Note: Descriptions are shown in the official language in which they were submitted.
29,416
ELECTRODES, ELECTRO-CHE~ICAL CELLS
CONTAINING SAID ELECTRODES, AND
PROCESSES FOR FORMING AND UTILIZING
SUCH ELECTRODES
The present invention relates to electrodes
having fibers which provide large surface areas, high
bond strength between the core fibers and metallic
coatin~s thereon, an~ efficient electrical connections;
c electro-chemical cells including such electrodes; and
processes for forming and utilizing the elec~rodes
and cells of the invention.
~ .7~rAr
~,J~ 3~ q
BACKGROUN~ OF THE I~ TION
Efficiency in electro-chemical processes,
such as electrolysis, electroplating, electrowinnin~,
electro-orqanic synthesis, and waste recoverv, depends
to a substantial e~:tent upon the surface area of the
electrode. Electrodes have been constructed with
ridges or convolutions to increase the surface area.
Sandblasting also has been used to roughen the electrode
surface, and thus provide a larger surface area. These
known techniques have been found to have limited effec-
tiveness in increasing the surface area.
More recently carbon fibers for electrodes
which provide large surface areas have been described in
United States Letters Patent Nos. 4,046,663, 4,108,754
and 4,108,757. The electrodes comprise a plurality of
carbon fibers arranged ~enerally parallel to one another
and clamped at one end to an electrical connection.
Although the5e electrodes may have large surface areas,
they provide relatively poor electrical connections.
Specifically, a large number of carbon fibers invariably
break as a tow of such fibers is clamped into an electrical
connection. This breakage of fibers adversely affects the
electrical effectiveness of the tow. Additionally, the
mechanical connection of carbon fibers results in an
undesirably ~igh electrical resis~ance at the connection.
Consequently, the theoretical efficiencies of the
electrodes are not attainable because of the mechanically
destructive and inefficient electrical connections.
The electrodes shown in U.S. Patent Nos. 4,046,663
4,046,664, 4,108,754 and 4,108,757 also act as a wic~,
causing the electrolyte to be drawn up into the area o~
the terminal. When the electrol~te evaporates, a salt
3~
-- 3
residue re~ains which affects the electrical connection.
The salt deposits thermally shield the terminal causina
heat buildup, increased resistance, and eventually ter-
minal failure by bridging. Even if wicking and fiber
damage could be controlled, there would be poor electrical
connection to the fibers in the center of the bundle.
Several attem~ts have been made to place
metallic coatings on the carbon fibers so that tows of
the plated carbon fibers can be used more efficiently
as electrodes in various electro-chemical processes.
In most instances, the plating applied to these carbon
fibexs has been discontinuous, brittle, and expensive to
ap~ly. For example, United States Letters Patent Mo.
4,132,828 shows the ~acuum deposition of nickel onto
carbon fibers. The coatino taught by this patent, how-
ever, is not continuously in contact with the carbon
fibers and will easily break and all off ic the fiber
is twisted.
Electroless nickel baths also have been
employed to plate carbon fibers. However, this platlng
process is slow, expensive to carry out, and agalr
results in inferior discontinuous coatings. Another
undesirable coated fiber is shown in United States
Letters Patent No. 3,622,283.
In view of the above, it is an object of the
present invention to provide fiber containina electrodes
having large surface areas, efficient electrical connec-
tions, and continuous metal coatings on fibers with hinh
bond strengths therebetween.
It is a further object of the subject inventlon
to provide plated and unplated fiber electrodes which can
~\
~ ~3~
- ~ - 61109~7299
be bent, wrapped, woven or knitted into a variety of
configurations for efficient use in electro-chemical cells~
The invention seeks to provide electro-chemical cells
and processes with electrically conductive fibers constructed into
electrodes without the drawbacks of -the prior art electrodes.
SUMMARY O_ THE I~VE~TION
The present invention relates to an electrode comprising
a tow of a plurality of continuous fibers, wherein each of said
fibers has a thin firmly adherent, metallic coating thereon, said
electrode being provided with a terminal at the end of each metal
coated fiber, and an electrically conductive metal which extends
between and joins said metallic coatings at said fiber ends to one
another and to said terminal to provide an integral metal matrix
that produces an efficient electrical connection between said
metal coated fibers and said terminal. The coating preferably is
continuous and is bonded so well that if the metal coated fiber is
bent, the coating may fracture, but will not peel off. The fibers
for the electrodes of the invention can be semi-metallic, such as
carbon and silicon carbide fibers, or non conductive, such as
nylon, polyester and/or aramides fibers.
The fiber-containing electrode of the invention can be
made by a process comprising providing continuous lengths of
plurality of core fibers, plating at least an end of each of the
core fibers with substantially uniform, firmly adherent coatin~ of
metal, positioning a terminal for contact with the metal coated
ends of said core fibers, and joining the metal
_ 5 - 61]09-1299
coated ends and the -terminal by an electricalLy conductive metal
which extends between said metal coated fiber ends to form an
integral fiber/metal matrix which provides an efficien-t electrical
connection.
The fibers formed by the described method will have a
metal to core bond strength sufficient to provide that if the
fiber is bent, the coating may fracture, but it will not peel off.
Moreover, in preferred fibers, the bond strength is more than
sufficient to permit the -fibers to be knotted without substantial,
i.e.; more than 5 percent by volume, separation and flaking of the
coating.
~ hen the fibers are non conductive, nylon, polyester
and/or aramides, and the like, they are first rendered conductive
by providing an extremely thin metallic interlayer and then coated
with a metallic layer.
Whether the core fibers are semi-metallic or non
me-tallic, the electrode of the invention preferably is formed from
fibers which are metal coated adjacent the connection of the elec-
~11."~?J~
-- 6 --
trode to a power source~ The metal coating of the fibers enablesthe connection to the power source to be made by means such as
soldering to create a continuous Eiber/metal matrix adjacent to
the electrical connection, thereby avoicling the mechanical connec-
tions such as crimping, which damage fibers and reduce the effec-
tiveness of the electrode. Additionally, the soldered connection
and the resultant continuous fiber/metal matrix avoids wicking
which has been prevalent with prior art mechanical connections,
and which rapidly deteriorates the quality of the electrical con-
nection. Also, the soldered connection and the resultant continu-
ous fiber/metal matrix encapsulates all the fibers to the metal
for low contact resistance even to the center of a mass of 100,000
fibers.
The subject electrode can be formed by metal plating
only the portion of a fiber tow which will be adjacent the elec-
trical connection. The electrode also can be formed from a fiber
tow which is entirely metal coated, and which is subsequently
stripped of part of the metal coating prior -to use as an
electrode. In most electro-chemical applications, the electrode
with plating only near the electrical connection, preferably would
function as an anode.
The subject invention further includes an array of
fibers with each fiber in the array being continuously coated with
metal along their entire lengths. These coated fi~ers provide a
3~ia~ i~r
large surface of high electrical conduc-tivity. They are electric-
ally connected to a power source by a means such as soldering to
create an integral carbon/me-tal matrix adjacenk -the electrical
connection. As explained above, this continuous matrix avoids
damage to fibers and substan-tially prevents wicking. The elec~
trode formed with plating along the entire length of each fiber,
-typically is used as a cathode.
As a result of the enhanced coating of the fibers, it is
possible to form the subject fiber electrode into a variety of
useful configurations which heretofore had been unavailable.
Specifically, a metal coated fiber tow can be wound around a flow-
through support with little or no possibility of having -the metal
coatings breaking from the fibers. Other plated electrode config-
urations include woven mats, which can be supported in a planar
configuration or wrapped around a flow-through support, and
knitted tubular configurations, which can be posi-tioned around a
cylindrical flow-through support.
As a result of the flexibility of the subject elec-
trodes, several unique cell s-tructures and processes
7~3~
are made availa~le. For example, anodes and cathodes
mounted around flow-through supports can be alternately
arranged in one or more cells. The electxolyte then can
be passed through the cells in such a manner as to ensure
maximum contact with the carbon fibers. In one embodi-
ment, each cell can contain an anode o~ a flow-through
support and a cathode on a flow-through support. Each
such cell could be separated by a non-conductive barrier,
with each barrier having electrolyte passageways in the
form of one or more holes. To achieve the desired flow
pattern, the passageways ln the barriers would be
alternately located in a bottom corner or an opposed top
corner. Holes disposed in this manner in the barriers
help to achieve maximum contact of the electrolyte with
the electrodes.
In another embodiment of the above described
construction, each cell can include a plurality of the
fiber containing anodes and cathodes wrapped around flow-
through supports. A plurality of such multi-electrode
cells can be arranged in series, with the connections
between the cells constructed to ensure maximum contac-t
of the electrolyte with the electrodes. As noted pre-
viously, this electrolyte flow pattern can be achieved
by alternately locating holes in the barriers between
cells in the top and bottom corners of the barriers.
Other electro-chemical cells of different
configurations also are include~d in the present inventior..
For example, porous metal plates can be used as the
cathodes and arranged alternately with the above described
anodes. In still another embodiment a discretionary cell
can be provided utilizing a small anode, such as a
platinum wire, in conjunction with a large area metal
plated fiber cathode to plate soecific metals onto the
? ~
g
cathode while leavin~ other metals in solution~ To
ensure optimum electrolyte contact with the electrodes of
the above described discretionary cell, the metal plated
fiber cathode can be formed into a cylindrical configura-
tion with the cylinder being disposed concentricallyabout the anode. The cylindrical fiber cathode can be
formed either by helically wrapping a fiber tow about a
porous cylindrical form or by knitting a tubular structure
from the metal plated fiber. Another cell of the inven-
lG tion which can be used for oxidation-reduction reactions
in a bipolar cell, includes an alternate arranaement of
anodes and cathodes in a cell containing both solutions,
wherein one of the interconnected electrodes of the
present invention is positioned in one solution while the
other interconnected cell is positioned in the other
solution.
In each of the described embodi~ents, the
electrodes of the invention provide large surface areas,
2~ efficient electrical connections and hi~h bond stren~th
between the core fibers and the metal coatinas thereon.
- 10 -
BRIEF DESCRIPTION OF THE DRAW~NGS
The following is a detailed description together
with accompanying drawings of illustrative embodiments
of the invention. I~ is to be understood that the inven-
tion is capable of modification and variation apparent
to those skilled in the art within the spirit and scope
of the invention.
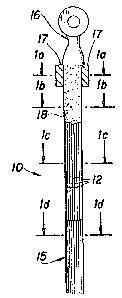
FIGURE 1 is an elevational view, partly in
section, of an electrode of the present invention includ-
ing a tow of partially plated fibers and an integral fiber/
metal matrix at its terminali
FIGURE la is an enlarged cross-sectional view
of FIGURE 1 taken along lines la-la thereof;
FIGURE lb is an enlarged cross-sectional view
of FIGURE 1 taken along lines lb-lb thereoE;
FIGURE lc is an enlarged cross-sectional view
of FIGURE 1 taken along lines lc-lc thereof;
FIGURE ld is an enlarged cross-sectional view
of FIGURE 1 taken along lines ld-ld thereof;
FIGURE 2 is an enlarged elevational view,
partly in section, of a single fiber of the electrode
shown in FIGURE l;
FIGURE 3 is an elevational view, partly in
section, of the electrode of FIGURE 1 used in conjunction
with a protective tube;
FIGURE 4 is an elevational view, partly in
3~ ? ~
section, of another electrode of the subject invention
including a tow of fully plated fibers;
FIGUP~ 4a is an enlarged cross~sectional view
5 of FIGURE 4 taken along lines 4a-4a thereof;
FIGURE 4b is an enlarged cross-sectional view
of FIGURE 4 taken along lines 4b-4b thereof;
FIGURE 4c is an enlarged cross-sectional view
of FIGURE 4 taken along lines 4c-4c thereof;
FIGURE 5 is an enlarged elevational view, partly
in section, of a single fiber of the electrode shown in
FIGURE 4;
FIGURE 6 is an elevational view, partly in
section, of the electrode of FIGURE 4 used in conjunction
with a protective tube;
FIGURE 7 is an exploded perspective view of
another embodiment of the electrode of the subject
invention wherein the tow of fibers are wrapped about a
flow support;
FIGURE 8 is an elevational view, partly in
section, of the electrode shown in FIGURE ,;
FIGURE 9 is a side view, partly in section, of
the electrode showr. in FIGURE 7;
FIGURE 10 is a side view, partly in section,
of an electro-chemical cell of the subject invention
includin~ the electrode of FIGURES 7-9
.s~J,~3~
~ IGURE 11 is a perspecti~e view of the divider
panels used in the electro-chemical cell of ~IGURE 10;
FIGURE 12 is a perspective view of the flow-
through spacer positioned between the electxodes of FIGURE10;
FIGU~E 13 is a schematic diagram of an electro-
chemical system including an electro-chemical cell of
the subject in~ention,
FIGURE 14 is a plan view of the electro-chemical
cell shown in FIGURE 13,
FIGURE 15 is a side sectional view of FIGURE 14
taken along lines 15-15 thereof;
FIGURE 16 is a cross-sectional view of FIGUP~E
14 taken along lines 16-16 thereof;
FIGURE 17 is an elevational view, partly in
section, of a discretionary electro-chemical cell of the
subject invention;
FIGURE 18 is a perspective view of the cell
shown in FIGURE 17;
FIGURE 19 is a side elevational view, partly in
section, of an electro-chemical cell of the invention,
including a porous plate electrode;
FIGURE 20 is a perspective view of the porous
plate electrode of the cell of FIGURE 19;
FIGURE 21 is a side elevational view, partly in
r
~ 7
section, of a bipolar electro-chemical cell of the
subject invention; and
FIGURE 22 is a perspecti~e view of the divider
and the active electrodes of the cell of ~IGURE 21.
DETAILED DESCRIPTION OF THE
PREFERRED EMBODIMENT
The electrode of the subject invention is
indicated generally by the numeral 10 in FIGS. 1, la,
lb, lc, ld and 2. The electrode 10 is formed from a
plurality of fibers 12, each including a central, prefer-
ably carbon, fiber 13, e g., about 7 to 11 microns, and
a thin concentric continuous layer 14 of nic~el or other
plated metal, e.g., about 0.5 microns. The plated
fibers 12 are formed into a tow 15, which is a generally
parallel array of numerous plated ~ibers 12, e .a., about
40,000 to 50,000 fibers, wherein the tow typically has a
20 diameter of about 0.125 inch. A tow 15 o:f the desired
length is placed in an electrical connector 16 such that
the clamping arms 17 of the electrical connector 16 are
engaged about one end of the tow 15. More particularly
the arms 17 of the electrical connector 16 are engaged
about the tow 15 with sufficient force to loosely retain
the tow 15, but yet ensurina that the plated fibers 12
are not damaged. This force exerted by the clamping arms
17 on the tow 15 is substantially less than the force
that normally would be utilized if this mechanical
connection were to be relied upon for the conduction of
the electricity.
Once the tow 15 has been engaged by the
electrical connector 16, the combination of the connector
16 and tow 15 is dipped in a bath of molten metal, such
` "` 3-~73~ *
as solder of about 60% tin and about 40% lead~ Solder
18 wicks into the area between adjacent plated fibers 12
and the area between the electrical connector 16 and the
plated fibers 12 to form what is effectively a carbon/
metal matrix at the end of the electrode 10 thereby
defining an efficient electrically conductive connection
between the tow 15 and the connector 16. The desired
wicking of the plated fibers 12 can be accomplished in
a matter of seconds, typical~y in about 10 seconds.
The metal plating 14 on the portion of each
fiber 13 away from the connector 16 then is stripped off,
for example, by dipping in a bath of nitric acid. More
particularly r the plating 14 is stripped so as to leave
a short section of plating 14 extending away from the
solder 18. Preferably the plating 14 extends between
one-half inch and two inches from the solder 18, as
indicated by dimension "x" in FIG. 2. Thus, as
illustrated in FIG. la, the uppermost portion of electrode
10 defines an intearal carbon/metal matrix com~rising
the carbon fibers 13, the plating 14, the solder 18 and
the arms 17 of connector 16. Slightly away from con-
nector 16, as shown in FIG. lb, the integral carbon/metal
matrix comprises the carbon fiber 13, the pl~ting 14 and
the solder 18. Still further away from the connector
16, as shown in FIG. lc, the electrode 10 includes
carbon fibers 13 and plating 14 but no solder lBo This
plating 14 without the solder provides a step resistance
for current averaging from terminal 16 to stripped fiber
13, In so doing a current gradient is provided to pre-
vent a surge area which would more rapidly be attacked
by any electrolyte in contact therewith. ~inally, as
shown in FIG. ld, in the remainder of the electrode lD
the fibers 13 are loosely arranged in tow 15 with no
plating, and the electrolyte, indicated generally by
-15 -
c~
arrows 19, can flow freely between and achieve maximum
contact with fibers 13. These carbon fibers are graphite
and generally free of amorphous carbon.
Turning to FIG. 3, the electrode 10 is used
in conjunction with a non-conducting protective tube 20
formed from plastic or other inert material. The tube
20 is loosely fit over the electrode 10 and extends
generally from the connector 16 to a point alonq electrode
10 which will be dis~osed several inches below the surface
of the electrolyte with which the electrode 10 is used.
The protective t~be 20 reflects the fact that the most
aggressive damaging electrolytic reactions take place
within the area immediately below the surface of the
electrolyte. The protective tube 20 thus minimizes the
damaging effects in this critical area of the electrolyte.
To further minimize the effects of the transition between
the electrolyte and the electrode 10, the protective -tube
20 is provided with a plurality of small holes 21 at the
end of the protective tube 20 most distant from the
electrical connector 16 to e~fectively create a transition
zone of current gradient to minimize an area of current
surge and electrolyte attack.
Another elect~ode 22 of the invention is illus~
trated in FIGS. 4, 4a, 4b, 4c and 5. The electrode 22 is
similar in construction to the above described electrode
10 except that electrode 22 includes platin~ 14 disposed
continuously along the entire length of each fiber 13.
Thus, as illustrated in FIG. 4a the portion of electrode
22 adjacent connector 16 defines an integral carbon/metal
matrix comprising carbon fibers 13, metal plating 14,
solder 18 and arms 17 of connector 16. At a location on
electrode 22 spaced sightly from connector 16, the integral
carbon/metal matrix comprises carbon fibers 13, metal
7~3~ f
plating 14 and solder 18 as shown in FIG. 4b. Further
away from connector 16 and extending to the opposite end
of electrode 22, the fibers 13 each include metal plating
14, but, as indicated by arrows 19, the electrolyte may
freely flow through the electrode 22. These metal plated
fibers have a high electrical conductivity.
FIG. 6 lllustrates electrode 22 used in con-
junction with protective tube 20, which, as noted above,
minimizes the damaging effects of the electrolyte at the
boundary between the electrolyte and the ambient surround-
ings. In most electro-chemical applications the electrodes
shown in FIGS. 4-6 are used as cathodes.
FIGS. 7-9 show a generally planar electrode
30 incorporating the subject invention. The electrode
30 is formed from an elongated tow 32 which is wrapped
around a generally rectangular flow-through inert support
34 and which is held in position on the support 34 by
an inert screen 36. The tow 32 can be either stripped
of most plating as shown in FIGS. 1-3 or can be entirely
plated as shown in FIGS~ 4-6. All electrodes 30, whether
used as anodes or cathodes, include a metal plated area
38. This metal plated area 38 enables the application of
solder 40 to attach th~ electrical connector 42 to the
tow 32 and thus forming an integral carbon/metal matrix.
As explained above, the metal plated area 38 preferably
extends beyond the limits of the soldered area 40, and
on certain electrodes would extend throughout the entire
iength of the tow 32. The electrode 30 further includes
a protective tube 44 which typically extends from a
location above the interface between the air and the
electrolyte to a location preferably 3 or 4 inches into
the electrolyte. Although the protective tubing 44
could terminate above the flow-throuah support 34, it
preferably extends into an area adjacent ~he flow-through
~ ~3~
- 17 - 61109-72g9
support 34 to facilitate mounting of the tow 32 on support 34.
As illustrated most clearly in FIG. 7, the flow-through support
34 has numerous apertures 48 and can include an elongated cut out
portion or groove 46 into which the tube 44 is placed. The tow
32 then can be threaded through an aperture in the flow-through
support 34, and wrapped around the support 34 in a contiguous
manner. Although a single tow 32 terminated at each end is
shown in FIG. 8, multiple tows and terminations can be used in the
practice of the invention. The tow 32 is held in position on
the support 34, and is protected from damage by the screen 36,
which is folded around the combined flow-through support 34 and
tow 32. The screen 36, which can be made of nylon or glass fiber,
also prevents stray fibers from one electrode from contacting
another electrode.
When the electrode 30 is used as a cathode, the entire tow
32 typically is maintained in its plated condition. With the
preferred plating, as described above, the plating will remain
intact on the fibers of tow 32 despite the many sharp bends which
are made in tow 32 during the formation of electrode 30.
When the electrode 30 is employed as an anode, the plating
typically is removed from the tow 32 for all areas of the tow 32
except the areas near the solder connection 40 of tow 32 to the
electrical connector 42. This removal of the plating f~o~- tow 32
can be carried out either before or after the mounting of tow 32
on the flow through support 34.
In the illustrative embodiments of FIGS. l-9 the fibers
12, which form the core of the electrodes lO or 30, are carbon.
In addition/ the fibers 12 ~an be
3~
- 18
formed from other semi metallic fibers, such as silicon
carbon, or non conducti~e fibers, such as nylons, polyesters
and/or aramides and the like, which are rendered electri-
cally conductive by a thin intermetallic layer of silver,
copper, nickel and the li3~e.
The metal coating 14 can be formed from a wide
variety of metals including nickel, copper, silver, lead,
zinc, the platinum group and other metals depending upon
the application. Also, the metal coating can be multi-
layered, e.g., an inner layer of nickel and an outer layer
of silver.
With respect to the matrix, the term solder as
used herein, includes alloys, such as tin and lead or
copper and silver, as well as pure metals, e.g., copper.
The solder matrix creates an electrical bridge between
the walls of the terminal and each and every fiber 12.
The length of the tow o the ibers ]2 will
depend upon the width and lenqth required for the
electrode 10 or 30 and can be wrapped as shown in FIGS.
7-9 or woven or knitted. Illustratively, tows from a
few inches to over 40 feet in length have been satis-
factorily used in the practice of the invention.
One of the features of the present invention
is the large surface area made possible by the electrodes
in a small volume of solution which effects a low current
density while yielding a hiqh total current for the
Farraday equivalents. Illustratively, a fiber of 7
microns which results in a coated fiber of 8 microns
ater plating in a tow of 40,000 (40K~ fibers equals ~0
s~uare inches of area per inch of lenath of to~.
. . .. .
.
Furthermore, the resistance of the electro-
plated fibers is so law that the potential of the
tow is substantially uniform even at a substantial
distance from the terminals.
The electrodes of the present invention can
be used in the removal and recovery of soluble metals
in dilute solutions, such as process streams from plating,
hydrometallurgy of mining, waste streams from mining,
as well as wherever metals are present in dilute solutions,
such as in photographic and catalytic processes. As has
been described, the electrodes of the present invention
have large effective areas. As a result, effective
winning currents and discretionary voltages can be
achieved for the selective recovery of metals and removal
of impurities. Moreover, the electrodes can be used in
bipolar cell systems for effective oxidation and
reduction in se?arated chambers for solute recovery and
electro-organic chemistry.
In the ensuing embodiments, electro-chemical
cells and processes are described utilizing the
electrodes of the present invention.
2~ A typical application of the electrodes 30
described ahove is shown in FIG, 10 which illustrates a
tank 52 used for an electrochemical process such as the
removal or recovery of metals from an electrolyte 54.
The electrodes which are used as anodes are identified
as 30A, while the electrodes used as cathodes are
identified as 30C. Anodes 30A and cathodes 30C are
arranged alternately in the tank 52 with flow-through
spacer panels 56 disposed intermediate adjacent anodes
30A and cathodes 30C. The tank 52 includes a plurality
3~ of cells, with each cell includinq one anode 30A,
one flow-through spacer panel 56 and one cathode 30C.
~r~r~ 3~ r
- 20
o
The anodes 30A and cathodes 30C are electrically
connected to a power source 58 by standard circuitry as
sho~n in FIG. 10. The voltage differential provided by
the power source i5 a function of the current/voltage
relationship for the particular electrolytic solutions.
The preferred voltage would correspond to the appropriate
"knee" in the current/voltage curve for the particular
metal which is to be removed or recovered.
Each cell in tank 52 is defined by a pair of
divider panels 60 as shown in FIG. 11. Each panel 60
is formed from an inert see-through material such as
polymethyl methacrylate and includes a plurality of holes
62 adjacent one corner of panel 60 and illustratively
arranged in a vertical row. Preferably the total area of
holes 62 is about 50% greater than the area of outflow
conduit 66. The holes 62 can be about 0.625 inch in
diameter and are spaced approximately 0.5 inch apart.
The holes 62 are provided to enable the flow of electrolyte
2~ 54 from one cell to the next cell in the tank 52. More
particularly, the panels 60 are rotated 180 within the
plane of the panel 60. As a result, one panel 60 will
have holes 62 in a bottom corner, while the adjacent panel
60 will have holes 62 disposed in the opposite top corner.
In operation the electrolyte 54 is directed
into the tank 52 throu~h inflow conduit 64 which is
located adjacent the upper edge of the tank 52. The-
~electrolyte 54 initially enters an accumulation area
55 prior to passing through the holes 62 in the first
divider panel 60. This construction ensures the desired
flow pattern of electrolyte 54 into and through the first
cell. The accumulation area 55 also functions as a surge
averager and collects any sediment that may be in the
electrolyte 54. The el~ctrolyte 54 is ultimately
7.3~ ?~
-21 -
urged out of tank 52 through outflow conduit 66~ The
arrangement of holes 62 in panels 60 throughout the
tank 52 causes the electrolyte 54 to alternately flow
upwardly and downwardly and across from one cell to
the next. This general flow pattern of the electrolyte
54 throug~ tank 52 which is illustrated gra~hically by
the arrows 68 causes the electrolyte to cascade in
length and width relative to the tank 52 to maximize
residence time of the electrolyte in the tank 52 and
contact time with the electrodes 30,all to optimize
recovery or removal of the metal from the solution.
Thus, the construction of the anodes 30A and cathodes
30C as described above, provides an extremely large sur-
face area, while the construction of the tank assures
maximum contact of the electrolyte 54 with the anodes
30A and cathodes 30C.
The metal to be recovered or removed is plated
onto the cathode 30C. Periodically, therefore, it is
necessary to remove the cathodes 30C from the cell to
win the metal. This winning of recovered metal from the
cathode 30C typicallv can be accomplished by diaestion,
pyrometallurgv or by making the cathode anodic in a con-
centration cell.
The electrochemical and structural principles
described above can be incorporated into a system, as
shown in FIG. 13, for treatment of a process stream
which incorporates an electrochemical cell shown in
detail in ~IGS. 14 and 16. In this system, the process
stream is pumped into storage tank 70, and then is
directed into the multi-cell tank 72. The process stream
is denuded of metal in tank 72 and discharged through
conduit 98 to an accumulator 97. The effluent in accu-
mulator 97 is pumped by pump ~9 to the neutralizer tank
r
100 containing limestone, where it is neutralized andthen discharged as waste via conduit 101.
Referring to FIGS. 14-16, the process stream
containing a dilute acid solution of a metal, e-g. t
nickel, tin, lead, copper, etc., is directed throuah the
inflow conduit 76 into an accumulator 78. The illus-
trated tank 72 is rectangular and a divider panel 80
extends across its width at one end thereof to form a
chamber which serves as the accumulator 78. The divider
panel 80 between the accumulator 78 and the first cell
82A of the tank 72 includes a channel 84 which allows the
process stream 74 to flow into the upper portion of the
surge control area 86. More particularly, the surge
control area 86 is defined by a surge panel 88 which
extends thereacross at one end thereof above the level
of process stream 74 in the first cell 82A to a point
spaced from the bottom wall 90 of the bath 72. This
provides a bottom channel 91 through which the process
stream flows into the first cell 82A. The first cell
82A is provided with an alternating and repetitive array
comprising an anode 30A, a flow-throuah spacer panel 56,
` a cathode 30C and a second flow-through sDacer panel
56. This arrangement repeats itself such that each
cell 82A through 82D includes a plurality of alternating
anodes 30A and cathodes 30C. As shown in FIG. 15, the
anodes 30A and cathodes 30C are spaced from the bottom
wall 90 and supported on members 93 to allow for the col-
lection of sediment. As shown in FIG. 1', the anodes 30A
and cathodes 30C are connected to a variable power source
by standard circuitry, such as common bus bars. For
clarity, the electrical connectors are not shown in FIGS.
14-16. As already described, the voltage for the operation
of the system is selected to optimize the recovery or re-
moval of metals Erom the electrolyte 54.
~,73~ 7
- 23-
D
The cells 82A through 82D extend across the
tank 7Z parallel to t~e accumulator 78 and are separated
from one another by divider panels 94. Each divider
panel includes one or more holes 96 located in one corner
of the divider panel 94. As described above, the divider
panels 94 are alternately rotated 180 within their plane
such that the holes 96 are alternately in opposed top and
bottom corners. Thus~ the divider panel 94 between cells
82A and 82B has holes 96 located in the corner most
distant from the bottom wall 92 and the surge panel 88.
It follows that the divider panel 94 between cells 82B
and 82C is disposed in the corner nearest the bottom wall
92 and the surge panel 88. This particular construction
ensures an end-to-end flow pattern of the process stream
within each cell 82A through 82D along with either a top-
to-bottom flow pattern or a bottom-to-top flow pattern.
As previously described, this flow pattern optimizes
residence time of the process stream in the tank 72
while minimizing channeling. The process stream 74 is
ultimately removed from the bath 72 through the outflow
conduit 98 which is located near the bottom wall 9~ of
the bath 72.
The electrochemical cells and processes shown
in FIGS. 10-12 and 13-1~ are suited for the removal and
recovery of metals, including semiprecious and precious
metals, from process or waste streams to less than about
1.0 ppm. For example, the system of FIGS. 1~-16 can be used
to remove in a single pass about 50% of the nickel in a
process stream containing 30 ppm nickel flowing at the
rate of 5 gallons per minute in a 50 gallon multi cell
tank 72. To remove additional nickel the process can be
repeated until the nickel is reduced to a satisfactory
level for discharge. This can be done by recycling the
3~
_ 24-
I
process stream from the conduit 98 to the conduit 76 ~ia
conduit 103 before the stream is ultimately fed to the
neutralizer.
The described cells also can be used to dis-
associate the solution to render soluble salts, e.g.0
municipal waste, insoluble for filtration from the
effluent.
Furthermore, the subject electrodes can be
utilized in a discretionary cell, as shown in FIGS. 17
and 18 where the voltage is varied to a particular pre-
cise selected level for causing a desired metal to deposit
on the cathode while metals higher in the electromotive
series remain in solution. To accomplish this type of dis-
cretionary plating it is necessary to employ a cathode
having a large surface area. This objective is
achieved within a small space and with a small amount
of electrolyte by the discretionary cell 100 shown in
FIGS. 17 and 18 which employs a carbon fiber cathode 102
with a thin single wire anode 104 such as platinum.
The electrolyte 106 is directed into the discretionary
cell 100 through an inflow conduit 108 which is located
approximately centrally within the discretionary cell 100.
The inflow conduit 108 is mounted in a deflector socket110 and includes a plu~ality of holes 111 which cause
the electrolyte 106 to be dispersed uniformly about
the cell 100. The single wire anode 104 is wrapped
helically around the inflow conduit 108. The cathode 102
then is disposed concentrically around, but spaced from,
the anode 104. As a result of this construction, the
~ ~73~
- 25 -
electrolyte 106 flowing out of the inflow conduit 108
is urged past the anode 104 and through the cathode 102
to carry out the desired plating of the metals on the
cathode 102.
The cathode 102 used in the discretionary cell
100 is a nickel plated carbon fiber electrode. The
concentric mounting of the cathode 102 about the anode
104 is achieved hy uniformly winding the cathode 102
about a generally cylindrical plastic flow-through support
or grid 112. The flow-through support 112 can be formed,
for example, from a sturdy but flexible plastic screen
bent and secured into a cylindrical configuration. The
cathode 102 is secured on the support 112 by a porous outer
screen 114. The flow-through characteristics of the
support 112 and the screen 114 readily permits the flow
of electrolyte 106 through the cathode 102 and into
contact with the many nickel plated carbon fibers which
comprise the cathode 102.
Althouah the cathode 102 is shown as being
uniformly wound about the grid 112, it is understood
that the cathode 102 could be knitted into a cylindrical
configuration or woven into a mat which in turn would
be wrapped around the grid 112 or other flo~ through
structural support. Outflow conduit 116 also is provided
to remove the electrolyte 106 from the discretionary
cell 100. Typically, the discretionary cell 100 defines
a closed system with outlet 116 and inflow
conduit 108 being in communication with a common source
of electrolytic solution from which metal will be removed.
The discretionary cell 100 can be constructed to any
size. For example, cell 100 can be a small unit mounted
over a larger tank containing the electrolytic solution.
In a typical application 40K nickel plated tow cathode
102 was formed into a cylinder, as shown in FIG~ 17,
having a diameter of about four inches and a length
of about 12 inches. This cathode 102 provides a surface
area of about 100 squaxe feet, and can be mounted in a
discretionary cell with a volume of less than one gallon.
Alternatively much larger tanks can be constructed.
As described above, after a suitable amount of the metal
in the electrolyte 106 has been deposited on the cathode
102, the process is stopped temporarily to remove and/or
replace the cathode 10~ so that the metal deposited thereon
can be suitably removed where the metal is a contaminant
or recovered where the metal has value.
Turniny to FIGS. 19 and 20, this electro-chemical
cell includes a tank 122 which is substantially identical
to the tank 52 shown in FIG. 10. The tank 122 includes
a plurality of dividers 124 each of which includes a
plurality of apertures 126 through which the electrolyte
128 can pass. As described, the holes 126 are disposed
adjacent a corner of the panel 124, and the panels 124
are alternately rotated 180 through their plane~to
create the desired up-and-down and side-to-side flo~
pattern of the electrolyte 128 through and across the tank
122.
As previously described, the panels 124 se-parate
the various cells from one another, wherein cell 130 in-
cludes an anode 132 and a cathode 134 separated by a
flow-through spacer panel 136~ The anode 132 includes
a carbon fiber tow 138 wrapped around a flow-through
support 1~0. ~ore particularly, the carbon fiber tow 138
of each anode 132 is form~d from a plurality of carbon
fibers each of which is metal plated adjacent the electri-
cal connection, but is unplated or stripped of plating
more distant from the electrica~ connection. Thus, each
. ~ .
'3J 73~ ~
. - 27-
O
anode 132 is of substantially the same configuration as
the anode 30A described above~
As shown in FIG. ~.0, the cathodes 134 are flow-
through or porous metallic plates which include a pluralityof apertures 142~ In this embodiment the plate 134 is
stainless steel. The flow-through spacer panels 136
which are disposed intermediate each anode 132 and cathode
134 are substantially ide~tical to the already described
flow-through spacer panels 56.
Illustratively, the cell of this embodiment of
the invention can be used to remove and recover cyanide
and other alkaline electrolytes which contain metals,
such as silver, copper, zinc, cadium and tin.
Another embodiment of the invention which is
particularly useful in electro-organic chemistry and
synthesis and in the treatment of organic residues is
illustrated in FIGS. 21 and 22. Referring to FTG. 21,
the cell includes a tank 150 divided by a porous membrane
152 into two chambers for t~o distinct electrolyte solu-
tions 154 and 156. The cell also includes an anode 15
and a cathode 160 which are substantially identical to
the already described anodes and cathodes 30A and 30C.
The anode 158 and cathode 160 are passive electrodes
which are electrically connected to one another at point
164, but are not electrically connected to an outside
power source, thus becoming bipolar. The anode 158 and
cathode 160 preferably are separated from one another
by the porous membrane 152 so that the anode 160 is in the
chamber containina the electrolyte 154 and the cathode
is in the chamber containing the electrolyte 156. The
membrane 152 includes a hollow support 161 about which is
secured a porous member 168, such as canvas. As shown,
3 . ~ 3 ~ r
- 28-
the membrane 152 is filled with solution 165, which can
be neutral. The active,electrodes for the cell are the
plate cathode 166 in the electrolyte 154 and the pla~e
anode 168 in the electrolyte 156~ As stated, the
electrolyte solutions 154 and 156 are distinct, one of
which is acidic and the other one of which is basic.
The solution 154 is oxidized at the anode 158 while the
solution 156 is reduced at the cathode 160 without the
fiber electrodes polarizing~ In practice the porous
membrane 152 need not be ion selective. Consequently,
the membrane 152 is relatively lnexpensive and does not
require high electrical energy.
.
The bipolar cell is particularly well suited
for use where the anolyte and catholyte are to be kept
separate, where oxidation or xeduction in either side of
the cell may be ionic, or where a polarized electrode
is desired.
The invention in its broader aspects is not
limited to the specific described embodiments and depar-
tures may be made therefrom wlthin the scope of the
accompanying claims without departina from the principles
of the invention and without sacrificing its chief
advantages.