Note: Descriptions are shown in the official language in which they were submitted.
The present invention concerns a causticizing process wherein of
soda liquor and of unslaked lime is produced white liquor and
lime sludging over lime slaking and a subsequent causticizing
reaction proper.
The spent liquor from sulphate cellulose digesting is recovered
mainly in the form of soda liquor containing sodium carbonate, or
of green liquor, which is causticized with lime to become white
liquor containing mainly sodium hydroxide. The white liquor is
reused, possibly after intermediate storage, in the cellulose
digestion. The other product from the causticizlng reaction is
lime sludge consisting mainly of sodium carbonate, and this is
calcinated in a lime sludge kiln to become unslaked lime that can
be reused in the causticizing process.
The first step in the causticizing process is slaking of the lime
taking place by effect of water:
CaO -~ H2O ~Ca(OH)2
The reaction is fast, and a considerable quantity of heat is
liberated in connection therewith. Next, the causticizing
reaction proper takes place, in which the slaked lime reacts with
the sodium carbonate contained in soda liquor:
Ca(OH)2 + Na2C03; ~ 2NaOH + CaC03
The causticizing reaction is slow and it ends at a certain
equilibrium between sodium hydroxide and lime sludge, which are
the reaction products, and the starting substances. The reaction
is endothermic, although the heat quantity which is bound is
substantially less than that released in the lime slaking
process.
In existing procedures, causticizing is usually accomplished in
that the soda liquor and unslaked lime are combined, whereby the
water contained in the soda liquor causes slaking of the lime,
whereafter the causticizing reaction proper takes place between
the slaked lime and the sodium carbonate in the soda liquor.
However, the problem embarrassing this procedure is the tendency
of the soda liquor to form foam at the slaking step, the process
being disturbed as its consequence. The foam formation is due to
solid and/or solved impurities in the soda liquor, such as carbon
and organic substances derived from black liquor, which have a
surface-active effect.
The object of the present invention is to devise a problem
solution by which said known drawback of the causticizing methods
of prior art can be avoided. The invention is characterized in
that lime slaking is carried out using white liquor, whereupon
the causticizing reaction proper is allowed to take place between
the slaked lime and the soda liquor combined therewith.
Since white liquor is a substantially pure, and free from surface
active impurities, product mainly consisting of sodium hydroxide,
no foaming whatsoever takes place in lime slaking performed with
its aid. Since white liquor constitutes one of the two end
products of the causticizing process, the required white liquor
is simply obtainable by separating part of the white liquor that
has been produced and recirculating it to the slaking step. The
liquor quantity that is circulated may be 5 to 50%, preferably
about 20% of the liquor quantity obtained in the process after
lime sludge separation. Thanks to said recirculation of white
liquor, it is further possible to achieve a higher degree of
causticizing in the process than before.
Instead of recirculated white liquor, it is naturally possible to
use in the process other white liquor as well. The white liquor
produced by the causticizing process is typically a strong alkali
with NaOH its main component, and which may in addition contain
~3~
small quantities of Na2S, Na2CO3, Na2SO4, Na2SO3 and NaCl. The
total alkali of the liquor, which is calculated in grams Na2O per
litre, is within the limits of 70-180 g, most appropriately 110-
145 g. Any sodium-based liquor consistent with these values as
to its concentration can thus be contemplated, that is white
liquor recirculated in the process or added in the slaking step
of the process, or pure NaOH.
The most advantageous design, from the viewpoint of the foamlng
problem, is that in which lime slaking is accomplished
exclusively with white liquor, the whole of the soda liquor
quantity to be causticized being combined with the lime only
after the slaking step. However, an improvement in comparison
with prior art techniques is also achieved if only part of the
soda liquor used towards slaking according to prior art technique
is replaced with white liquor. It is thus understood that in
such an embodiment of the procedure soda liquor is used on the
side of white liquor to slake the lime, the soda liquor quantity
to be causticized being divided into two parts of which one is
conducted to lime slaking and the other is combined with the lime
after slaking.
It is also possible in connection with the invention to enhance
the utilization of the thermal energy released at slaking, by
carrying out the entire causticizing process, or preferably only
its slaking step, in known manner under elevated pressure. If
pressure is only applied at the slaking step, the thermal energy
released can be utilized by means of the steam which is
separated, by conducting this steam to a suitable point of
consumption, or it may be recovered by heat transfer to an
external fluid.
The invention is described in the following in greater detail
with the aid of examples, referring to the attached drawing,
wherein:
~3~
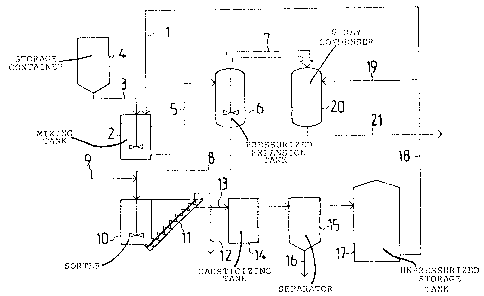
Fig. 1 presents an apparatus intended for applying the invention,
and
Fig. 2 shows part of another apparatus intended for applying the
invention.
Slaking the unslaked slime for use in causticizing soda, or
green, liquor is accomplished in the apparatus depicted in Fig.
1, with white liquor obtained as end product in the causticizing
process. White liquor is circulated by the lime 1 to a mixing
tank 2, into which further is supplied unslaked lime by the line
3 from the storage container 4. In the mixing take 2, the
unslaked lime and white liquor are rapidly mixed together and
they are immediately thereafter pumped through the line 5 into a
pressurized expansion tank 6. In the expansion tank 6 the
exothermal slaking reaction takes place between the unslaked lime
and the water contained in the white liquor, and the pressure in
this tank is so controlled that the mixture of lime and white
liquor is first heated up to the boiling temperature consistent
with the pressure, i.e. to 120C, and thereafter the rest of the
thermal energy set free in the reaction is consumed in vaporizing
the water in the mixture. The steam thus obtained, which is at
115C, goes to the line 7, and the white liquor containing the
slaked lime departs from the tank 6 into the line 8.
The next step in the causticizing process is the causticizing
reaction proper between the slaked lime and the sodium carbonate
contained in the soda, or a green, liquor that is being
causticized. The green liquor, which is at 95C, is forwarded
into an apparatus as depicted in Fig. 1, through the incoming
line 9, which joins the line 8 coming from the expansion tank 6.
The green liquor and the white liquor containing the slaked lime
become mixed and they go, to begin with, to the sorter 10. The
sorter 10 is unpressurized and the temperature of the mixture to
be causticized which is introduced therein is 104C, that is,
lower than the boiling point of the mixture. The purpose with
-~3'7S~
conveyor 11 to the line 12. The sorter lo is by a line 13
connected with the causticizing tank proper, 14, where the
causticizing reaction mainly takes place. Upon causticlzing, the
lime sludge is separated from the white liquor that has been
obtained, in the separator 15, and it is removed to the line 16.
The white liquor is transferred from the separator 15 to an
unpressurized storage tank 17.
of the white liquor that has been obtained and that is stored in
the tank 17, part is returned, as has been described, to the lime
slaking step, and the rest is used in the cellulose digesting
process. The portion which is returned to the slaking step is 5
to 50%, preferably about 20%, of the liquor quantity stored in
the tank. The line 18 starting from the storage tank 17 branches
into a recirculation line 1 leading to the white liquor and lime
mixing tank 2 and a line 19, which conducts the white liquor
going to cellulose digestion into a spray condenser 20 serving as
preheater for the white liquor. This heating is accomplished
with the hot steam generated in the lime slaking step and carried
to the condenser 20 by the line 7, this steam condensing and at
the same time heating the white liquor to 110C, at which
temperature it is conducted by the line 21 to the cellulose
digester.
In the embodiment of the invention depicted in Fig. 1, white
liquor is exclusively used towards lime slaking. Fig. 2
illustrates an alternative design in which green liquor is used
in addition to white liquor towards lime slaking. To this end,
the green liquor to be causticized is divided into two parts, one
part being supplied to the lime slaking step and the other part
being combined with the lime after the slaking step. It is thus
understood that the incoming green liquor line 9 branches into
two lines 22, 23 one of them leading to the liquor and unslaked
lime mixing tank 2 and the other ~oining the line 8 transporting
.
~3~
slaked lime from the expansion tank 6 to the sorter 10. In other
parts, the apparatus of Fig. 2 is equivalent to that of Fig. 1.
In the apparatus of Fig. 2, the green liquor conducted to the
lime slaking step may in some instances cause slight foaming,
which the invention is meant to prevent. However, the foaming is
substantially less than in the case that the lime is slaked, as
taught by prior art, with green liquor alone.
It is obvious to a person skilled in the art that different
embodiments of the invention are not confined to the examples
presented and that they may, rather, vary within the scope of the
claims following below.
- 6 -
,...
"~,,
,