 g) and the mixture is stirred
until the ice has melted. The crude product is col-
lected by filtration and crystallized twice from an
ethyl acetate-hexane mixture (1/2) affording diethyl
5-(bromoacetyl)~1,6-dihydro-6-oxo-2,3-pyridinedicarbo-
xylate with mp 141-142C.
Utilizing the the above procedure using diethyl
2-methylpropionyl-2-pyridone-dicarboxylate yields diethyl
5-(2-bromo-2-methylpropionyl)-1,6-dihydro-6-oxo~2,3-
pyridinedicarboxylate, mp 124-126C.
w75
EXAMPLE 27
_reparation of d;e~hyl 5-(2-bromo-l_hydroxyet:hyl)-1,6-
dihydro-6~oxo-2,3-py~_dinedicarboxylate and diethy~
2,3-di~ydro-3-hydroxy~furo[2,3-b]pyridine-5,6-dicarbo-
BrCH2-C ~ 02C2Hs N~BH4
0 ~ ~ 02C2H5 i-C3H70H
H
~H
BrCH2-CH ~ 2C2H~ (C2H5)3N
o=l~ o2C2H5
1'
H
OH~C02C2H5
20~ 0 1~N ~ o2C2H5
Sodium borohydride ~2.54 g, 0.066 mol) is
added in portions over a 30 minute period to a stirred
suspension of diethyl 5-(bromoacetyl)-1,6-dihydro-6-
oxo-2,3-pyridinedicarboxylate (57.2 g, 0.159 mol) at
10-20C. Upon completion of the sodium borohydride
addition, the reaction mixture is stirred while attain-
ing room temperature. Ice (100 g) is added and the
mixture stirred until the ice has melted. The mixture
is then concentrated in vacuo and the residue crystal-
lized twice from an ethyl acetate-hexane mixture to
give pure diethyl 5-(2-bromo-1-hydroxyethyl)-1,6-
dihydro-6-oxo-2,3-pyridinedicarboxylate mp 134-138C.
`~
';7~
-76-
Stirring this compound with triethylamine (1.0 mL/g of
solid) in methylene chloride for one hour, followed by
washing the organic solution with dilute hydrochloric
acid, water9 brine and drying over anhydrous MgS04
yields the crude furo[2,3-b]pyridine as an oil upon
removing the solvent in vacuo. Crystallization Erom a
cyclohexane-toluene mixture affords pure diethyl 2,3-
dihydro-3-hydroxy-furo[2,3-b]pyridine-5,6 dicarboxylate
~p 73-77C.
'7'~
-77-
EXAMPLE 28
_
Preparation of diethyl 2,3-dihydro-3-methoxyfuro[273-.bl-
~g~'~
Hl~
~C02C2~15
co2C2Hs
O N
NaH
THF
CH3I
.~
~C~3
~C02Et
C02Et
0 N
Sodium hydride, 60% dispersion in mineral oil
(2005 g, 0.0512 mol) and iodomethane (7.9 mL, 0.128 mol)
are added to a solution of the hydroxy diester of
Example 27 (12.00 g, 0.0427 mol) in tetrahydrofuran
t400 mL) under N2. After stirring overnight at room
temperature, the reaction is heated to 50C under a
stream o N2 to remove excess iodomethane. The reaction
is then cooled, filtered, stripped and chromatographed
over silica gel. Eluting with hexane/ethyl acetate
(4:1) gives the product as a yellow oil in 57.3% yield.
Calcd. for C14Hl7N06; C, 56.94; 4,5.80, N, 4.74. Found:
C, 56.93; H, 5.59; N, 4.83.
t7~7~3
-7~-
EXAMPLE 29
r ~ ~dlne=~LL ~_ arbo
_ _ .
xylate
H0 ~ ~ 02C2H5 P toluene sulf~nic acid
O~C2Hs~ xylene
Q N
CC) 2C 2 H 5
02C2H5
0 N
A xylene solution of the hydroxy-furo com-
pound obtained in Example 23, (3.7 ~) containing ~
toluene sulfonic acid (0.01 g) is heated at reflux for
two hours. The solution is cooled and the xylene solu-
tion decanted off. The residue is extracted with ether
- and the extracts combined with the xylene. Distillation
- of the solvents gives a yellow solid which is crystal-
lized from a cyclohexane-~oluene mixture to give pure
diethyl furo[293-b]pyridine-5,6-dicarboxylate mp 66-77C.
- 79 - 61l09-7287D
EXAMPL,E_30
Prepa ation o-f dimethyl_?. _e~yl-furo ~ ~3-
5,6-dicarboxylate
I _ ~ CO2CH3
- 0=~ , C02CH3
H
CH3C--CH
CuI DMSO
Pd(P~3)2C12 (C2H5)3N
~' /~--C02CH3
CH3 ~ 0 / ~ N/ ~ C02CH3
Propyne (3.0 mLs, 0.045 mol) is condensed in a graduated
cylinder in a dry ice/acetone bath and added to a stirred sus-
pension of dimethyl 1,6-dihydro-5-iodo-6-oxo-2,3-pyridinedi-
carboxylate ~13.48 g, 0.04 mol), cuprous iodide (0.38 g, 0.002
mol) and bis(triphenylphosphine)palladium II) chlor~de (2.81 g,
0.004 mol) in 150 mLs DMS0 and 50 mLs triethylamine at 10C.
After addition of the propyne the reaction mixture is stirred at
room temperature ~or 60 hours. Water is added and the mixture is
extracted with ethyl acetate. The ethyl acetate solution is
washed with water and dried over anhydrous MgS04~ then concent-
rated ln vacuo to give a mixture of materials. The crude product
is isolated by flash column chromatography using 9:1 methylene
chloride, ethyl acetate. The fractions containing the title pro-
duct are concentrated in vacuo and the residue is recrystallized
from cyclohexane to give the pure compound mp 115-118C.
~,.
. ~ .
1~7~7;~B
-~o -
Utilizing the above procedure and substituting
the appropriate substituted acetylene yields the com-
pounds illustrated below.
~ C02CH3
R ~ ~ /~ C02CH3
0 N
R ~
C2H5 147-149
Phenyl 152-153
:~ 20
-81-
EXAMPL,E 31
Pre~_rat;on of diethyl 2,3-d~ydro-3~3-dimethylfur
[2,3-~]pyridine-5,6-dicarbo~ylate
5(CH3)2f----c ~ 2C2~5
Br o ~ ~ C02C2H~ 3 ~2toluene
H sulfonic acid
CH3 ~02C2H5 HO-~02C2H5
02C2H5 CH3 02C2H5
0 N 0 N
CH3
15(major) (minor)
The 5-(2-bromo-2-methylpropy ketone diester
of Example 26 (20 g, 0.052 mol) is dissolved in 200 mL
absolute ethanol and 3.0 g sodium borohydride (0.078 mol)
is added at 0C, the temperature is then allowed to
rise gradually to 15C. After the mixture is stirred
for one additional hour, the ethanol is removed in vacuo.
The solidified mass is treated with water and extracted
with methylene chloride. The organic extract is then
washed wi~h water and a saturated sodium chloride solution,
dried and concentrated. The residue, weighing 12 g is
redissolved in xylene and 1.0 g p-toluenesulfonic acid
is added. The solution is refluxed for 12 hours then
cooled. The xylene solution is decanted and the residue
washed with several portions of ether. The combined
organic solutions are concentrated then chromatographed
with 9:1 methylene chloride/ethyl acetate to obtain
3.2 g of the oily diester ~21~/o); mass spectrum M ~ l/e
= 294.
3S
-82-
From a later chromatographic Eraction is
obtained 1.4 g of 2,2-dimethyl-3-hydroxyfuro[2,3-b]-
pyridine-5,6~dicarboxylate (11.5%).
~7
-83-
EXAMPLF, 32
Pr~ tion of diethyl 3-bromofuro[2,3-_]pyridine-5,6-
dicarboxylate
~ ~ 1. Br r~ 5
O N 2C2~15 2. DBU o N O~C2H5
The diester of Example 29 (6.0 g, 0.0228 mol)
is dissolved in 200 mL methylene chloride containing
4.6 g sodium acetate, and bromine is added (7.3 g,
0.0556 mol) at reflux. Following the addition, the
mixture was stirred for 15 minutes at reflux, then
eooled, washed with aqueous sodium bisulfite, dried
with sodium sulÇate and filtered. The filtrate was
stripped to 8.8 g o~ the crude dibromo compound which
was redissolved in methylene c hloride and treated with
3.16 g DBU (0.021 mol) at room temperature for 30
minutes. The mixture was then concentrated in vacuo,
and chromatographed over silica gel with hexane-
ethylacetate to give 7~1 g (90%) of the diethyl 3-bromo-
furo[2,3-b]pyridine-5,6-dicarboxylate mono bromo diester
mp 49.5-52C.
-84 -
EXAMPLE 33
5 7 6-dicarboxylate
Br
2C2H5
02C2H5
O N
NclOAc ¦
Br2
CH 2C1 2
Br
Br~(~cC02C2H5
B r~ C02C2 H5
H N
¦DBU
Br I ~ \`rC02C2Hs
Br~~ J~ /; C02C2Hs
O N
-~5-
Sodium acetate (2.40 g, 0.0292 mol) and
bromine (7.5 mL, 0.146 mol) are added to a solution of
die~hyl 3-bromofuro[2,3-b]pyridine-5,6-dicarboxylate
(5.00 g, 0.0146 mol) in methylene chloride (150 mL).
The mixture is stirred at room temperature for four
days then washed with aqueous sodium bisulfate to
remove unreacted bromine. The aqueous solutian is then
back extracted with methylene chloride. The organic
solu~ion is combined, dried over sodium sulfate and
filtered. To the filtrate is added 1,8-diazabicyclo-
[5.4.0]undec-7-ene(4.4 mL, 0.032 mol), and the mixture
is st-rred at room temperature for one hour. The solu-
tion is then concentrated in vacuo. The residue is
chromatographed over silica gel eluting with 20% ethyl
acetate in hexane, yielding the crude 2,3-dibromofuro-
12,3-b]pyridine as a white solid in 95% yields recrystal-
lization from a methylene chloride-hexane mixture affords
diethyl 2,3-dibromofurol2,3-b]pyridine-5,6-dicarboxylate
mp 96-98C in 75.9% recrystallized yield.
2~
, .... . . ~
2'~7
-86-
EXAMPLE 34
_reparation of diethyl 3-trifluoromethyl~
pyridine-5,6-dicarbox~late
5~1 ~ C02Et
LC02Et CF3CO2Nc
O N
10N-methylpyrrolidone ¦CUI
CF3 r ~ CO~Et
C02Et
15 0 N
To a solution of sodium trifluoroacetate
; (0.0234 mol) in N-methylpyrrolidone (50 mL) is added
3-bromofuropyridine of Example 32 (2.02, 0.00585 mol)
and cuprous iodide (2.2 g, 0.0119 mol). The mixture is
heated to 160C for three hours under N2, cooled to
room temperature, treated with EtOAc (100 mL) and
hexene (100 mL), and filtered. The filtrate is washed
with H20 (4x~00 mL), dried over Na2S04 and stripped ~o
an oil which is chromaEographed over silica gel with
hexane-ETOAc (7:3) elution. The product is collected
as a pale yellow solid; mp 50-55C.
..
~L~ 7~ d3~
EXAMPL,E 3 5
Preparation of turo[2,3-~]~ ridine-5,6-dicarboxylic
acid
_
r~ 02c2~15 K011 ~ 02~1
Potassium hydroxide (5.60 g, 85%, 0.087 mol)
in water (5 mL) is added ~o a stirred suspension of
diethyl furo[2,3-b]pyridine-5,6-dicarboxylate (9.3 g,
0.035 mol) in absolute ethanol (100 mL). The reaction
mixture is heated at 60C for one hour, then cooled and
anhydrous acetone added. The precipitate is ~iltered
off, dried, suspended in dry acetone and treated with
hydrogen chloride to adjust to a pH of 2. Crystal-
lization of the isolated solids from an ethyl ace~ate-
acetone mixture affords furo[2,3-b]pyridine-5,6-dicar-
boxylic acid mp 189-192C.
-88-
EXAMPLE 36
Pre~aration_of furo[2,3-b3~yridine-5,6-dicarboxylic
anhydride
C02H ~cetic_anhydride
/,LCO2H
0 N
D N ~
Furo[2,3-b]pyridine-5,6-dicarboxylic acid
(6.7 g, 0.032 mol) is heated at 60C for 30 minutes in
acetic anhydride (150 mL~. The reaction mixture is
cooled to room temperature and concentrated ln vacuo
and the residue triturated with cyclohexane-ether(5:1),
filtered off and dried to give 5.35 g furo[2,3-b]-
pyridine-5,6-dicarboxylic acid anhydride.
7~47V~;~
-89-
EXAM LE 37
~5~lLa~r~ f ~ carbamoy~ 2-dimethylpropyl)-
carbamoyl]=furo[2,3-b]pyridine-5-carboxylic acid
R
~: ~ fH3
~ ~ J " ~ NH2--cl~ONH2
O N ~ CH(CH3)2
02H fH3
ONH--Ç--CONH2
O N CH(cH3)2
2-Amino-2,3-dimethylbutyramide (2.1 g,
0.016 mol~ is added to a stirred suspension of furo-
~: [213-b~pyridine-5,6-dicarboxylic acid anhydride (3.0 ~,
0.016 mol) in tetrahydrofuran (7.5 mL) and the mixture
allowed to stir at room temperature for 16 hours. The
reaction mixture is then stirred at 60C for one hour,
cooled to room temperature, et~er added, and the solid
filtered off and dried to give 5 g of 6-[(1-carbamoyl-
1,2-dimethylpropyl)carbamoyl]furo[2,3-b]pyridine-5-
carboxylic acid mp 192-196C (dec).
7~1~
-so -
EXAMPLE 38
Preparation of 6-(4-iso~ropyl-4-methyl~S-oxo-2-imidazo-
lin-2-yl)furo~2,3-b]pyridine-5-carboxylic acid
~co2H fH3 H20
o ~ N/~CONH--CI~ONH2 Nc10H
CH ( CH3 ) 2
~02H H3
~ o~ /,L</N~H(CH3)2
H
A solution containing 6-[(1-carbamoyl-1,2-
dimethylpropyl)carbamoyl]furo[2,3-b~pyridine-5-carbo-
xylic acid (3.8 g, 0.012 mol) ;n aqueous sodium
hydroxide 2.4 g, 0.06 mol) in water (40 mL) is stirred
at 65C for three hours. The reaction mixture is t~en
heated at 75C for one hour, a:Llowed to cool, poured
into ice, acidified to pH 2-3 and the resulting solid
filtered off and dried. Crystallization from an
acetone-methanol mixture affords pure 6-t4-isopropyl-4-
methyl-5-oxo-2-imidazolin~2-yl)furo[2,3-blpyridine-5-
carboxylic acid mp 237-244C.
- 91 - 61109-7287D
EXAMPLE 39
Preparation of 6-(4-isopropyl-4-methyl-5-oxo-2-imidazolin-
2-yl)-3-nit ofuro[2,3-b~pyridine-5-carboxylic acid
COOH
\O ~ N J ~ N ~ CI-I(CH3)2
N --- O
2 6
Sulfolane
N02 ~
COOH
O~N~ E(CH3)2
I . O
H
~ itryl hexafluorophosphate (0.75 g, 0.00391 mol) is
added to a suspension of 6-(4-isopropyl-4-methyl-5-oxo-2-imidazo-
lin-2-yl)-furo[2,3-b] pyridine-5-carboxylic acid (1.07 g, 0.00355
mol) in sulfolane (63 mL) under nitrogen. The temperature of the
reaction is maintained between 64C and 8$C for three days during
which time the solids dissolve. The mixture is cooled to 30C and
chromatographed over silica gel. Elution with 1:1 hexane to ethyl
acetate removes the sulfolane. Elution with 1% to 10 methanol in
methylene chloride followed by recrystalli2ation from acetone-
hexane yields 6-(4-isopropyl-4-methyl-5-oxo-2-imida~olin-2-yl)-3-
nitro-furoC2,3-b]pyridine-5-carboxylic acid, mp 220-237C
~L~ B
-92-
EXAMPLE 40
Pre aration of 2 iso ro 1-2-methyl-5H-furo[2,3-b]-
. . P _ . P PY
im dazo[2',1'.5,1]pyrrolo[3,4-~]pyridine-3(2H),5-dione
~C02H
O N ~N --o
N= ~CH ( CH 3 ) 2
CH3
Ac20
DME
Pyr dine
G ~ ~ ~ H(CH3)2
CH3
To a suspension of 6-(4-isopropyl-4-methyl-
5-oxo-2-imidazolin-2-yl)-furo[2,3-b]-pyridine-5-car-
boxylic acid (11.7 g, 0.039 mol) in lO0 mL dimethoxy-
ethane (DME) is added 7.3 mL acetic anhydride and
3.9 mL pyridine. After stirring 24 hours at room
temperature, the solids are filtered and washed wi~h
ether and the mother liquor is concentrated by adding
xylene to aid removal of pyridine. The residue is
triturated with ether to obtain solids which are
combined with the first crop to give 11.1 g (100%) of
product. Recrystallization from 2:1 ethyl acetate-
hexane gives an analytical sample mp 193-205C.
~ 7
-93-
EXAMPLE 41
Preparation of methyl 6-(4-isopropyl-4~methy~5-oxo-2-
imidazolin-2-yl)-furo[2,3-b]pyridine-5-carboxylate
~CH(CH3):Z
¦ MeOH
l NaOMe
~ -CO~CH3
0 N ~ \ ~ CH(CH3)~
CH3
The compound prepared in Example 40 (10.5 g,
0.037 mol) is suspended in 150 mL absolute methanol and
4.0 g sodium methoxide is added. After stirring for 72
hours at room temperature the mixture is poured onto
ice containing acetic acid to maintain the pH at 3-4.
A white solid forms and is filtered yielding 9.8 g
(84%) of the title compound with mp 134-137G.
~w~
_9~,_
EXAMPLE 42
Preparation of methyl 3-chloro-6-(4~ o~_____ethyl-
5-oxo-2-imidazolin-2-yl)-furo[2_3-~]pyridine-5-carboxylate
~1 Cl2 l~N2H
N ~0 2. DBU O N =
N- I CH(CH3)2 N ¦- CH(C~13)2
CH3 CH3
The methyl ester described in Example 40 is
dissolved (1.4 g, 4.4 mmol) in 20 mL acetic acid and
sodium acetate (1.0 g, 12.2 mmol) is added. Gaseous
chlorine is passed into the stirred solution for two
hours during which time the temperature reaches 40C.
After cooling and pouring onto 50 g ice, the mixture is
extracted with ethyl acetate, washed with distilled
water then with saturated sodium carbonate solution.
The organic layer is then concentrated to a foam, re-
dissolved in 20 mL methylene chloride and treated with
10 mL diazabicyclo-[5.4.0]undec-5-ene (DBU). After ten
minutes, the mixture is treated with 20 nlL cold dilute
hydrochloric acid. The methylene chloride layer is
removed, dried over anhydrous magnesium sulfate. The
solution is then passed through a one-quarter inch pad
of silica gel and concentrated in vacuo. Recrystalliza-
tion from hexane-ethyl acetate gives 0.85 g (57%) of
the 3-chloro compound mp 150-156C.
.2~'7;~
- 9 5 -
EXAMPLE 43
Preparation of 3-chloro-6-(4-isopropyl-4~ y~
oxo 2-imidazolin-2-~,1)-furol2,3-b~ pyridine-5-carbox~'lic
acid
2cH3 C02H
1. NaOH I I ~ H
O N ~ \ ~ 2. Mel O N ~ N---lt-0
N t CH(CH3)2 N ¦-CH(CH3)2
CH3 C~3
The ester from Example 42 ~0.55 g, 1.157 mmol)
is dissolved in 10 mL 95% ethanol and 0.28 g 50% sodium
hydroxide solution is added. After one hour the mix-
: 20 ture is treated with 10% hydrochloric acid to pH 2, and
the product separates as a solid, which is filtered,
dried and recrystallized from acetone to give 0.35 g
(67.3%) mp 239-240C.
. .
-96--
EXAMPLE 4 4
Preparation of (+)-6-(4-isopropyl-4-methy~-S-oxo-2-
imidazolin 2-~1?-furo[2,3-~]~yridine-5-carboxylic acid
~ C02H ~H3
CH(CH3)2
O N N___L=o
H
(~)-iso~er
Furo~2,3-b]pyridine-5,6-Dicarboxylic anhydride
(2.50 g, 0.013 mol~ is suspended in 40 mLs anhydrous
THF and (+)-2-amino-2,3-dimethylbutyramide (1.72 g,
0.013 mol) is added. The mixture is stirred under N2
a~ room temperature for 16 hours. The solution is
poured into 150 mLs anhydrous ether, and the resulting
solid is collected in 88.6% crude yield. The unpuri-
fied adduct is converted to the indicated product in
the manner described in Example 38 for the racemic
mixture. The (~)-isomer is recrystallized from
absolute ethanol, and is isolated in 27.0% yield ~rom
the adduct.,mp 244-245C E~ = 44 5
~-
~ 7
-97-
EXAMPLE 45
Pre.para_ion_of sodium 6-(4-isopropyl-4-methyl-5-oxo-2-
imidazolin-2-yl)furo[_2,3-b]pyridine-5-carboxylate
Il ~02H
O N ~ - o
N - ~ H(CH3)2
CH3
¦NaOH
MeOH
r ~ 2- Na~
O N ~ ~ = O
H(CH3)2
CH3
NaOH ~0.13 g, .0033 mol) is dissolved in
50 mLs anhydrous methanol under N2. The Eree car-
boxylic acid (1.00 g, .0033 mol) is added, dissolving
to give a yellow solution. The solvent is removed
in vacuo to give a yellow oil. The oil is dissolved in
anhydrous ethanol and the solvent removed in vacuo to
give a solid. The solid is dissolved in anhydrous
ethanol and reprecipitated with anhydrous ether9 giving
0.60 g (56.1%) of the title sodium salt as a yellow
solid, mp 240->2S0C.
'7
-98~
EXAMPLE 46
Preparation of 6-(4-isopropy1-4 methyl-5-oxo-2-imidazo-
lin-2-yl)furo~2,3-b]pyridine-5-carboxy~ate ~compound
with isopropylamine (1:1)
. COOH
N ~H3 ~ NH2CH(CH3~2
O N ~/ ~CH(CH3) 2
~ ,1 o
H
CH30H Acetone
~COO H3NCH ( CH3 ) 2
O N'~ CH(CH3)2
N O
H
Isopropylamine (0.25 mL, 0.00266 mol) is
added to a suspension of the carboxylic acid (0.80 g,
0.00266 mL) and methanol (SO mL). The reaction is
stirred at room temperature for one-half hour, during
which time all the solids dissolve. The solvent is
removed in vacuo, and the residue is slurried in ether
and filtered, yielding the isopropyl amine salt in
78.1% yield, mp 100-220C with slow decomposition.
' 35
.r
-99--
EXAMPLE 47
Preparation oE imldazolin-2-yl thieno- and ~uro-
~ridines
Utilizing the procedures oE the preceeding
examples and substituting the appropriate thieno or
furol3,2-b~pyridine or thieno or furo~2,3-b]pyridine
compounds, yields the compounds illustrated below.
Z T~C02R3 H ( CH3 ) 2
Y~ X ~J~ N /~L</~
X Y Z R3 mpC
S H H CH3 215-217
S H H H 220-223.5
(dec)
S H H -CH2C_CH 188-189.5
S H H ~ 14G-142
-C~2-~ O ~
~H3
S H H -CH2~=CH2 108-110
S CH3 H H 225.5-227.5
S H Br H 274-276
S H Cl H 266-267
O H H H 237-244
S H No2 CH3 201-202.5
S H NO2 H 260 (dec~
S Cl Cl H
S H N(CH3)2 H
S H SC6Hs H
S H SCH3 H
S H OCH3 H
S H OCHF2 H
7~3
- 1 o o -
X Y Z R3 mpC
_~ _ _ ____
0 H Cl H 239-240
0 H M CH3 134-137
0 H Br H 239-245
O CH3 H H 174-177
: 0 C2H5 H H 170-172
O C6Hs H H 244 245
S Cl H H 268 (dec)
0 H C1 CH3 137-141
S H CH3 H 255-757
S - t CH2) 4 ~ H 234 - 237
O H CF3 H
O H SC~Hs H
0 H H - CH2 - - ~ J 137-141
O
O H H -CH2C-CH 150-156
: S Cl H ~NH3-CH(CH3) 2 Anal:
ok f or
S and Cl
.
S H S02N(CH3) 2 H
S C6H~ H H
S -(CH2)3- H
S -(CH)4- H
S H C6H5 H
S H OC6H5 H
S CF3 H H
, S C2H5 H H
.. H C2H5 H
S H I H
S H F H
S H CHO H
S H CH2C1 H
S H CF3 H
O H N0~ H 220-237 (dec)
-101-
X Y Z R3 rnpC
O H N(CH3) 2
O H SC113 H
O H OCH3 H
O Cl H H -
0 Cl Cl H
O H CH3 H
O CH3 CH3 H
O H OCHF2 H
O H CN .H
0 H S02N~CH3)2 H
O -(CH2)4- H
O -(CH2)~ - H
- (CH) 4- H
O H C6Hs H
0 H OC6Hs H
O CF3 H H
o H C2H5 H
O H I H
O H F H
0 H CHO H
O H CH2Cl H
127:~7~1
-102-
y ~ ~ 02R3 H(CH3)2
Z ~ / ~ ~ ~ CH3
H
X Y Z R3 ~IpC
S H H H 242-244
S H Cl H 238~239
S H Br H 226-227
S H H ~ 156-157
~H2l~ J
o
O H H H 214-223
S Cl H H 266-267
O H Cl H
O H CH3 H
: O CH3 H H
o C2H5 H H
O H C2H5 H
CH3 ~H3 H
S H CN H
S H CH3 H
S CH3 H H
S CH3 CH3 H
S H No2 H
S H N(CH3)2
S H SCH3 H
: S H OCH3 H
S H OCHF2 H
S Cl Cl H
',~
-103-
X Y Z R3 mpC
__
S H S02N(CH3) 2 H
S C6H5 H ' H
S H C6Hs H
S -(CH2)3- H
S - (CH2)4- H
S - (CH) 4 w H
S H OC~Hs H
S CF3 H H
S C2H5 H H
S H C2H5 H
S H I H
S H F H
S H CHO H
S H CH2Cl H
S H CF3 H
S H SC6Hs H
~.
,
~0
V~2'~
-104-
EXAMPLE 48
Preparation of 6--(4-isopropyl-4-methyl-5-thioxo-2-lmida-
zolin-2-yl)-furo[2,3-b]pyridine-5-carboxylic acid
OOH H3
H
5,6-Dicarboxylic anhydride-furo[2,3-b]pyridine
(1.35 g, 0.0071 mol) is suspended in 25 mLs anhydrous
THF and 2-amino-2,3-dimethylthiobutyramide (1.04 g,
0.0071 mol) is added. The mixture is stirred under N2
at room temperature for three hours. The suspended
solid is collected and the filtrate is stripped to a
solid. The combined yield is 2.30 g ~96~2D/o)~ Both
solids arc added together to KOH (1.91 g, 0.034 mol) in
20 mLs water. The solution is warmed to 60C for four
hours, then stirred at room temperature for 16 hours.
The slightly cloudy solution is filtered to give a
clear filtrate, which is acidified to pH 4 with 10%
HCl. The resulting yellow solid is collected and re-
fluxed in 100 mLs xylene for 16 hours. The indicated
product crystallized from the xylene solution in 28.0%
yield, mp 231-232C dec.
In the same manner as described above for the
furo[2,3-b]pyridine compound, 6-(4-isopropyl-4-methyl-5-
thioxo-2-imidazolin-2-yl)-thieno[2,3-b]pyridine-5-carbo-
xylic acid is prepared in 37% yield having a mp 242C.
-105-
EXAMPLE 49
Preparation of 2,3-dihydro-6-(4-isopropyl-4-methyl-5-
oxo-2-imidazolin-2-yl)furo[2,3 b]~yridine-5-carboxylic
acid
~ ~ H(CH3)2 H2
~ 02H H3
~o 1\N~ CH(CH3)2
A solution of 6-(4-isopropyl-4-methyl-5-oxo-2-
imidazolin-2-yl)furo[2,3-D3pyridine-5-carboxylic acid
(1.7 g 0.056 mol) and 1.0 g (0.0972 mol) potassium
carbonate in 200 mL 9:1 ethanol:water is added to 100 mg
5% palladium on carbon catalyst in a 500 mL pressure
bottle. The bottle is ~itted to a Parr hydrogenation
apparatus, pressurized to 30 psi, with hydrogen, then
shaken at room ~emperature for 10 hours. The catalyst
is removed by filtration through a sintered ~lass fun-
nel, and the filtrate concentrated in vacuo to 10 mL.
Acidification of the residue to pH 2 gives a white
precipitate which is removed by filtration, washed with
water and air dried to give 1.0 g (63%) of 2,3-dihydro-
6-(4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)furo-
[2,3-b]pyridine-5-carboxylic acid as an off-white solid,
mp 189-192C.
-106-
By the above procedure, a solution of 5-(4-
isopropyl~4-methyl-5-oxo-2-imidazolin-2-yl)furo[3,2-b]-
pyridine (400 mg) may be converted to 2,3-dihydro-5-(4-
isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)furo[3,2-b]-
pyridine-6-carboxylic acid, mp 205-206C.
-107-
EXAMPLE .50
Preparation of 4-mer~5s~ EL~
11
CH3CSH + BrCH2CH2CH2CN
R
CH3~C--S~ H2CH2CH2CN
Thiolacetic acid (49 mL, 0.69 mol) is added
to potassium carbonate (93.4 g, 0.68 mol) dissolved in
water (150 mL~. Ethanol (260 mI.) is added and then
4-bromobutyronitrile is added at 15 to 28C and the
reaction mixture stirred at room temperature for 16
hours. The resulting inorganic solids are filtered oEf
and the filtrate extracted with toluene. The organic
layer is separated, dried over anhydrous Na2S04 and
concentrated to give the desired 4-mercaptoacetyl
butyronitrile as a yellow oil~
,7
-108-
EXAMPLE 51
Preparation of dihydrothiophenimine hydrochloride
CH3--C--S--CH2--CH2CH~CN HCl
r~
~ S ,~NH HCl
Hydrogen chloride is introduced to a cooled
solution of the nitrile in methanol (220 mL) for one
hour and the mixture then stirred at room temperature
for 16 hours. The resulting product is filtered off,
washed with ether and dried to give 55.38 g of dihydro-
thiophenimine hydrochloride, mp 189-195C.
~DV ,~,
-109-
EXAMPLE 52
P paration of dimeth~ [(tetrahydro-2-th enylidene)-
amino]fumarate (and maleate)acid
1~ fO2CH3
~ ~ NH HCl ~ C f
C02CH3
fj,"~:O2CH3
,C--C02CH3
Dimethylacetylenedicarboxylate (0.45 mL,
15 0.037 mol) is added to a stirred solution of dihydro-
thiophenimine hydrochloride (0.5 g, 0.0036 mol) in
methanol (60 mL) containing sodium acetate (0.3 g,
O.OQ36 mol) under an inert N2 atmosphere at -15C.
After stirring for 16 hours at room temperature, the
20 solvent is removed on a rotary evaporator and the
resulting mixture separated by column chromatography on
~ silica gel eluting with a methylene chloride-aceto-
: nitrile mixture (19:1) yielding 0.68 g (78% yield) of
the desired mixed isomeric acid esters as a yellow oil.
t' Y ~
- 1 1 0 -'
EXAMPLE 53
Preparation o dimethyl 2L3-dih~drothieno[2,3-b]-
pyridine-5,6-dicarboxylate
~02CH3 oxal yl chlorLde
_ 1I DMF
S ~ N ~CO 2CH3
~ co2CH3
S N 02CH3
A Vilsmeier reagent is prepared by adding
oxalyl chloride (0.25 mL, 0.0028 mol) to a stirred
solution of DMF (0.22 mL, 0.0028 mol) in 1,2-dichloro-
ethane (50 mL) at room temperature in an inert N2 at-
mosphere. A 1,2-dichloroethane (50 mL) solution of
dimethyl {(tetrahydro-2-thienylidine)amino]fumarate
(and maleate) (0.0028 mol) is added to the Vilsmeier
reagent and the reaction mixture heated at reflux for
four hours. The reaction mixture is quenched with
water, made basic with sodium bicarbonate and the
organic layer separated and dried over anhydrous
Na2SO4.
The solvent is removed in vacuo and the
residue purified by column chromatography on silica
gel, eluting with a methylene chloride-acetonitrile
mixture (19:1). Crystallization from toluene-hexane
affords dimethyl 2,3-dihydrothieno[2,3-b]pyridine-5,6-
dicarboxylate as a white solid with mp 102-103.5C.
"~
- 1 1 1
EXAMPLE_54
Prepara~ion oE 2,3-dihydro-6-(5-isopropyl-5-methyl-4-
oxo-2~imidazolin-2-yl)thieno[2,3-b]pyridine-S-carboxylic
acid, l-oxide
~C02H
S N ~ N ~Fo
N TCH(CH3)2
CH3
CH2Cl 2
m~CPBA
CH30H
~\ C02H CH3
.~ S N /~ CH ( CH3 )
~=0
0 H
m-Chloroperbenzoic acid (2.0 g, 0.0094 mol)
is added to a solution of the dihydro thieno pyridine
in methylene chloride (400 mL) and methanol (40 mL) at
0C under a nitrogen atmosphere. After stirring for 16
hours while attaining 18C, water 100 mL is added,
followed by the addition of 1~0 mL of a saturated
NaHC03 solution. The aqueous layer is separated off
and washed with methylene chloride. Acidification with
concentrated HCl, precipitates m-chlorobenzoic acid
; 30 which is removed by filtration prior to adjusting the
pH of the aqueous layer to pH 1. Extraction of this
acidified layer with methylene chloride and removal of
the solvent yields the title product as a white solid,
mp 216-218C dec.
~ff~ ~
- 1 1 2 --
EXAMPLE 55
Preearation of diethyl d~hydrothieno[3,2-b]pyridine-
S=L
--O
Piperidine ¦
`~ N~ ~ =~ N~
~ I ) _ ( I I )
~C2 H5
HC~
~ f_CO2C2H5
,c~
O C02C2H5
. I
~02C2H5
o2C2H5
* ~ ~7
-113-
~o a solution o te~rahydrothiophene-3-one
tMaybridge Chem. Co; 20.0 g, 0.196 mol) in benzene
(100 mL), stirred at room temperature, is added piperi-
dine (16.7 g, 0.196 mol) and ~-toluenesulfonic acid
monohydrate (0.20 g, 0.001 mol). The mixture is heated
at reflux under a Dean-Stark trap for four hours, cooled
and stripped to a dark brown oil consisting of a 1:1
mixture of 2,3- and 2,5-dihydrothiophene enamines (I
and II; Recl. Trav. Chim., 92, 865(1973).
To the above enamine mixture is added ethanol
(lOQ mL) and diethyl ethoxymethylene oxalacetic carbo
xylate (72.1 g, 0.294 mol) and the mixture is stirred
for 45 minutes. Ammonium acetate (45.3 g, 0.588 mol)
is added in one portion and the mixture is heated at
reflux for 45 minutes. After cooling, the solvents are
stripped and the yellow oily diethyl dihydrothieno[3,2-b]-
pyridine-5,6-dicarboxylate product is obtained by
chromatography after eluting with hexane-ethyl acetate.
The mass spectrum shows the parent peak tm+l/e) at 282.
~d~ 7~ 72
-114-
EXAMPLE 56
Preparation of diethyl 5,7-dihydrothieno[3,4-~]pyridine-
2,3-dicarboxylate and diethyl-2,3-dihydrofuro[3,2-bJ -
pyridine-5,6-dicarboxyl~te
0~
O
Piperidine¦
=L N~' ~ ~LN~>
C2H5
f_CO2C2H5
" ~
co2C2H5
= 2C2H5
02C2H5
- ,,, ~ ,. . -, .
~L~7Z'7
-115-
To a solution of tetrahydrofuran-3-one (J.
Pharm. Sci, 59 1678(1970); 46.55 g, 0.540 mol) in
benzene (250 mL), stirred at room temperature, is added
piperidine (45.98 g, 0.540 mol) and ~-toluenesulfonic
acid monohydrate (0.46 g, 0.002 mol). The mixture is
heated at reElux under a Dean-Stark trap for four
hours, cooled and stripped to a dark brown oil con-
sisting of a 1:1 mixture of 2,3- and 2,5-dihydrothio-
phene enamines. Then ethanol (500 mL) and diethyl
ethoxymethylene oxalacetic carboxylate (178.79 g, 1.35 mol)
is added and stirring continued for 45 minutes. Ammo-
nium acetate (124.87 g (1.62 mol) is added and the
mixture is heated at reflux for 45 minutes. After
cooling, the solvents are removed and the yellow oily
diethyl dihydrofuro[3,2-b]pyridine-5,6-carboxylate is
purified by chromatography on silica gel, eluting with
hexane-ethyl acetate. The mass spectrum shows the
parent peak (m+l/e) at 266.
:, . '' ''~
`:t!h.............................................. .. _~
'.~1
-11.6-
EXAMPLE 57
.
Preparation oE 2,3~dihydro-5 and 6-(4-isopropyl-4-
methyl-5-oxo-2-imidazolin-2-yl) furo and thieno-[2,3-b]
and [372-b]pyridines
Utilizing the procedures of Examples 8, 10,
12, 15, 18, 27, 35, 36, 37, 38, 49, 53, 54, 5S an 56
yields the dihydro compou~ds illustra~ed below.
z~C02R3 H(cH3)2
X N ~
Y'
X Y Y' Z Z' R3 mpC
S H H H H H 224-227
S=0 H H H H H 216-218
O H H H H H 189-lg2
: 20 0 H H CH3 CH3 ~ 193-198
0 H H HO H H 170-173
0 H H CH30 H H 140-143
Y'
y ~ X ~ C2R3 CH(CH3)2
1, N ~ t ~
H
; X Y Y' Z Z' R3 mpC
S H H H H H 239-241
0 H H H H H 205-206
` 'NW
~ 7
-117-
EXAMPLE 58
Postemergence herbicidal evaluation of test compounds
The postemergence herbicidal activiey of the
compounds of the present invention is demonstrated by
the following tests, wherein a variety of monocotyledo
nous and dicotyledonous plants are treated with test
compounds dispersed in aqueous acetone mixtures. In
the tests, seedling plants are grown in jiffy flats for
about two weeks. The test compounds are dispersed in
50/50 acetone/water mixtures containing 0.5% TWEEN~ 20,
a polyoxyethylene sorbitan monolaurate surfactant of
Atlas Chemical Industries, in sufficient quantities to
provide the equivale~t of about 0.16 kg to 10 kg per
hectare of active compound when applied to the plants
through a spray nozzle operating at 40 psig for a pre-
determined time. After spraying, the plants are placedon greenhouse benches and are cared for in the usual
manner, commensurate with conventional greenhouse prac~
tices. From four to five weeks after treatment, the
seedling plants, are examined and rated according to
20 the rating system provided below. The data obtained
are recorded in Table I below.
% DiEference in Growth
Rating Systemfrom the Check*
_
0 - No Effect 0
2~ 1 Possible effect 1-10
2 - Slight effect 11-25
3 - Moderate effect 26-40
5 - Definite injury 41-60
6 - Herbicidal effect 61-75
t 30 7 - Good herbicidal effect76-90
8 - Approaching complete kill91-99
9 Complete kill 100
4 - Abnormal growth, that is, a definite physiological
malformation but with an over-all effect less than
a 5 on the rating scale.
~2'~2 ~Z8
-118--
In most cases the data are for a single testl but in
several instances, they are average values obtained
from more than one test.
Plant Speeies Used
Barnyardgrass (Echinochloa crusgalli)
Green foxtail (Setaria viridis)
Purple Nutsedge (Cyperus rotundus L.)
Wild Oats (Avena fatua)
Quack~rass (Agropyron repens)
Field Bindweed (Convolvulus arvensis L.)
Cocklebur (Xanthium pensylvanicum)
Morningglory (Ipomoea purpurea)
Ragweed (Ambrosia artemisiifolia)
Velvetleaf (Abutilon th_~phrasti)
Barley (Hordeum vul~are)
Corn (Zea mays)
Rice (Oryza sativa)
Soybean (Glycine max)
Sunflower (Helianthus annus)
Wheat (Triticum aestivum)
- l:L9 - 61109--72~37D
~1ou~ooooo oou~ool~ oooooo
u~loooooo ~ i~;iooo
~3~ oInooooo Inlnoul~o oooooo
ooooooo ~r~_i_l~i ~,i~_ioo
E'i 9 o ~n o ~ u~ o o u~ In o o o o o o o o
2 ooooooo ooou~oo oooooo
Z C~~ ~ o o o o ~ a~
~1ou~u~oooo oooou~o oooooo
' ` ~ ~ ~
oou~oooo oooooo oooooo
u~ ~OOOoO ~ ~,i,io
o o~oooo ooou~o~ oooooo
~ ~ 1 ~~ ~ ~ Ci~ r ,i o
C~ I oU~ o o U~ o o o o o o
oooo ~ io ~oooo
~i Ooooooo oooulnln oooooO
~ ~9oooooo a ~ ioooo
Lq ~1o u~ou~ooo ooolnoo oooo o
o o o o a~
o ~ooooo oooooo oooooo
C~ i o O O O ~
~n o o u~ ~ O u~ U~ O O O O u~ u~ O o o o o o
oU~OOOOO ou~ou~u~o ooOooo
a~~i o o o o O o~ ~ ~ o
~&1ou~ou~ooo ooou~ou~ ooc)ooo
'~1 CD~0000 ~C~ ~o
~1 ooo~ooo oou~u~ou~ oooooo
~ o o o o ~
8 ~1 0 ul ~ 7 N O O O 11~
~ I ~8 ~ ~ 'D ~ ~ o o o u~ ~ ~ o o
,,~ .
7~
~ 120 - 61109-7287D
~1oooooo oooooo oooooo oooooo
i ,i o o ~i .-; o o O O ~ ~ o o o 0 o o o o o
oooooo oooooo ooo oo oooooo
oooo ~i~ oo oooooo
oooooo oooooo oooooo oooooo
I
,i ao r r ~ ~i o 0 r~
~1oooooo oooooo oooooo oooooo
oooooo oooooo oooooo oooooo
~0 Co~ ioo ~a~s~oo co~i~oo
I
~ o o a~ ~D c~) 0 0 a~ i o
.C ~3 g3 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 -- ~ ~ N O O d' N --i O O O d ' N ~i 0 0 0 0 0 0 0 0 0
1
9 N ~i 0 0 0 CD ~ i O O 1~ ~ _I O O O ~D N O O O O
~1oooooo oooo o oooooo oooooo
~1oooooo oooooo oooooo oooooo
co 1` ~ 0 CD ~ ~i 0 0 cs~ N _( O
OOOOOO OOOOOO OOOOOO OOOOOO
~i ~ ~ CO a:~ ~ N ~ CO C~ '1 N _I ~ ~ ~ t- ~ N CO ~ ~ N N
1 oooooo oooooo oooooo oooooo
Z ~ .. ,.,. ,,,,~, ...... ......
CO Ot) CO 9 ~ O 1~ D O O O 0~ r N O c~ ~D N O o O
oooooo oooooo oooooo oooooo
~d 0 ~` ~ N N O ~ di' '~ N .-~ O ~ ~ d' N ~ O ~` ~D ~ N O O
oooooo oooooo oooooo oooooo
Ot~ ~D d' ~ ~ /5~ V~ N ~i ~ /5` a~ ~ ~ N t~ d' N O
8 u~ ~ N It~D N 8 8 In U ~ N 8 8 u~ N ~ ~)
~, 1 In N _I O O It~ N ~'1 0 0 0 ~n N ~1 O O O In N r l O O
r~ 9e 8 ~ c ~
~ ~ ~rl ~SJ N ~ i Ir) N ~N ~
.
~ iL~7~d~
-121
I
o Q o o o o D O O O O O IC~ O 1:~ 0 0 0
IO Cl O o o O N N N ~1 o O ~1~ 1~ Ul ~ N N al 0 1`~ 1`. r~i 0;
el o c~ o ~ O o o o o o o c~ o o o ~a ,o Cll O :1' 0
o~ ~Z I " ~ ~ o c:l o o ~:i o ~ o o o o o 1~ U~ ~ ~ ~ ~
I~U O ~ ~ O C~ O ~ O O ~ O . . . ~ .
, ~ o o o e- o o ~
O ~r ~ ~ O O N r~ o o o o O~ ~ r o~
o o C~ o o o o s~ o o o o , ':' '~ ~
~ ~ !
:1~ J I
~ ~ I O O O O O O O O D O O O O o O o o " O O. O. O, O. O,
~--)I ooC-OOO OOOOOO r~ ooo
~ ~ ~ O O O O O O O o 1~ o 0. O, O, O, o o.
G~ ~ I ' r~ ~ o o ~i ~ O O O a~ ~ ~ ~ ~ r o o o
~n
t~utI OOOOOO r~oOOOO oCOOO<~ .~,,
tt ~ ~1 ~ ~o ~r o o o ~ J o o o o ~ t~ 3 r~ d
t~Ioooo~o oooooo ot~oooo ~.,.,
~I~~ u I O O O O O N _l o o o o O' at N Cr~ ~ O'
Ot~ OOOOOO OOOOoo OOOOOO ...
_ Z ~ t O; N O O O N ~ O o ô o ~ ~ dr ~:r O O 0' 7` ¢I N
r~t
U '1 1 0 0 0 0 0 0 0 o O o o o O O ~I O O O . . .
~D ~ti5 0 I O O O N O O O O O 1~ r dr O O ~ ~r Ul r~ rl N
~ 2nvl t oOoooo oooooo oe:loOOO oooooo
~ O I 1~ ~t N O O O O O o O o o ~r ~ ICt r; 1~ C ' ~ ~ In
2 V I o o o o o o o ~ o o o o t, o o o s~ o . , , . 'r.
W iO o o o o O N O O O O O N ~ O O O O
~~ Vl t e~ o o Q O O O O O O O ~ O o o o o O O, e- O. O~
t j~t r~ o o o o ~ _l o o o o 1~ r~ Lr~ n o o ~ t ~t O
t~ O O O O O O O Q cl O O O O O O O O ~ ~ . ,
~ < I P~ ~ ~ t~ ` ~O dr N O O e~ O rt o
O O O US rl N O O O Ut rt N O O Cj Ul r ~ o o ~
r ~o o In ~ ~ ~ ~ O o ul N _ o ~ O. IA
C
o ~ c- ~1,8 o I 1:: D¦8 I E ~ -- ~
_ N c .~ I ~ L ~ N I Cl la
--~ N t~ C) 3 ~ N ~ C~ 3 ~ N N Cl D, O I D 1 Cl
c I a~ I O X ~
3~ ~ .~ C ~ . I E ~ o~ , ~ I E C) C I ~ CQ- --~ '~
C~Q 3 E a~ Q) C,) 1 3 E~ a) ~ C.l 1 3 E a.s a~ C.) O U~ I N X
EL I _ -~ C a O I ~-- r C ~ O I -- -- C tll U~ I N ~ Q
~3 ~ 1 CJ O ~ N ~) '1:1 C) O ~ . C r L
~ o o I s ~ o o I ~. _ m OQ O I ~ ,5~ - a) c~
I C X ~ ~ I C X ~ ~ I L X ~ a~ ) O S I
C~, O :~ D. X ~ O. O ~ Cl. X ~1 ~ O ~ x ~o ~ N ~
~". , . ,",~,
- I 2 2 -
~1~ OC I
., ~ . ~ O O O o o ~ '~ o 11::1 0
I N ~- rl O Cl O ~ O ~ ? N
Ul ~Y I .
O q O O O O O O ~ 1 o o o , C~ o o o Q Cl a
N r~ J N r; O N ~q O O ~ 7 N ~ r. ~ 1 rl r;
U ) - I O O C:l o o o ,~ o o O O C~ o ~ ~ O O O O O
O
1-- ~Cl ~ ~.. ~7OO
~J 2 ~ ~ ~ ~ N ~ tt~ ~ O` ~ N ~ 0~ U~
a l
. o ~ o o o o o o
3 J ~
:", ~ I o o c~ o o o o o ~:~ O O O e~ O ~1 D O O o o o
N O O ~5 rl O C I Cl O 0~ O~ C 1~
O O O o 9 o
~ < n I ~ i O ~ o~ a~ ~a ~ o~
u~
njO~,OOOO OOOOOO, OoO oe~C~ooo
~ 0 r~ r~ ~ O r- 0 o o o o c ~ o~ c o~ o r a~ a~ r r~ ~r
._ I
! ~. .
,S;~ U I ~ ~ ~i o o o a~ ~ o a~ ,o o G a~ r ~ o ~ ~ r ~ ; o
O ~ O O I o. o; O. O. O, O. o ~. a~ a r' a~ ~ ' '
S I o o o o o o o o o o. o o ~ a~ r a ~ Ci o~ r a~ D~ o ~
O~~OOOOOO OOOOOO OCJOOOO oooooo
~ < ai a ~ .o :r o a ~ a a ~ ~ ~ ~ a o~ a a o a a o a
30 ~
2 ~ I O O O O o o o o ~ c~ o o o o O O O O ~ O
O~ C~ a ~ C ~ ~ ~ O. ~ O O
~Iooooe~o oooooo C>ooooo
X ~ I a ~ ~ o o ~ o r~ o o o ~ ~ a a~ a a~ a ~ a a o ~o
~t~Ioc:~oooo oooooo oooooo OOOOclO
a o~ r~ o a a a~ ~ ~ o a; o~ o~ a 0~ ~ a; o~ o~ ~ a; a~
ID ~ I
o ~ a 1~ o o u~ " ~ O '~ ~ ~ o o o n ~
t-- I O 1~ O Cl O Ir~ N ~ O O Cl U~ N _ O O o lltl " _ o o
O ~ I I I O Lt~ I I I I '-- ~ I :~.~--~ I
V~ I r ~ o o7 I r ~ o ~ _ I 1 3 S ~ ~)I Q
~ _ N C _ ~ L ~ ~ C
J ~ o LJ ~ ~r s o ~. ~ ~ E t~
--~ N o c~ O
c ~ ~ E ~ O In ~ O--~ L C) CO 3 ~
a ~ 3 E s a) 0 3 E s ~ o o L ~ I a ~ O
E ~ I ~ S:: L I ~_ ~ r ~ S C~ 1 ~ r ~a
o ~ -- I I -- a~ o ~ I x -- o
C_) 3 ~ , L I O ~ L O ^ -o C)
o O ~ O O ~ 3 S ~-- ~ D ~ O O ~ c _
3 ~ X I ~ ~ X I ~ ~ I ql O ~ ~ ^ u~
h. Cl. O N C~. X ~ CL O ~ ~ X ~O ~ N ~ ~ O. x
S~ f,S~
-123-
F.XAMPLE 59
Preemer ence herbicidal evaluation of test compounds
_ g . _ _.__ _ _
The preemergence herbicidal activity of the
compounds of the present invention is exemplified by
the following tests in which the seeds of a variety of
monocotyledonous and dicotyledonous plants are separa-
tely mixed with potting soil and planted on top of
approximately one inch of soil in separate pint cups.
After planting, the cups are sprayed with the selected
aqueous acetone solution containing test compound in
sufficient quantity to provide the equivalent of about
0.016 to 10 kg per hectare of test compound per cup.
The treated cups are then placed on greenhouse benches,
watered and cared for in accordance with conventional
greenhouse procedures. From four to five weeks after
treatment, the tests are terminated and each cup is
examined and rated according to the rating system set
forth above. The herbicidal proficiency of the active
ingredients of the present invention is evident from
the test results which are recorded in Table II below.
Where more than one test is involved for a given com-
pound, the data are averaged.
7~ ~
- 124 - 611.09~7287D
ooo u~ooomu~ oooooo oooooo
i o o o o o 11') Il~ d' N,--i 0 ~ ~ ~`i ~ ~i 0 O~ i 0 0
)011~000 oo~ o oooooo oooooo
, o o o o ui ~ t~i O o o ~ i ~ ~ ,i o o o
oolr OOOOU71r 000000 000000
ommlnm ooou~oo oooooo oooooo
~1 omol~ o oooooo oooooo oooooo
~ loooooo oooooo oooooo oooooo
3 ~I u) ~ o O O O ~ a) CO ~D U~ ~ a~ CD Ct~ I` u~ tY~ ~ ~ CO ~9 ~`i O
ou~ooo ooou lno oooooo oooooo
I OU~U~OOO ~OU~OOO 000000 ~00000
i 0 _i aD C4 CO U~ t`~ O o~ o o ~ ~ 'd' O
~1oou~ooo u~ 00 oooooo oooooo
oo u7000u~u~ oooooo oooooo
HI ~ O d ' ~ O O O ~D CO OD 1` U'7 1") a~ 0~ a~ ~D ~ r-i tSl Gt~ 0 ~D LO ~r
000000 000000 000000 000000
0011~ 00 OOOOlf-lt) 000000 000000
o~ ~ ~ 0 cu ~ l~ ~ ~ o
U H ~ . O, O O O O 0 1~ O O O O O O O O O O O O O O
oou~om ooou~oo oooooo oooooo
N .-~ O ~ ~ a~ ) ~ Cr. ~ ~ ~ D
g~ u~ OOuO O~OOu~O OOOOOO OOOOOO
H 0 li') ~ _i O O C~ CD CO ~ N O ~ 0 I` ~ O O ~ I` d' N O
noou~u~o ou~mou~o ooooOO OoOOOO
cot~ Oo ~u~ o ~a~oo a~a~oo
o o u~ ~ ~ ~ 8 Ir~ ~) N ~D 8 O 11-) ~) N ~ 8 o 117 ~ N ~
Il~ ~ ~ O O O U7 N ~ O O O In N r-l O O O Ll') N ~1 8 o o
~ ...... ...... ...... ......
b ' b ~ q 1 ~
b~ N
N y ~ o I a) llj~ ~ L~
N
- 125 ~ 61109-72~7D
~1oooo~o oooooo oooooo oooooo
¦ In ~ N N N N d' N N ~ i O r-l O O O O O t~ ~r) ~i O O O
~1~1oooooo oooooo oooooo oooooo
~1~ ~ ~-~
000000 000000 000000 000000
tS~ ~ r Ir) d ' N a~ ~ I` li ~i ~i r` ~ .--i 0 0 0 0~ N H O
I
æ l ~ o ~ O O o o CO Co ~D ~ o o
31 oooooo oooooo oooooo oooooo
') N ~ ~ ~ N ~ N d ' N N _i _I O 0 I~ I~ ~ N N
oooooo oooooo oooooo~ ~ ~ o o ~ ~ N O O O 1-) 0 0 0 0 0
000000 000000 000000 000000
a~ ~o OD~D~O t~D~OOO C~l`~OOO
000000 0000 0 000000 0000
o ~ a~ i o Cl~ ~) O O O O ct) ~ ~s ~
~&1 oooooo oooooo oooooo oooooo
'D N ~i 0 0 0 ~ -i 0 0 0 0 0 0 0 0 0 0 ~ 00 1` N O O
i OOOOOO OOOOOO OOOOOO OOOOOO
a~ o o a~ d' d' O O O 1` ~ 0 0 0 0
~ 000000 000000 000000 000000
H ~ H a~ r` O O t~ ~ N r-i O O
8 ~1
n ~ o ~ ~D ~ ~ O O l` r~ ~D ~ O O
au, oooooo oooooo oooooo oooooo
~d ~ co ~ N ci~ c~ ~ ~) N ~i 0 0 0 0 0 0 (D ~9 N N --I O
000000 0000 0 000000 000000
¦ (~ ~ ~ `D 1~ ~9 ~ ~ ~ ~D N ~ 1 0 0 r~ r~ N N N O
~1oooooo oooooo oooooo oooooo
D u~ O O a~ ~ CD ~ O O 0~ ~ O O O O ~ ~ O O O O
oooooo oooooo oooooo oooooo
a 1~oo a~NOO ~NOOOO NN.'~i00
~ ¦ ~ D N ~D o L~O'l N ~') N ~D o ~ N ~ N ~D o o
e ~ ~ 9 U~ ~ t`7 ~ U') 1~ N _I
N ~ N ~ Irl N N rl O
- 126 - 6~1.109-7287D
.~1oooooo oooooo oooooo oooooo
1 a~
~1
¦ ~ N t`~ O O O ~`i ~ ~ N ~ d' d' t`i ~`i ~`i ~`i ~`i ~
~0 000000 oOoooo 000000 000000
oooooo oooo ooooo oooooo
~1
9 o o o c~ D o~ r r. ~ N
000000 000000 000000 000000
r~i o o O a~ r o ~ ~ ~ co ~ ~ o~
000000 000000 000000 000000
D O O O O~ a~ 0 C~ P a) CO a) ~ ~ CO N
1
D Irl o o r~ ~ ~ ~ ~ ~ (r~ ~ ~D O~ ~ I~
~1
N _i O O O ~ a~ D O ) OD CO CO ~ N Cl:) 0~ 1` 1` ~9 N
0 000 000000 000000 00 000
H t~ ~æ ~ ~ ~ ~ ~0
~ ~ 0 000 000000 000000 00000
O O O ~ r` tO O O O ~ ~ ~ C~ D (:~ ~ r--~D (`
~sq oooooo oooooo oooooo oooooo
~o N ,-1 O O O a~ 0 OD ~D a~ D ~
~1oooooo oooooo oooooo oooooo
~ ,......... ...... ...... ...
P.~ u~ oo COI`I`~OO COC~0~'~ 0CD~COC~
~1oooooo oooooo ooo oo oooooo
D 1~ ~`i O O O ~ ~ `i ~ O ~ O~ ~D ~ ~D ~ ~ ~ `9 ~D ~
~1 oooooo oooooo oooooo oooooo
~¦ 8 ~ ~ ~ ~ O ~ ~ $ ~ D oO o ~ D o u~ D~
P~ .a' ~ æ~ æ~,
-127-
~,
O ~ ~ ~ ~ a ~ ~
ul a, ~ co ,~ .~ ~ N
W rr I
O O e:l o e~ eJ O O O o C~ o o
2 ~
U i O O ~ O O C~ Cl O O O O O C:l
~ ~ I ct c~ c~ c~
O
~^ I O o c~ O ~ O o cl o ~ o O O O O O
~A~ T I ~ ` ` ~ ` O` ~ O- c~ C~ O O~ cr O-
:~ I
u~I C~
I
~c _l
lU u. I
~<I ~ooooo ooooo oo~ooO
~ ru ~ c~ C~ ~ c~
Y
2 ~ o o o o c~ c> o o o o ~ c~ o o o o
J ~ I
L~ c~ c; ,~ cJ~ ~ O O O O O O o o o
L ~ ~ ~ ' ~ ` C; ~ Cr~ ~ m
~" C2 i C~
C ~ ~ I t~ t~ tO 0 ~ ~ c~ 0~ r ~ o o
C ~n o I
~ OCI ooooo~ oc~oc:~o oooooo
t~ c~ r o ~ c~
-1 t~
u~ :c o I
~2~w ~tI c~.~ oooc~o oooooo
~ ~y O ~ C~
c ~
ov~I oooooo oooos~ oooooo
~ O I ~r o~ c; rr ~ ~r o~ o~ ~ cr 0 o~ o~ cr ~ o~ t
~- u~ I
2 ~ I o o o o o o o o o o o o o c~ o o o
~L U- ~ 10 ~ ~ ~ r~ o o~ c; cr c; c; c; o~ o ~r o~ ~r
c cL I
o~oooo c~o~:>O
~ I ~ ~ r~ cr C~ cr o~ ~ o cr .o ~r
~ ~ I o c~ o c~ c~ c~ o o o o o o o o o O O
o r. ~ c; rr. c~. c; c; ~ ~ cr c~ cr
O O ~ t~ N 0 0 IA ~ N ~ O O 111 ~ N ~0
.J _~ O C~ O ~ _ O O O O 1 N ~ r~ _
D~j '''' ..... .;
O ~ ~ I I I C) I ~ U~
V) I ~. Q ~ ~~ 3 S I
~ ~ I a) N o c --
J S ~ CL O ~ a. O
C I ~ I I I O X I r_ O O a~
;~ ~ ~: C ~ Lr~ ~ L O C D¦ L S_ I E I L c
e~ 0 3 ~ O 11'~
c~i S_ I O ~ O ^ I C CL~ ~
_ ~ --~ N ~ U~ ~ L ~J I D
~ _ 3 ~: ~ O a~ ~ I ~ O I ~) L
I ~ E S >1 :~ 1 0 E 3 ~ C~ J CC
~ Q X ~O E ~
-1~8-
EXAMPLE 60
P t_~rowth re~ulant evaluation of the compounds
The plant growth regulant activity of the
compounds of the present invention is demonstrated by
the following test, wherein barley (H_rdeum ~ ) is
treated with a test compound dissolved in aqueous ace-
tone. In the test., the compound dissolved acetone/
water mixtures S0/50 containing 0.25% by volume of
Colloidal Multi-film X-77~surfactant (alkylarylpolyoxy
ethyleneglycols, free fatty acids and isopropanol) of
Colloidal Products Corporation (Petaluma, California)
in sufficient quantities to provide the equivalent of
about 0.00625 kg to 0.10 kg per hectare of active com-
pound when applied to the plants when the first node is
detectable (Zadok~30). After spraying, the plants are
lS placed on greenhouse benches and are cared for in the
usual manner, commensurate with conventional greenhouse
practices. From 11 to 12 weeks after treatment the
plants are measured and harvested. The heads are
removed and dried for 48 hours at 85 to 90C and weights
recorded~ The data obtained are recorded in Table III
below
~a27~
-129-
TABLE III
Plant growth regulant activity test
Total %
Rate Head Increase
Compound ~ Replicate Dry wtlg Head wt
Untreated control - 1 56.7
- 2 55.9
- 3 60.6
Average 57.7
Furfuryl 6-(4-iso- 0.10 1 64.6
pro w1-4-methyl-5- 2 68.2
oxu-2-imidazolin- 3 66.1
2-yl)-thieno[2,3-bj- Average 66.3 14.9
pyridine;-5-carboxylate
0.05 1 66.6
2 74.1
3 77.7
Average 72.8 26.1
: 0.025 1 5~.9
2 70.6
:: 3 68.7
Average 64.0 10.9
0.0125 1 64.~
2 72.6
~5 3 75~5
Average 71.0 23.0
0.00625 1 58.6
2 67.5
3 68.4
Average 64.9 12.5