Note: Descriptions are shown in the official language in which they were submitted.
3~3~
.~
- 1 - 21489-6789
This is a divisional application of application Serial
No. 490,301 filed September 10, 1985.
The parent application relates to a novel process for
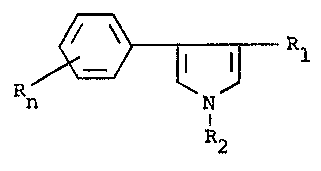
the preparation of 4-phenylpyrrole derivatives of the formula I
Rn \ ~ (I)
N
R2
wherein Rl is CN, CHO or COO(Cl-C6)alkyl, R2 is hydrogen, CH2CH2CN
or CH2CH2COO(Cl-C6)alkyl, R is halogen, Cl-C6alkyl or Cl-C6halo-
alkyl, and n is 0, 1 or 2.
This application relates to novel compounds of formula
Ia
n ~` ` ~ R1 (Ia)
Ni
R2
wherein R is halogen, Cl-C6 alkyl or Cl-C6haloalkyl, n is 0,1 or 2,
and,where Rl is CHO or COO(Cl-C6)alkyl, R2 is at the same time
hydrogen, CH2CH2CN or CH2CH2COO(Cl-C6)alkyl, or, where Rl is CN,
R2 is at the same time CH2CH2CN or CH2CH2COO~Cl-C6)alkyl.
It should be noted that in this application the -term
"invention" includes the subject matter of both the parent and
the divisional applications.
Depending on the indicated number of carbon atoms,
alkyl by itself or as moiety of another substituent, such as halo-
~q,~
3~
- la - 214~9-6789F
alkyl and the like,comprises e.g. the following straight chain
or branched groups: methyl, ethyl, propyl, butyl, pentyl, hexyl,
etc., and the isomers thereof, e.g. isopropyl, isobutyl, tert-
butyl, isopentyl etc. Throughout this specification, a sub-
stituent prefixed by "halo" will be understood as meaning that
said substituent may be monohalogenated or perhalogenated.
Halogen and halo signify in particular fluorine, chlorine or
bromine. Hence haloalkyl denotes a monohalogenated to perhalo-
genated alkyl radical, e.g. C~Cl2, CH2F, CCl3, CH2Cl, CHF2,
CH2CH2Br, C2Cl5, CHBr, CHBrCl etc., with CF3 being preferred.
'73~3~i
-- 2 --
4-Phenylpyrrole deriv~tive~ of formula I, wherein n 18 O, 1 or 2,
R~ 19 cyano and R2 18 hydrogen or acatyl, ar~ known a~ plant
fungicldes from Germsn Offenlegungsschrift 29 2~ 480 ). AB will be
shown below, co~pound~3 o~ formula I, wherein R~ i~ CHO or COO(Cl-C6)-
alkyl or Rz ia CHzCH2CN or CH2CH2COO(Cl-C6)alkyl, can be converted
in ~imple mann0r into the known funglcidal 4-phenyl-3-cyanopyrrola~
~nd thus have the character of intermedlatea.
A process for the preparation of 4-phenyl-3-cyanopyrrole derlvative6
which iB known irom Tetrahedron Lett~rs No. 52, pp. 5337-5340,
19722) 9 iB disclosed in German Off~nlegungsschrift 29 27 480 ). In
thl~ process, known as the To~MIC proc~s~, a cinna~lc acid darl-
vatlve of for~ula X
(X) ~ /--CH~CH CN
n _ CH3~ SOzN ~ ~o-l - il-CN
...
(XX) CH3--~ SOzCH2NC
18 cycll~ed with to~yl methyl l~ocyanide (XX) [To~MIC], ln th0
pre~enc0 of a Dtrong base, a.g. sodlum hydrlde, to giv0 4-phenyl-
3-cyanopyrrol0 d~rlvatlvea of for~ula (XXX). In the above formulae,
R lu a9 deflned for formula I ant n i8 O, 1 or 2.
Although numerous pyrrola ~yntheaes sre known (q.v. J.M. Patter30n,
Synthesl~ 1976, pp. 281-3043)), only the To0MIC proce~a outlined
above ha~ 60 far led direct to the fungicidally u~eful 4-phenyl-
3-cyanopyrrole derlvatlve~. Howev~r, referenc~ 2) lndlcate~ for tha
preparctlon of 4~phenyl-3-cyanopyrrole 8 yl~ld of only 35 70, which
i~ low for industrial purposss. It has been found th~t the r~ag~nt
To~MIC ha~ grave di~3advantage~ for lnductrial ~ynth~se~. For
example, at elavated temperatures above 90C (normal drying con
dltlons), TosMIC ha~ the propensity to d~compo~e ~xplo~lvely. On ths
.~ - 3 -
othe~ hand, resldu~l moisture consumes 30me of the baae smployed
(danger of hydrolysis/reduction in yiold). Further, To0MIC ha~
physlological ha~ardfl and cau~e~ ~avere irritatlon to the eye~ and
- akin.
The ~hortcoming~ referred to above ~how that u~eful laboratory
~ethods are un~ultable for the indu~trial production of 4~phanyl-
pyrrole derivatl~e~. A novel, more econom~c and envlronmentally more
acceptable proce~s for the prap~ration of these compounds ln
su~prlsingly hlgh yleld ha~ now been found.
The novel process of thla lnvention for the praparatlon of tha
4-phenylpyrrolq dsrivstives of the formula I as defined at the
out~et comprl~os ~eacting B phenacylamine of formula II
~ CHz-~N (II)
R 2
in the form of ~n acid addition ~alt, wlth a compound of formula III
T-CH~CH-Rl tIII~
to give an intermediate of for~ula IV
~ CH2-~-CH-Ch-Rl ~IY)
R ~- 2
and cycllslng thls compound of formuls IV, in the prasanca af a
bas0, to a compound of for~ula I. In the formula~ II, III and IV
abovn, the ~ubatituent~ Rl, R2 and Rn ~re a~ defin~d for formula I,
T iB a group selected from -OZ, -N(R3?(R4), -OCORa, -OSO2~ , -SRC or
halogen, whera Z iB C~-C6alkyl, unsubstituted or ~ubatituted phenyl,
an alkali metal atom or an alkaline earth metal atom, each of R and
independently of the other i8 Cl-C6elkyl or un~ub~tituted or
~ubatltuted phenyl, R~ iB Cl-C3alkyl, Cl-C~haloalkyl or un~ub-
~titut~d or ~ubatltuted phenyl and each of Rl and R4 i~d~pendently
~ 3~
.
- 4 -
of ths other ld Cl~C6alkyl or, togsther wlth the nmine nltrogen
atom, form a saturated 5- or 6-memb0red heterocycllc rlng which
contalns, as hetaro atom, elthsr only the amine nltrogen ato~ or a
f~rther hstero atem.
An unsubutltuted or ~ubstituted ph0nyl group i8 in partlcular ph0nyl
or phenyl whlch 1~ sub~tltuted in the para-posltlon by halogen,
preferably chlorine or bromine, and by Cl-C3alkyl, prefRrably
methyl. Alkali metal atoms or alkaline earth metal atoms may be Li,
Na and K, preferably Na and K, or Mg, Ca, Sr and Ba, preferably Hg,
Ca and Ba. Where the -N(R3)(R4~ group d~notas a ~aturated 5- or
6-membered heterocyclic ring containlng N as hetero atom or a
further hetero atom, said rlng may be selected from the following
heterocycllc rlng system6: pysrolldine, piperazine, perhydro-
thiazine, morpholine, pip0ra~ine, oxa~olidine, thlazolldine,
imidazolldina, pyrazoline and the like. A further hstero atom i~
preferably N, O or S.
In the process o thi~ invantlon lt 18 not necessary to lsolatQ the
intermediate (IV) first and th2n to cycli~e ~t to compounds o
:Eormula I. To the contrary, the reactlon of (II) wlth (III) may al~o
be carrled out direct in tho precence of a baae, utlll~l~g a ~lngle
reaction ve~sel fer both steps, to give the flnal productA. In thls
procedure, ~he intermedlate (IV~ i~ further proce~ed direct without
isolation. On th0 other hand, it may be convenient to prapare the
intermedlate (IV) first ln sspeclally pure form, e.g. by repeated
r0crystalllsatlon, and then to cycli~e lt to ~ compound oE formula
I. A preferred embodiment of th0 process of thi~ invention accord-
ingly comprises reacting the phenacylamine II ln the for~ of an
acld addltlon salt, in the presence of a ba~e, dir~ct wlth
compound of for~ula III to give the flnal product I.
The ~econd preferred e~bodiment of the proce~ cD~prises flr~t
reacting the phenacyla~ine II ln the form of an acid addition ~alt,
ln the absence of a base, to glve the intermedlate (IY) and then
converting (IV) to (I) by cyclisstion ln the preaenc~ of a ba~e.
~ ~363~:i
. .
- 5 -
The reHcesnts (II), (III) and, where sppropriAte, tIV). are con-
venlently e~ployed in 0qulmolar ~mount~. It i9 preferred to add an
equlmolar amount or sn eXCQB8 of b&~e.
Typlcal representatiVQ~ of th0 compounds of formula III, the 113t of
which 18 not axhau~elve~ are the following compounds a) to t), of
which compound~ a) to 1) arQ pArticularly adv~ntsgeou~ snd therefore
preferret:
g) (CB3)2N-CH~CH-CN
b) (CZHs)2N-cHucH-cN
._ .
c) ~ CH~CH-CN
._~
.
d) I ~ -CH~CH-CN
._.
._ .
e3 O~ ~ -CH-CH-CN
f) NaO-CH~CH-CN
8) Ko-c~cH-cN
h) (CH3)2N-CH'CH-COOCH3
i) (C2Hs)2N-CH~CH-COOCH3
k) (CH3)2N-CHnCH-CHO
1) (C2Hs)2 N~CH~CH-CHO
~) Cl-CH~CH-CN
n) Cl-CH~CH-COOCH3
o) CH3O2SO-CH~CH-CN
p) [c6H4cH3(4)]-cH~cH-cN
q) CH30-CH~CH-CN
r) C2HsO-CH~CH-CN
8) C3H70-CH~CH-CN
t) ~ C6H4C1( 4) ¦O-CH.CH-COOCH3
3~
- 6 -
The process of thl~ lnventlon 18 convaniently car~l~d out ln an
inert solvent or mlxtur~ of ~olventa. Thus one or ~o~o inert
solvonts or diluents m~y ba employed. Examples of sultable ~olve~ts
and diluents are: allphatic and arom~tlc hydrocarbona such as
benzene, toluene, xylenes, petroleum ether; halogenated hydroc~rbons
such a~ chlorobenzene, methylene chlorlde, ethylene chlorlds,
chloroform, carbon tetrachloride, tetrachloroethylena 2thers snd
ethere~l co~pounds ~uch a8 dialkyl ether~ (dlethyl a~her, dliso-
propyl ethsr, tert-butylMethyl ether etc.), ani~ole, dioxane,
tetrahydrofuran; nltriles such a~ acetonitrllo and propionltrile;
N,N-dlalkylated amldes such as dimathylformamlde; dimsthylsulfoxlde;
ketones such as acetone, dlethyl ketone, methyl ethyl ketone;
alcohola, in partlcular methanol, sthanol, propanols, butdnols and
the llke; and water and aqueous two-phasQ mlxtures and mlxtures of
the above solvents.
The followlng solvents for example ars sultabl~ for the organlc
water lm~lscible phase: sliphatlc and sromatic hydrocarbons such as
pentana, xylenes etc.; halogenated hydrocarbonn such as dichloro-
methane, chloroform, carbon tetrachlorlda, ethylene dichloride,
l,2-dlchloroethane, tetrachloroethylena ~nd tlle llke, or allphatic
ethers ~uch a~ diethyl ether, diisopropyl ether, tert-butylmethyl
ether and tha like. The addltion of a phase tranafer cataly~t may be
advantageous. Example~ of suitflble phase transfer catalysts are:
tetr~alkylammonlum halides, hydrogen sulfate~ or hydroxides, e.g.
tetrabutylammonlum chloride, tetrabutylammonlum bromids, tetrabutyl
a~monium lodide, triethylbenzylammonlum chloride or triethylben~yl-
ammonluM bromide, tetrapropylsmmonium chloride, tetrapropylammonium
bromlde or tetrapropylammonium iodlda etc. Suitabl~ phase transfer
catalysts are also phosphonium salts. The ammonium salt of for-
mula II ltself act~ a0 phase tran0fer catalyat.
Particularly suitable solventa are nitrilea and lowar alkanols,
preferably acetonitrlle and eth~nol, aR w~ll aa mlxture~ of
alkanol/water (ethanol/water).
~ ~ ~73~3~i
-- 7 --
In &11 partlQl step~ snd ln the single ve~3sel reaction, the reaction
temperatures are generally ln thc range from o9 to +120C, pre-
fesably ~rom +30 to ~80C.
Owlng to the reduced thermal ~tabllity of the ~tarting phenacyl-
amlne, the compound of formula II ls employed in the form of it~
more stable ammonium t3alt, which can be obtained by conventional
additlon of ~n organic or inorganic acid to the free amin0.
ExAmples of ealt-forming aclda ara inorganlc acld~, e.g. hydrohalic
scids i3uch 8~ hydrofluoric acid, hydrochloric acid, hydrobromic ncld
or hydriodic acid, as well as aulfurlc acld, phosphorlc acid,
phosphoroufl ~cld, nitric acid and the like; and orgsnlc acids such
a8 acetic acid, trifluoroacetic acid, trlchloroacstic ~eid, pro-
pionlc acid, glycollic acid, lactic acid, 3uccinic acld, benzoic
acid, cinnamic acid, oxalic acid, formic aoid, benzenesulfo~ic acld,
p-toluenesulfonic acid, methanesulfonic acld, salicyclic acid,
2-phenoxybenzoic acid or 2-acetoxybenzoic acld and the like.
Preferred ~alt-forming acids are ~trong acid~ ~uch as the hydrohallc
acids, phosphoric scid, nitric acld; and the ~ulfonic acid~ such a~
p-toluene~uleonic acld. Nydrochlorlc acid i8 eBpeCially pr0ferred.
The reactlon of (II) with (III) direct to ~lve (I), or of (IV) to
give (I), i~ conducted in tha presence of a base. Example~ of
suitable bases are lnorganic base~ ~uch a~ the oxldes, hydrid~a,
hydroxides, carbonste~, carboxylic acid salt~ and alcoholatss of
alkaline earth metals, preferably o~ alkall ma~als, in p~r~icular of
~odium and potas~ium [e.g. NsH, NaOH, KOH, Na2CO3, KzCO3~ CaC03,
CH3COONa, C2HsCOOK, C2HsONa, CH30Na and the lik~], preferably the
alkali metal alcoholates such ~3 sodium ethylate or sodlum
methylste. Suitable organic ba~e~3 are e.g. triethylamina,
plperidine, pyridlne, 4-dimethylaminopyridine and the like.
~. ~7~
In the proce~seD of thls invent~on, intermediatea and final producta
~ay be iflolated from the raaction medlum and, if desired, purifled
by one of the commonly employed mothods, for examp}e by oxtraction,
crystalll~ation, chromato~raphy, distillation and the like. However,
the preparation o ~he compounds of formula I can be carried out
generally ln good yield and in excellent purlty utlli~ing a s1ngle
ve~el for both reaction step~ without isolation of lntermedistes.
Proferred embodimenta of tho procesn of thi~ lnventlon ~re e.g.
those whlch co~prise:
a) the u~e o~ atartlng materlala of fDrmula II, wherein R i8
hslogen, prefarably fluorine, chlorino or bromlne, most pre-
ferably chlorine, n i8 1 or pref~rably 2, with the proviso that,
if n is 2, the orti)o- and meta-positions are partlcularly
preferred, Rl 1~ CN and R2 is hydrogen;
b) the usa of reagents of formula III, wherein T i~ a group selected
from -N(CH3)2, -N(CzHs)z,
0 , -OK or -ONa,
snd R~ 1~ CN, COOCH3 or CHO, preferably CN;
c) the u~e of intermediates of formula IV, whorein Rl t R2 and Rn are
~8 dafin~d in a) and b) abova;
d) the u~e of acld addition salts of formula II, which contaln, as
acid component, 8 hydrohalic acid, preferably hydrochloric acid,
a sulfonlc acid~ preferably bcnzenesulfonic or p-tolusnesulfon1c
acld, or sulfuric acid;
e~ carrying out the reaction of (II~ with (III) ~uch that the
intermediate IV is further proce~aed direct without iflolation;
~;~7
f) carry~ng out the procea~ in the tempesHture range from ~30 to
+8~C
Accordlngly, a particularly preferred embodlment of the proce~ of
the invention comprl~es reactlng 2,3-dlchlorophenacylamine ln the
form of sn acid addltion salt, prsferably in the form of the
hydrochloride, wlth ~ compound of formula III, wherein Rl i9 CN and
T 1D ~ group ~elacted from -N(CHI)2,
-N(C2Hs)z, ~ ~ 0~ or -ONa,
~ O
preferably -N(CH3)2, ~ ~ or ~ ~0
to give 3-(2,3-dlchlorophenacylamino)acrylonltr~le, and cycli~ing
this intarmadiaee~ either as ~ubBtance or preferably in situ, in the
presence of a ba~a, preferably of ~ lower alkanolate, ~odlum
hydroxlde, potas~lum hydroxide, ~odium scetate, pota~sium acetate or
a tri-lowQr slkylamine, to give 4-(2,3-dichlorophenyl)-3-cyano-
pyrrole.
Most of the starting material~ of formula II are known or can be
prepared ln ~imilar manner to the known representative~. However,
2,3-dlchlorophenacylamlne and the acld addition ~alt~ thereof are
novel. In vlew of its structure, thls compound is dsstlned for u~e
a~ intermediate for the preparation of fungicldally actiYa 4-(2,3-
dichlorophenyl)-3-cyanopyrrol~ and therefore constitute~ an ob~ect
of thi~ invention. Its preparatlon will be descrlbed e~pllcitly
below.
Compounds of formula II, wher~ln R2 1~ CH2CH2CN or CH2CH2COO(Cl-Cs)-
alkyl, can be prepared e.g. a~ follows from the startin~ phenecyl-
amlnes (II) (~z ~ H): The acld addition ~alt (e.g. the HCl salt)of
an N-sub~tltuted phenacylamins of formula II i~ reect~d, in the
~t73~
-- 10 --
presenCQ of an oquimolar amount o acrylonltrile or o~ n Cl-C6alkyl
ester of acryllc acid, preferably in the preaenco of on~ of the
ba~ peclfied above and under the cond~tlon~ for the reactlon of
(II~, with (III) to giva (I).
Wlthin the ~cope of the present lnventlon~ typlcal rapro~ent~tive~
of compounds of formula I ara for example the compounds li~ted ln
Table 1.
Table 1: Compoùnds of for~ula II
~ -CH2-NH-R2 (II)
R~
Cu:poand Rn .
1.2 3-Cl H
1.3 2,4-C12 H
1.4 4-Cl
1.5 4-F H
1.6 3-CH3 H
l.7 3-~ H
1.8 3-Br N
1.9 3-C~3 H
1.10 2-Cl H
1 ~ 1 1 2 1 3 C1 2
1.12 2,5-C12
1.13 2-Br H
1.14 2,6-C12 H
1.15 H CH2CH2CN
1.16 3-Cl CH2CH2CN
1.17 2 Cl CH2CH2CN
~.~73~
-- 11 ~
Table 1 (continustlon)
Corpoond K . _ _
_ . n _ __ ~_
1.18 2,3-C12C~IzCH~CN
1.19 3-F CHzCH2CN
1.20 3-ClCHzCH~COOCH3
1.21 2,3-ClzCN2CH2COOCH3
1.22 2-ClCH2CH2COOCH3
1.23 2,3-C12CH2CH2COOC2Hs
1.24 2,3-C12CH2CHzCOOC3H7
1. 25 2-Br /CN~CNzCOOCN~
The compounds of formula III are in general co~mercially available
and thua known sub~tances or compounds which can be prepared ln
similar mannar to their knDwn rapresentAtives.
The preparatlon of the intsrmediates of formula IV 1~ an obJect of
the prasent invention and ha~ been described in datail abovs. These
intermediates IV can be converted by slmpls bRslc cycllGatlon into
the u~eful fungicide~ of formula I, have the~selve~ fungicidal
actlvity, and accordingly constltute an es~ential ob~ect of the
prssont inv~ntlon.
Within the ~cope of thi~ invention, typical representativea o~
intermediates of formula IY are:
Table 2: Compounds of the formula
~-cH~c~-Rl (IV)
cn- 2
, ~
Compound R Rz R
n _ _ . .
2.1 . H H CN
2.2 3-Cl H CN
_ ~ .. . ~ __
73~ 6
2 -
Table 2: (contin~ation)
Compound Rn R2 .
2.3 2,4-Cl H CN
2.4 4~Cl H CN
2.5 4~F H CN
2.6 3-CH3 H CN
2.7 3-F N CN
2.8 3-Br H CN
2.9 3-CF3 . H CN
2.10 2-Cl H CN
2.11 2,3-C12 H CN
2.12 2,5-C12 H CN
2.13 2-Br H CN
2.14 2,6-C12 H CN
2.15 2,3-C12 H COOCH3
2.16 H H CHO
2.17 3-Cl H COOCH 3
2.18 3,4-Clz H COOCH3
2.19 2-Cl H COOCH3
2.20 2,3-C12 H COOC3H7
2.21 2,3-Clz CH2CH2COOCH3 CN
2.22 2,3-C12 CH2CH2CN CN
2.23 H CH2CH2CN CN
2.24 3-Cl CHzCH2CN CN
2.25 2 Cl CHzCH2CN CN
2.26 2,3-C12 CN2CH2COOC2Hs CN
2.27 3-F CHzCHzCN CN
2.28 3 Cl CR2CH2COOCH3 CN
2.29 2-Cl CH2CH2CO0CH3 CN
2.30 2,3-C12 CH2CH2COOC3H7 CN
2.31 2-Br CHzCH2COOCH3 CN
2 . 3Z 2,3-C12 CH2CH2CN C30
3 ~73fj~,
- l3 -
A~ mentloned at the outset, ~omc of the compounds of formulA I hAve
the character of intermediates. These compounds are the represen-
tatlves of formula I herelnaftar referred to ~B subgroup Ia, wher~ln
Rn 18 as definod for formula I; and in tho~e compounda ln whlch Rl
i8 CHO or COO~C~-G6)alkyl~ Rz 18 at the sama time hydrogen, CH2CH2CN
or CH2CHzCOO(Cl-C6)alkyl~ or ln those compounds in which R~ i~ CN,
R2 i9 at the sam~ time CH2CH2CN or CHzcH2coo(cl-cs)alkyl~ These
novel pyrrole derivatives, whieh al~o hsve fungicidal properties,
can be converted ~n simpla manner into the fungicidal 4-phenyl-3-
cyanopyrroles known from German O~f~nlegung~chrlft 29 27 430, a8
CHO and COO(Cl-C6)alkyl can be conv~rted into CN, and CH2CH2CN and
CH2CN2COO(Cl-C6)alkyl as substituents at the pyrrole nitrogen atom
~re ca9~ ly removable groups. On account of these Advantageous
propertles, the compounds of ~ubgroup IA con~tltute a further ob~ect
of the pre~ent invention.
Typical examples of compounds of subgroup Ia ar~ listed b~low.
Table 3: Compounds of formula Ia
~ Il~Rl (Ia)
Compound Rn Rl R2
. .. . ~
3.1 H CHO H
3.2 3-Cl CHO H
3.3 2,4-C12 CHO H
3.4 4-Cl CHO H
3.5 4-F CHO H
3.6 3-CH3 CHO
3.7 3-F CHO H
3.8 3-CY3 C~O H
3.9 2,3-C12 CNO _ _
~ ~ ~73~
.
- 14 -
Table 3 (continuatlon)
Compound R ~1 RZ
3.10 2,6-C12 CHO H
3.12 3-Cl COOCH3
3.13 2-Cl COOCH3 H
3.14 4-F COOCH3 H
3.15 2,3 Cl2 COOCH3 H
3.16 3-Cl C~ CHzCH2CN
3.17 2-Cl CN CH2CH2CN
3.18 3-CH3 CN CHzCH2CN
3.19 2,3-C12 CN CH2CH2CN
3.20 4-F CN CH2CH2COOCH3
3.21 2-Cl CN CH2CH2COOCH3
. 3.22 2,3-C12 CN CH2CH2COOCH3
3.23 2~3-Clz CHO CH2CH~COOCH3
3.2b 2,3-C12 COOCH3 CH2CH2CN
The conver~lon of CHO into CN can be effected ln a mann~r known per
~e, for example aR follows: An aldehyde of formula I (Rl - CHO) 1~
oonverted at 0 to 100C~ ln an lnert aolvent (e.g. an clcohol, an
ether, pyrldln~, trlethylamine and the like) lnto the corresponding
oxlm0 (0yn/nntl mixture), which i~ converted into the nitrile by
treatment with 8 dehydrating agent (e.g. acstic anhydride, cyanurlc
chlorlde/pyridlne, (PNCl2)3, dicyclohexyldlcarbodllmldelCuCl2/trieth-
yla~ine, P20s, tosyl chloride/pyridine, TlCl4/pyridine ~nd the
like).
If it l~ da~ired to convert the ester group COO(C1-C6)alkyl into the
CN group, a start i~ best made from the free acid, whlch l~ prepsred .
ln a manner known per se by e~ter hydroly~i~ with an aqueou~ mineral
acid (e.g. HCl/H20), in the presence of a colublli~er (e.g. alcohol,
dioxane, tetrahydrofuran and the like), mo~t convenlently under
reflux temperature. The frea acid ic then convarted lnto the acld
smide either direct with ammonla ~t elevatad temparatur~ or via the
~ 73~
..
- 15 - 214~9-6789
acld chloride ~-COOH + thlonyl chloridQ ~ -COCl) wlth a~monle ~t
room t~mper~ture, and the acld amlde i8 conver~ed to th~ nitrils
with on0 of the prevlously mentloned dehydrating ~gent~ in the
~emperaturo r~n8e from 80~ to 220C.
I~ it 1~ deslred to form the frea pyrrole by removal of tha CH2CH2CN
or CH2CHzCOO(Cl-C6)alkyl radlcal, thls may bs done e.g. by treatment
wlth ~ ba~e ln ~he temparatura r~nge from -20 to ~180C, in R
~uitable lnert ~olvent. Exempl~ry of ~uitable reaction conditions
nre:
A) Bodlum hydrlde in dimethylormamide at 0C
b) ammonlalwater/dioxane At 180C
c) pota~ium hydroxlde/water/alcohol at 100C.
PreParstory ~Axamples
Example P1: Prepnretlon of
B-CH2-NH2 HCl bnse ~., ~ ll li CN
(CH~)2N-CH~CH-CN
4-(2,3-Dlchlorphenyl)-3-cynnopYrrole
a) Prepar~tlon of the precureor:
N-Acetyl-2,3-dichlorophenacylemlna
150 g of 2,3-dlchloroben~oyl cyanide aro hydrogenated with elament-
al hydrogen under normal prassuro at 70C in 1.5 ~ of glacial acetic
~cld and 84.15 g o~ acetlc anhydrlde o~or 5 g of PtO2. Aftcr
sbsorptlon of 112 % of tha c~lcul~tst amount of hydrogfln (~ime
taken: c. 5 hours), the hydrogon~tion 18 di~continued, tho rs~ction
m~xtur~ 13 flltered ~nd tha flltrete is concentrat~d by e~por~tlon.
The re~idual yellow oll 1~ cry~t~ ed by sddition of hexane/
diethyl ethar. The cry~talline product i8 i~olated by filtratlon ~nd
~ ~3~
- 16 -
drled. M.p. 107-109C. IR (~olld~XBr) in cm ; 3300 (NH); 1735 (C0);
16S0 ~C0). IH-NMR (CDCl3) ln pp~: 2.08 (s,3H); 4.55 (d,2H); 6.2-6.6
(broad 0,lH); 7.25 (m,3H).
b) Preparation of the precur~or:
2.3-dichlorophenacYlamlne h~drochlorlde
50.0 g of the N-acetyl-2,3-dichlorophenscylamine obtained ln a) ar~
heated for 2 hous3 under reflux ln S00 ml of hydrochloric acld. The
~lightly turbid resction flolution ia concentrated by eVaporAtion and
the resldue 18 digested wlth ethyl acetateO The crystalllne 2,3-di-
chlorophenacylamine hydrochloride i~ isolated by filtratlon and
drled. Malting point: 217-218C. IR (~olld/KBr) ln cm : 1695 (C0).
(Another crystsl modificstion 3how~ two carbonyl resonance bands at
1690 and 1705 cm 1) ~H-NMR (DMS0, d6) ln ppm: 4.54 (8, 2H); 7.6 (t,
lH); 7.9 (~, 2H); 8.6 (8, 3H, r~placeable ~lth D20).
c) Preparatlon of the final product
4-(2,3-dichlorophenyl)-3-cyanopyrrole
20.0 g of 2,3-dlchlorophenacylamine hydrochloride and 10.0 g of
3-dimethyla~lnoacrylonitrlle are heated for 1 hour under reflux in
300 ml of ethanol. Then an ethanolic solution of sodium ethylate,
prepared from 2.1 g of sodlum and 30 ml of ethanol, i~ rapidly added
dropwlse and the reactlon mlxture i8 stirred or another 10 minut0~
under reflux. The reactlo~ mixture 1B cooled to room temporature and
then poured into ice/hydrochlorlc acld and the resultant mlxture 18
~tirred for 1 112 hours. The precipitata is lsolated by filtratlon,
washed with water and dried, affordlng 1S.4 g (78 % of theory) of
title compound with a melting polnt of 152-154C.
~73
7 -
Examples P2 to P4: Pr0paration of
P2: O~ ~ -CH~CH-SN C~ ~Cl Cl
`. . + .~ 9. -CH2-NH2 HCl C~ ,~
P3: ¦ ~ -CH~CH-CN ba~e ) ~ ~~ -CN
P4: ~ ~ -CH~CH-CN
4-(2.3-Dichloro~henyl)-3-cYan~role
Followlng the proc0dure de~crlbcd in Exampla Plc), but replacing
3-dimethylamlnoacrylonltrlle by
N-plperldinyl~crylonltrile,
- N-pyrsolldlnylacrylonitrile, or
N-morpholinylscrylonltrile,
and increa~ing the reaction tlme from 1 hour to 3 to 4 hours, pure
4-(2,3-dlchlorophenyl)-3-cyanopyrrole i~ obtained in ~11 thre0
Exa~plec in yield~ ranging from 76 to 85 % of theory. M~l~ing point:
150-154C.
Exam~le P5: Praparatlon of
~ H-CH~CH-CN b~ C~
4-(2,3-dlchlorophenyl?-3-cYanopvrrole
a) Preparation of the intermediate:
3-~2,3-dlchlorophenacylamino~acr~lonitrile
20.0 g of 2,3-dichlorophenacylamine hydrochlorids and 10.0 g of
3-dimeehylaminoacrylonitrile ~re heated for 1 hour under reflux ln
300 ml of ethanol. After cooling it to roo~ temperature, the
reaction solutinn i~ pourad into a mixtura of ico/d~lute hydrochlor-
3~i~3
- 18 -
lc acld. Aft~r extractlon with sehyl acetate, the combined extract~
are drled over sodium aulfate, flltered, and the filtrate ia
concentrated, Tha olly roDldue ia purlf~ed by column chrom~tography
(~illca gel: olutlon with ~ 4:1 mixture of toluena/ethyl acotate).
M.p. 125-127C. I~ (solld/X~r) in cm : 3380 (NH), 2200 (CN),
1715 (CO) 1625 (C~C). IH-NMR (DMSOd6) in ppm: 4.09 (d, J - 15 H~,
1H~; 4.46 (d, J ~ 7 H~, 2H); 7.2 (q, lH); 7.4 (broad ~,lH~;
7.45-7.85 (m, 3H). MaD~ Bpectrum: ~olecular peB~ at 254.
b) Preparation of the final product
4-2,3-dlchloroehenyl)-3-cyanoeY~rrole
~o 4.2 g of the 3-(2,3-dlchlorophenacylamino)acrylonitrile obtained
ln a) lc added 0.5 g of ~odl~m ethyl~te in 50 ml of ethanol. The
reactlon mixture i~ heated to reflux temperature, cool~d to room
tomperature, pourod into a mxture of dllute hydrochlorlc ~cld and
lca, and ~tlrred for c. 1 hour. The preclpltate i8 i~olat~d by
filtration, ~a~hed wlth water and dried, sffordlng the title
compound in quantitatlv~ yleld. Meltlng point: 149-150C.
ExamPle P6: (Formulae, ~ee Ex. PS)
a) Preparation of the intermedlate
3-(2.3-dlchloroPhenncvlamlno)acrylonitrile
2 g of 2,3-dlchlorophenacylamlne hydrochlorlde, 1 g of 3-hydroxyacrylo-
nitrlle, ~odium salt, and 20 ml of ~thnnol sre heated for 2 hour~
undar rsflux. The reaction mlxture l~ concentrated by svaporatlon
and the olly r0~idue 1~ purlfled by column chromatography (~lllca
gel; elutlon wlth 8 4:1 mlxtur~ of toluene/ethyl ac0tato~, affordlng
3-(2,3-dlchlorophenacylamino)acrylonitrlle in the ci~/trans ratio of
S:l. Meltlng polnt: 122-125C.
b) Preparation of the final product
4-12,3-dlchlorophenyl)-3-cyanopyrrole
4.2 g of the 3-(2,3-dlchlorophenacylsmino~acrylonltrlle obtaln~d in
~) are reacted ln 50 ml of ethanol wlth 0.5 g of sodium ethyl3te a8
de~cribed ln E~ample P5 b), affording the tltle compount in qusn-
titatlve yleld. Meltlng point: 150-1S2C.
~ ~73~
. . .
-- 19 -
Example P7: Preparatlon of
~ -COOCH3 --~ -COOH - ~ ~ ~ CN
4-(2~3-dichlorophenyl~-3-cyanopyrrole
a) Preparation of 3-carbomathoxy-4-(2,3-dlchlorophenyl~pyrrole
10.7 g of 2,3-dichlorophenacylamine hydrochloride and 6 g of m0thyl
3-dlmethylamlnoacrylate are haated for 2 hour~ under reflux ln
120 ml of ethanol. Then a ~olution of 4 g of sodlum sthylate in
40 ml of ethanol 1B added dropwlse and the reaction mixture 1~
heated for another hour under reflux. The reaction mlxture i~ then
concentrated by evapor~tlon a~d the oily residue i8 purified by
column chromatography (silica gel; elution with a 3:1 mixture of
toluene/ethyl acetate. Helting polnt: 205-206C.
b) Preparation of the pracuraor
4-(2~3-dichlorophenYl)P~rrole-3-carboxY11c acid
3.2 g of the 3-carbometho~y-4-(2,3-dlchlorophenyl)pyrrole obtained
ln a) and 40 ml of a 1:1 mlxture of methanol and 5N HCl are ~tirred
for hours at 70C. After it has coled to room te~parature, th0
reaction mixture i~ poursd onto ic~ and extracted with ethyl
acetate. The ester phase i8 in turn extracted wlth 10 % 00dium
hydroxide solution. The aqueou~ extract i~ wa~had twice wlth ethyl
acatate, ~cidlfied with hydrochlorlc acid and extractd wlth ethyl
acatate. Tha organic phsse i~ wa~hed with water, dried over magnas-
lum sulfate and filtersd. The filtrats i~ concentrated by eYaporat-
ion and the resultant 4-(2,3-dichlorphenyl)pyrrole-3-carboxyllc acid
~elt~ at 180-182~C.
7 3
- 20 --
c) Preparation of the flnal product:
4-(2,3-dichlorophenvl)-3-cYano~yrrole
2.1 g of the free 4-(2,3-dlchlorophenyl~pyrrole-3-carboxyllc acid
obtained ln b) are dlssolved ln 30 ml of ethanol. The ~olution i8
made alkaline with concentrated ~mmonia and then evapor~ted to
dryness. The residue 1~ dl~olved ln 50 ml of ethanol. NH3 gn~ ln
added (20 atm) to ~hls Dolution at room temperature in an autoclave
and the react$on mlxturs 1~ kept for 15 hours at 220C. The renctlon
mlxture, which has coolsd to room temperature, 18 poured into
lce/HCl, the preclpitate i~ i~olated by flltratlon snd dried at
60C. The resultant powder i8 heated wlth 17 g of polypho~phorlc
acid ln an open ves~el at 180C, the hot mlxture i8 droppsd onto
lce, made alkaline wlth NaOH and extracted with ethyl acetate. The
comblned extracts are concentrated by evaporation and the renidue 1
purifled by column chromstography (sillca gel; elutlon with a 4:1
mixture of toluene/ethyl acetate), affordlng 4-(2,3-dlchlorophanyl)-
3-cyanopyrrole of m.p. 148-150C.
Example P8: Preparation of
C~ ~Cl C~ ~Cl
~ tl-CH --~ -CH~NOH ~ CN
4-~2,3-dichlorophenyl)-3-cyano~yrrole
8) Preparatlon of 3-formyl-4-(2,3-dichlorophanyl)pyrrole
5.4 g of 3-dimethylaminoacroleln, 3.2 g of ~,3-dichlorophsn~cylamlne
hydrochloride and 60 ml of ethanol are heated for 1 1/2 hour~ under
raflux. Then a ~olution of ~odium ethylste in ethanol (prepared from
1 g of sodlum and 15 ml of ethsnol) i~ added dropwlse and the
reactlon mixture i8 heated under reflux for another 30 minutes.
After lt ha~ cooled to room temperature, the reactlon mixture i~
poured onto lce/water ~nd neutralisad wlth hydrochloric scid. The
~.~t73~36
- 21 -
preclpitate 1B washed wlth water, dried ln vncuo, snd the dry
rQ~idUe iB purified by cnlumn chromatography (silica gel; clution
with a 4:1 mixture of toluene/ethyl acetat0). M~p. 152-154C.
IR (aolld/K~r) in cm : 1655 (CO). IH-NMR (CDCL3) in ppm: 7.0 (broad
B, lH); 7.3 ~m, 2H); 9.66 (B, lH); 11,9 (~, lh, H replaceable with
D2O). Mans psak at 204. Thi~ substance i~ novel, has fungicidal
activlty, and fall~ within the amblt of the inventlon.
b) Preparation of hYdroxYimlnomethyl-4-(2.3-dichlorophenyl)pyrrole
5.0 g of the 3-formyl-4-(2,3-dichlorophenyl)pyrrol0 obtsined in a),
1.7 g of hydroxylam~nQ hydrochlorlde and 2.4 g of sodlum acetate are
atlrred for 3 hour~ at 80C in 80 ml of ethanol. Afeer it hn~ coolsd
to room temperature, tha reaction mlxture iB poured onto ice and
~tirred for 30 minute~. The precipitate iB isolated by filtration,
wa~hed with water and drled, affordlng 5.02 g of 3-hydroxylmino-
methyl-4-(2,3~dichlorophenyl)pyrrola aB Bynlanti mixture of m.p.
158-160C. Thi~ sub~tance iB al~o novel, hss fungicldal activity
snd fall~ within the ambit of the invention.
c) Preparatlon of the final product
4-(2,3-dichlorophenyl)-3-cyanopyrrole
3.2 g of th0 3 hydroxylminomethyl-4-(2,3-dichlorophenyl)pyrrole
obtained in b) are kept for 5 hour~ at c~ 100C in 50 ml of acatlc
anhydrlde, then coolad to room temperature~ poured lnto ice/NaOH,
and the re~ultant mixtur~ i8 stirred for 2 hour~. Th0 precipitate i~
dlssolved in ethyl acetate, wa~hed wlth water, and the e~ter phase
iB dried over magnesium aulfate. The residue i~ puriflad by column
chromatography (~ilica gel; elution wlth a 4:1 mlxture of toluen~/
ethyl acetats). Melting point: 14~-151C.
The compound~ of formula I li~t~d ln Table 4 aro a1BO prepar~d by
method~ corre~pondlng to tho~e de~cribed above.
~ ~73~
- 22 ~
f ll 11 (I)
T~ble 4
Compound n _ _ m.p. [-C]
4.1 H CN H 120-123
4.2 3-Cl CN ~ 138-140
4.3 2,4-cl2 GN H 150-152
4.4 4-Cl CN H 153-155
4.5 4-F CN H 137-139
4.6 3-CH3 CN H 109-111
4.7 3-F CN H 138-139
4.8 3-Br CN H 132-134
4.9 3-CF3 CN H 87-89
4.10 2~Cl CN H 136-138
4.11 2,3-Gl2 CN H 152~154
4.12 2,5-Clz CN H 137-142
4.13 2-~r CN H 135-138
4.14 2,3-Clz COOCH3 H 205-206
4.15 2,3-C12 CHO H 152-154
4.16 3-Cl COOCH3 H 187-189
4.17 3,4-Clz COOCH3 H 183-186
4.18 2-Cl C0OCH3 H 198-~00
4.19 2,3-C12 COOC3H7-i H 153-156
4.20 2,3-C12 COOC2~s H 149-151
4.21 2,3-C12 CN GHzCH2CN
4.22 2,3-C12 CN CHzCH2C00CH
The dc~cribed procss~, includin~ all pArtisl ~tep~, constitute~ ~n
ob~ect of ~his lnvention.