Note: Descriptions are shown in the official language in which they were submitted.
1~74S~
O.Z 0050/37398
~enzyloxyalkylazoles and fung;c1des conta;n;ng
these compounds
The present ;nvent;on relates to novel benzyloxy-
alkylazoles, fung;c;des containing these compounds, and
processes for their preparation~
It has been disclosed that azole compounds, eg. 2,2-
dimethyl-4-~1,2,4-triazol-1-y~-5-phenylpentan-3-one, can
be used as fungicides (German Laid-Open Application DOS
2,638,470).
It is an ob ject of the present invention to provide
novel compounds and fungicides whose activity is superior
to that of the known compounds, particularly at low concen-
trations, or wh;ch can be put to an alternative use when
signs of resistance appear.
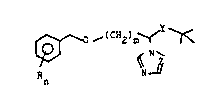
We have found that this object ;s achieved, and
that benzyloxyalkylazole compounds of the formula I
v
~(C 2)m ~ ~ Ir
R N
n
where R is halogen, alkyl, alkoxy, haloalkyl, unsubstituted
or substituted aryl, unsubstituted or substituted pyridyl,
ZO aralkyl, alkylthio or hydrogen, n is an integer from 1 to
5, m is an integer from 1 to 8, Y is a C=O or HCOH group
and Z is a CH group or an N atom, haYe a very good fungici-
dal action.
Preferred compounds are those in which R is halogen,
lower alkyl of 1 to 4 carbon atoms, haloalkyl of 1 or 2
carbon atoms and 1 to 3 halogen atoms, and hydrogen.
In formula I, R is, for example, halogen (fluorine,
chlorine, bromine or iodine), preferably fluorine, bromine
or chlorine, in particular 4-chloro, 4-fluoro, 2-chloro,
2-fluoro, 3-fluoro, 2-bromo, 4-bromo or 2,4-dichloro, lower
alkyl of 1 to 4 carbon atoms, preferably methyl, ethyl, n-
propyl, n-butyl or tert.-butyl, in particular methyl or
tert.-butyl, alkoxy where alkyl is of 1 or 2 carbon atoms,
~1, .
lZ7~5Z~
- Z - 0.Z. 0050/37398
preferably methoxy, or haloalkyl, in particular haloalkyl
in which alkyl ;s of 1 or 2 carbon atoms and which contains
1 to 5, preferably 1 to 3, halogen atoms, eg. fluorine,
chlor;ne or bromine, preferably f(uor;ne or chlorine. An
example of halomethyl is tr;fluoromethyl.
R may furthermore be unsubstituted or subst;tuted
aryl, for example unsubstituted or halogen-substituted
phenyl, preferably phenyl, 4-chlorophenyl or 4-
fluorophenyl. Finally, R may also be unsubstituted or
substituted pyridyl, in particular unsubstituted or
halogen-subst;tuted pyr;dyl, aralkyl ~benzyl), alkylthio
(of 1 to 4 carbon atoms, eg. methylthio) or hydrogen.
n may be an integer from 1 to 5, preferably 1 or 2.
m is an ;nteger from 1 to 8, preferably 2, 3, 4,
S or 6.
Y is a C=O or HCOH group.
In formula I, Z is an N atom or a C-H group.
The compounds of the formula I can be prepared
according to one of the schemes stated below:
Scheme A (intermediate)
~ 2)m ~ O ~ ~CH2~m-Hal
Rn Rn
Scheme B (intermediate)
~ Hal ~ HO-~CH2)m-OH ~ ~ (CH2)m-~H
Rn Rn
~ _~^~ ~ (CH ) -Hal
halogenation ~ ~ 2 m
Rm
' . ,, ~ '
127~S~l
- 3 - O.Z. OûSO/37398
Scheme C (intermediate)
(CH )
Ha~ m ~CHZ)m~
Rn Rn
Scheme D
t ~ ttH2)~-Hal b~se
reduction OH -
i f reguired ~ O ~ ~CHz)
Rn
2~
The starting compounds of the formula II may be
prepared by a conventionaL process by
a) alkylating a mono-(alkali metal) salt of an alpha,
omega-glycol w;th an appropr;ate benzyl halide and conver-
t;ng the result;ng alcohol to the bromide, for e~ample by
means of N-bromosuccinimide/triphenylphosphine ~Scheme 3)
or
b) alkylating a phenol with an alpha,omega-dihalo-
alkane (Sche0e A).
The intermediates below can be prepared in this
manner:
Rn . m X Physical data, bp
4-Cl 2 C~
4-Cl 2 Br 116C/0.1 mbar
15 4-Cl 3 Cl 108C/0.4 mbar
4-Cl 3 Br
4-Cl 4 Cl
lZ74SZl
- 4 - O.Z. 0050~37398
m X Ph sical data bo
R n -- -- . Y
4-Cl 4 8r
4-Cl 5 Cl 155-170C/0.4 mbar
4-Cl 5 8r
5 4-C~ 6 Cl
4-Cl -6 Br
4-C~ 7 Cl
4-C~ 7 Br
2-C~ 2 C~
10 2-Cl 2 Br
2-Cl 3 C~
2-Cl 3 Br
Z-C~ 4 C~
2-Cl 4 8r
1S 2-Cl 5 Cl
2-Cl 5 9r
2-C~ 6 C~
2-Cl 6 8r r
2-Cl 7 C~
20 2-Cl 7 Br
2,4-dichloro 2 Cl 111C/0.5 mbar
2,4-dichloro 2 8r
2,4-dichloro 3 Cl
2,4-dichloro 3 Br
25 2,4-dichloro 4 Cl 140C/0.5 mbar
2,4-dichloro 4 9r
2,4-dichloro 5 Cl
2,4-dichloro 5 8r
2,4-d;chloro 6 Cl
30 2,4-dichloro 6 Br
2,4-d;chloro 7 Cl
2,4-dichloro 7 8r
4-tert.-butyl 2 Cl
4-tert.-butyl 2 8r 112 - 119C/0.3 mbar
35 4-tert.-buty;1 3 Cl 140C/0.5 mbar
4-tert.-butyl 3 8r
4-tert.-butyl 4 Cl 14~C/0.5 mbar
127~S2~
- 5 - O.Z. ooso/37398
Rn- m ~ Physica~ data, bp
4-tert.-butyl 4 Br
4-tert.-buty~ 5 Cl 155C/0.4 mbar
4-tert.-buty~ 5 8r
4-tert.-butyl 6 Cl 160C/0.4 mbar
4-tert.-butyl 6 Br
4-tert.-butyl 7 Cl
4-tert.-butyl 7 3r
4-F 2 Cl
10 4-F 2 8r 88C/0.5 mbar
4-F 3 Cl
4-F 3 8r
4-F 4 Cl
4-F 4 Br
15 4-F 5 Cl
4-F 5 8r
4-F 6 Cl
4-f 6 Br
4-F 7 Cl
20 4-F 7 Br
2,4,6-trichloro 2 Cl
2,4,6-trichloro 2 Br
Z,4,6-tr;chloro 3 Cl
2,4,6-trichloro 3 8r
25 2,4,6-trichloro 4 Cl
2~4,6-trichloro 4 8r
2,4,6-trichloro 5 Cl
2,4,6-trichloro 5 Br
2,4,6-trichloro 6 Cl
30 2,4,6-tr;chloro 6 8r
Z,4,6-trichloro 7 Cl
2,4,6-trichloro 7 Br
4-methyl 2 Cl
4-methyl 2 Br 94C/0.4 mbar
35 4-methyl 3 Cl gsC/~.5 mbar
4-methyl 3 Br
4-methyl 4 Cl
~ z74521
- 6 - 0.Z. 0050/3739
Rn m X Physical data, bp
4-methyl 4 Br
4-methyl 5 Cl
4-methyL 5 8r
5 4-methyl 6 Cl
4-methyl -6 9r
4-methyl 7 Cl
4-methyl 7 Br
C/0.3 mbar
4-Br 2 8r 123-135
10 3-CH3 2 Br 92C/0.4 mbar
- 2-CF3 2 - ar 88C/0.7 mbar
Z-Br 2 3r 116-125C/0.4 mbar
3-f 2 Br 80C/0.5 mbar
4-CF3 3 Cl 95C/0.4 mbar
15 4-CF3 4 Cl 125C/0.5 mbar
4-CF3 5 Cl 140C/2.5 mbar
4-CF3 6 Cl 130C/0.5 mbar
The compounds of the general formula I contain one
or more centers of asymmetry; in the case of Y = CHOH, they
conta;n two or more centers of asymmetry and can therefore
occur as enant;omer m;xtures and/or d;astereomer m;xtures.
In the synthes;s accord;ng to the ;nvention, the compounds
are usually obta;ned in the form of m;xtures of stereo-
;somers; the pure ;somers can be obtained from these by
su;table separation operations. The present invention em-
braces both the ;nd;vidual ;somers and mixtures of these.
The ;somers of the novel alcohols of the formula I
may also be prepared d;rectly by selective synthesis, for
example by reduction of the novel ketones of the formula
I with the aid of su;table reduc;ng agents.
PREPARATION EXAMPLE 1
4.2 9 (0.14 mole) of an 80X strength suspension of
NaH in paraffin was suspended ;n 100 ml of dry d;methyl-
formamide, and 23.4 9 (0.14 mole~ of 1-~1,2,4-triazol-1-yl)-
3,3-dimethylbutan-3-one, dissolved ;n 50 ml of dry d;methyl-
formamide, ~ere added dropwise. When evolut;on of gas had
d;ed down, stirring was cont;nued for a further hour, after
- .
:
12745.Zl
- 7 - O.Z. 0050/37398
which a solution of 21 9 ~0.14 mole) of 3-benzyloxy-1-bromo-
propane, dissolved in 50 ml of dry dimethylformamide, was
added dropwise. Stirring was continued for 4 hours at 70C,
the mixture was left to cool and then poured into a mix-
ture of 500 ml of water and 500 ml of methylene chloride,
the phases were separated, the aqueous phase was extracted
w;th methylene chloride, the combined organic phases were
dried and the solvent was evaporated under reduced pressure.
The crude product W25 distilled to give 22.6 9 ~51X of
theory) of 7-benzyloxy-2,2-dimethyl-4-~1,2,4-tr;azol-1-yl~-
heptan-3-one.. IR: 1717, 1502, 1276, 1139, 1103 ~compound
No. 1).
PREPARATION EXAMPLE 2
15.0 9 ~0.048 mole) of compound No. 1 were dis-
solved in 50 ml of isopropanol, and a solution of 0.61 9
~0.016 mole) of sodium borohydride in isopropanol was
added. The mixture was stirred overnight at room tempera-
ture, after which it was poured onto water, the aqueous
phase was extracted with ethyl acetate, the extracts were
dried and the solvent was evaporated under reduced pres-
sure. The residue was distilled to give 12.0 9 ~80X of
theory) of product No. 2 ~7-benzyloxy-2,2-dimethyl-4-~1,2,4-
triazol-1-yl)-heptan-3-ol). IR: 2956, 2869, 1138, 1100,
1075.
The compounds listed below can be prepared in a
similar manner. The substances which are not characterized
by physical data serve to illustrate the w;de range of
possible syntheses. A and B are the two diastereomers.
lZ7(~S~l
- 8 ~ 0.~. 0050/~7~98
N~. ~h m Y Z Physical data IR bands
_ _ _
3 H 2 C~O N
4 H 2 C~O C~
05 S ~ 2 HCOH (A) N
6 H 2 HCOa (A) C~
7 ~ 2 HCOH (B) N
8 H 2 ~COE (B) CH
9 H 3 C~O C~
lo lo a 3 ~COH (A) C~ 1496, 1123, 1113, 1080, 742
11 H 3 HCOH (A) N 2956, 2869, 1275, 1136, 1101
12 H 3 ~CO~ (B) CH 2953, 2868, 1100, 1081, 745
13 H 4 C-O N 2935, 2860, 1716, 1501, 1102
14 ~ 4 C~O. C~
}5 15 H 4 HCO~ (A) N 2954, 2867, 1275, 1136, 1102
16 H 4 ~COH ~A) CH
17 ~ ~ HCO~ (B) N 2965, 2946, 1517, 1141, 699
18 H 4 HCO~ (B) ca
19 ~ 5 C-O N
20 H 5 C-O C~
21 H 5 HCO~ (A) N
22 ~ 5 HCOH (A) CH
23 a 5 . HCOH (B) N
24 a 5 HCOH (B) ca
25 a 6 C~O N
26 ~ 6 C'O C~
27 8 6 HCO~ (A) N
28 H 6 HCOH (A) C~
29 ~ 6 ~CO~ (B) .N 2963, 2939, 2859, 1518, 1142
30 30 a 6 NCOa (B) ca
31 H 7 C~O N
32 a 7 C-O ca
33 a 7 HCOH (A) N
34 a 7 aCOH (A) C~
35 35 a 7 ~cOu ( B) N
36 a 7 ~COH (B) C~
37 a 8 c-o N
38 H 8 C-O CH
39 ~ 8 ~CO~ (A) N
40 40 ~ 8 HCOH (A) ca
41 ~ 8 ~COH (B) N
42 a, 8 C~OH (B) C~
43 2-C1 2 C'O N
-
~2~4S~l
- 9 -O. Z. oo50/37398
NO. Rn~ m Y ZPhysi ca l data IR bands
44 2-C1 ` 2 C-O C~l
-45 2~1 2 ~COId (A) N
0546 2-C1 2 ~ oa (A) ca
47 2~1 2 aco~ (B) M
48 2-C1 2 ~CO}l (B) ca
49 2-C1 3 C~O N
2-Cl 3 C'O ca
1051 2--C1 3 ~COE (A) N
52 2-C1 3 ~coa (A) C~
53 2--C1 3 HCO~ (B) N
54 2-C1 3 acoa (B) ca
2--C1 4 C-(3 N
1556 2-C1 4 C-O CE
57 2-C1 4 acoa (A) N
58 2-C1 4 HCOI~ (A) ca
59 2-Cl 4 aco~ (B) N
2-C1 4 acoa (B) ca
2061 2--C1 5 C-O N
62 2-C1 5 C-O ca
63 2--C1 5 aco~ tA) N
64 2-C1 5 acoa (A) CH
2--C1 5 aco~ (B) N
2566 2-C1 5 acoa (B) ca
67 2-C1 6 C-O N
68 2-C1 6 C-O C~l
69 2-C1 6 acoa (A) N
2-C1 6 aco~ (A) CH
3071 2-C1 6 acoa (B) ~t
72 2-C1 6 acoa (B) ca
73 2-C1 7 C-O N
74 2-C1 7 C-O ca
2-C1 7 HCO~l (A) N
3576 2-C1 7 HCOH (A) CH
77 2-C1 7 acoa (B) N
78 2-C1 7 HCO}I (B) ca
79 2-C1 8 ~:-0 N
2-Cl 8 C-O CH
4081 2-C1 8 EICOIl (A) N
82 2-C1 8 HCO~ (A) C~
83 2-C1 8 ~coa (B) N
84 2-C1 8 acoa (B) ca
SZl
- 1 o o . z . 0050/ 37398
No. Rn m Y Z Ph~s;cal data IR bands
2, 4-C12 2 C-O N
86 2,4-C12 2 C~O ca
C5 87 2,4 - C12 2 aco~ (A) N ~p. - 220-C/0.15 ~b.
88 2,4-C12 2 ~CO~ (A) ca
89 2,4-C12 2 ~coa ( 8) N
2,4-C12 2 acoa (B) ca
91 2,4-C12 3 C-O N
92 2,4-ca2 3 C-O ca
93 2,4 - C12 3 ~coa (A) N
94 2,4 - Ca2 3 ~COH (A) C~
2,4 - C12 3 HCOa ( B) N
96 2,4-ca2 3 ac~ ~B) C~
lS 97 2,4-C12 4 C-O N
98 2,4-C12 4 C-O ca
99 2,4-C12 4 acoa (A) N 2955, 2868, 1473, 1135, 1096
100 2,4-ca2 4 aCOH (A) ca
101 2,4-C12 4 acoa (B) N
102 2,4-C12 4 acoa (B) ca
103 2,4-C12 5 C-O N
104 2,4-C12 5 C-O ca
105 2,4-C12 5 acoa (A) N
106 2,4-C12 ' 5 acoa (A) ca
107 2,4-C12 5 aCOH (B) N
108 2,4-C12 5 acoa (B) ca
109 2,4-C12 5 acoa (B) N
110 2,4-C12 6 C-O ca
111 2,4-C12 6 acoa (A) N
112 2,4-C12 6 acoa (A) ca
113 2,4-C12 6 acoa (B) N
114 2,4-C12 6 acoa (B) ca
115 2,4-C12 7 C-O N
116 2,4-C12 7 C~O ca
117 2,4-C12 7 acoa (A) N
: ~ 118 2,4-C12 7 acoH (A) ca
119 2,4-C12 7 acoa (B) N
i20 2,4-C12 7 acoa (B) ca
121 2,4-C12 8 C-O ' N
122 2,4-C12 8 C-O C~
:~ ~ : 123: 2,4-C12 B acoa (A) N
: 124 2,4-C12 8 acoa (A) ca
125 2,4-C12 8 acoa ( B) N
; ~
: ' :, '
~'
lZ'7~21
o. z . oo50/37398
Nt~. Rn m Y Z Physical data IR bands
-
126 2,4-C12 8 ~CO~ (B) CH
127 4-C1 2 C-O N 1717, 1502, 1492, 1090, 1015
05 128 4-C1 2 C-O C~
129 4-C1 2 ~COH (A) N 1134, 1123, 1097, 1087, 1014
130 4-C1 2 HCO~ (A) C6
131 4-C1 2 ~COH (B) N
132 4-C1 2 ~CO~ (B) C~
10 133 4-C1 3 C~O N 1717, 1492, 1097, 1089, 1015
134 4-C1 3 C-O ca 1714, 1492, 1108, 1096, 1087
135 4-C1 3 aCOH (A) N 2956, 1275, 1136, 1093, 1015
136 4-C1 3 acoa (A) C~ .
137 4-C1 3 aC~H (B) N 3320, 2963, 1510, 1146, 809
15 138 4-C1 3 aCOE (B) ca
139 4-C1 4 C-O N
140 4-Cl. 4 C'O CH
141 4-C1 4 acoa (A) N
142 4-C1 4 aco~ (A) C~
20 143 4-C1 4 aCOH (B) - N
144 4-C1 4 aco~ (B) ca
145 4-C1 5 C~O N 2936, 2863, 1716, 1492, 1090
146 4-Cl 5 C-O C~
147 4-C1 5 HCOa (A) N 2939, 2863, 1492, 1090, 1015
25 148 4-C1 5 HCOH (A) ca
149 4-Cl 5 ~coa .(B) N 2952, 2935, 2867, 2852, 813
150 4-C1 5 HCOH (B) ca
151 4-C1 6 C-O N
152 4-C1 6 C~O ca
30 153 4-C1 6- HCOa (A) N
154 4-C1 6 HCOH (A) C~
155 4-C1 6 aco~ (B) N
156 4-C1 6 ~COH (B) C~
157 4-C1 7 C-O N
35 158 4-C1 7 C'O CH
159 4-C1 7 ~COH (A) N
160 4-C1 7 acoa (A) ca
161 4-C1 7 aco~ (B) N
162 4-C1 7 HCO~ (B) CH
40 163 4-C1 8 C-O N
164 4-C1 8 C~O CH
165 4-C1 8 HCOa (A) N
166 4-C1 8 aCOH (A) . ca
.
.
.~s
lZ7~5Z~
- 1 2 o. z . 0050/37398
Nt1. Rn m Y ~ Physical data IR bands
_ _
167 4--C1 8 ~C0~ (B) N
168 4--C1 8 ~coa (B) CE
05 169 4-OCH3 2 C-0 N
170 4-OCE3 2 C~0 C~
171 4-oca3 2 ~coa (A) N
172 4--OCE3 2 ~COH (A ) C~
173 4-OCH3 2 aco~ (B) N
174 4--OCE3 2 ~COH (B ) CE
175 4-oca3 3 c~o N
176 4-OCE3 3
177 40C~3 3 ~COH (A) N
178 4-oca3 3 ~COH (A) CEl
15 179 4-oca3 3 ~co~ (B ) N
180 4-oca3 3 HCO~ (B) C~
181 4--OCE3 4 c~o N
132 4-OCH3 4
133 4-oca3 4 ~COH (A) N
20 184 40CE3 4 aCOE (A) CE
185 4-OCH3 4 ~coa (B) N
186 4--oca3 4 acoH (B ) CE
187 4-oca3 5 c~o N
188 4--OCH3 5 C-0 CE
2s 189 4-OCE3 5 EECOH (A) N
4-OCH3 5 HCOE (A) C~
-oca3 5 acoa (B) N
192 4--OCE3 5 aco~ (B) CE
193 4-OC~3 6 C~0 N
30 194 4-OCX3 6 C~0 C~
195 4-OCE3 6 ECOEI (A) N
196 4--oca3 6 acoE (A) CE
197 4--oca3 6 ~COE (B) N
198 4--oca3 6 ~COE (B) ca
199 4-OC~3 7 c~o N O
200 4--OCE3 7 c~o CE
201 4--OCE2 7 ECOE (A) N
202 4-OC~3 7 ~COEi (A) CE
20 3 4--OCE3 7 ~COE (B ) N
204 4--oca3 7 ~COE (B ) ca
20 5 4-oca3 8 c~o N
20 6 4-oca3 8 c~o CE
20 7 40C~3 8 ~COE (A ) N
1;~'7'15~
- 13 - O,Z. oo50/37398
N~ m Y Z Physi ca l data IR bands
.
208 4-OC~3 8 HCOH (A) CH
209 4-oCH3 8 ~CO~ (B)
05 210 4-oca3 8 HCOE (B) C~
211 4-CF3 2 C'O N 1718, 1327, 1164, 1124, 1067
212 4-CF3 2 C~O ca
213 4-CF3 2 aco~ (A) N 1326, 1164, 1128, 1125, 1067
214 4-CF3 2 Hcoa (A) . ca
10 215 4-CF3 2 ~CO~ (B) N 1327, 1164, 1124, 1067, 1018
216 4-CF3 2 HCOH (B) C~
217 4-CF3 3 C~O N 1717, 1327, 1164, 1124, 1108
218 4-CF3 3 C-O C~
219 4-CF3 3 HCOH ~A) N 1327, 1164, 1125, 1106, 1067
15 220 4-CF3 3 ~COH (A) C~
221 4-CF3 3 ~CO~ (B) N 1327, 1168, 1129, 1126, 1104
2Z2 4-CF3 3 ~COH (B) ca
223 4-CF3 4 C~O N 1326, 1124, 1607, 1105, 1164
224 4-CF3 4 C-O C~ .
20 225 4-CF3 4 ~CO~ (A) N 1326, 1164, 1125, 1105, 1067
226 4-CF3 4 ~coa (A) CH
227 4-CF3 4 ~CO~ (B) N 1328, 11~8, 1123, 1099, 1067
228 4-CF3 4 ~COH ~B) ca
229 4-CF3 5 C-O N 1327, 1164, 1124, 1105, 1067
25 230 4-CF3 5 C-O CH
231 4-CF3 5 ~CO~ (A) N 1236, 1164, 1125, 1106, 1067
232 4-CF3 5 ~coa (A) ca
233 4-CF3 5 ~CO~ (B) N 1327, 1164, 1125, 1105, 1067
234 4-CF3 5 HCOH (B) CH
30 235 4-CF3 6 C=O N
236 4-CF3 6 C-O CH
237 4-CF3 6 HCOH ~A) N 1327, 1164, 1125, 1104, 1067
238 4-CF3 6 ~COH (A) C~
239 4-CF3 6 ~CO~ (B) N
35 240 4-CF3 6 ~coa (B) C~
241 4-CF3 7 C-O N
242 4-CF3 7 C'O C~
243 4-CF3 7 ~COH (A) N
244 4-CF3 7 ~CO~ (A) C~
~0 245 4-CF3 7 ~COH (B) N
246 4-CF3 7 ~CO~ (B) CH
247 4-CF3 8 C~O N
248 4-CF3 8 C~O ca
1274SZl
- 1 4 -o . z . oo50/37398
Ntl. ~ m Y ZPhys;cal data IR bands
_
249 4-CF3 8 ~CO~ (A) N
2s0 4-CF3 8 ~COH (A) ca
05 251 4-CF3 8 ~CO~ ~B ) N
252 4-CF3 8 8CO~ (B) C8
253 4-C~3 2 c-o N 1718, 1502, 1276, 1139, 1096
254 4-ca3 2 c~o c~
255 4-C~3 2 ~co~ (A) N 1134, 1123, llll, 1~98, 809
256 4--ca3 2 ~CO~ (A) C~
257 4-CH3 2 ~co~ (B) N
258 4-C~3 2 ~co~ (B) ca
259 4-CH3 3 c~o N 2965, 2933, 2867, 1717, llOO
260 4-CH3 3 c~o ca
15 261 4CH3 3 aCOH (A) N
262 4-ca3 3 acoH (A) ca
263 4-ca3 3 acoa (B) N 2956, 2868, 1504, 1138, lO99
264 4-CH3 3 acoH (B ) ca
265 4-C~3 4 c~o N
20 266 4-ca3 4
267 4-ca3 4 acoa (A) N
268 4-ca3 4 Hcoa (A) ca
269 4-ca3 4 Hcoa (B) N
2-70 4-ca3 4 acoa (B) ca
25 271 4-ca3 5 c~o N
272 4-ca3 5 c~o ca
~73 4-CX3 5 ~coa (A) N
274 4-C~3 5 ~COH (A) C~
275 4-ca3 5 ~co~ (B) N
30 276 4-ca3 5 acoa (B) ca
277 4-ca3 6 c~o N
278 4-C~3 6 c~o ca
279 4-CH3 6 acoa (A) N
Z80 4-C~3 6 acoa (A) ca
3S 281 4-ca3 6 aCOH (B) N
282 4-ca3 6 acoa (B) ca
283 4-C~3 7 C-O N
284 4-ca3 7 C-O ca
285 4-ca3 7 ~coa (A) N
40 286 4-ca3 7 BCOa (A) ca
287 ~-ca3 7 aCoa (8)
288 4-ca3 7 acoa (B) C~
289 4-ca3 8 c~o N
,~ .
74S2~l
- 1 s - o . z . ooso/~73g8
N~ . m Y 2 Physi ca l data IR bands
290 4-CH3 8 C~O CH
291 4-CH3 8 HCOE (A) N
06 292 4-CX3 8 HCOH (A) C~
293 4-ca3 8 ~CO~ (B) N
294 4-CH3 8 HCOH (B) . CH
295 4-F 2 C-O N 1717, 1510, 1223, 1111, 1094
296 4-F 2 C~O CH
10 297 4-F 2 HCOH (A) N m.p. ~ 75-80-C
298 4-F 2 HCOH (A) CH
299 4-F 2 HCO~ (B) N
300 4-F 2 ~CO~ ts) ca
301 4-F 3 C-~O N
15 302 4-F 3 C-O ca
303 4-F 3 HCO~ (A) N
304 4-F 3 HCO~ (A) C~
305 4-F 3 HCO~ ( B) N
306 4-F 3 ~CO~ (B) C~
20 307 4-F 4 C~O N
308 4-F 4 C-O C~
309 4-F 4 HCOH (A) N
310 4-F 4 ~COH (A) CH
311 4-F 4 ~COH (B) N
25 312 4-F 4 acoa (B) C~
313 4-F 5 C=O N
314 4-F 5 C-O C~
315 4-F 5 HCOH (A) N
316 4-F 5 HCOH (A) ca
30 317 4-F 5 HCOH (B) N
318 4-F 5 HCOH (B) ca
319 4-F 6 C-O N
320 4-F 6 CAO C~
321 4-F 6 ~CO~ (A) N
35 322 4-F 6 ~COH (A) CH
323 4-F 6 ~COH (B) N
324 4-F 6 HCOH (B) CH
325 4-F 7 C-O N
326 4-F 7 C-O CH
40 327 4-~ 7 HCOH (A) N
328 4-F 7 HCOH (A) CH
329 4-F 7 HCOH (B) N
330 4-F 7 HCOH (B) CH
.. ~ .
.g
lZ745Zl
- 16 - o. z. 0050/37398
No. R~ m Y Z Phys;cal data IR bands
331 4-F 8 C~O N
332 4-F 8 C-O ca
OS 333 4-F 8 HCOH (A)
334 4-F 8 aCOH (A) C~
335 4-F 8 HCOH (B) N
336 4-F 8 HCOH (B) CH
337 4-C(CH3)3 2 C'O N . 2963, 2907, 2869, 1718, 1274, 1110
10338 4-C(C~3)3 2 C-O C~
339 4-C(Ca3)3 2 HCOH (A) N 2960, 2869, 1186, 1109, lOgO
340 4-C(Ca3)3 2 ~COH (A) C~
341 4-C(C~3)3 2 ~COH (B) N
342 4-c(Ca3)3 2 ~COH (B) CH
lS343 4-C(Ca3)3 3 C-O N 2963, 2868, 1717, 1275, 1109
344 4-C(Ca3)3 3 C~O CH
345 4-C(Ca3)3 3 HCOH (A) N 2959, 2908, 2868, 1108, 1100
346 4-C(CH3)3 3 HCOH (A) CH
347 4-C(Ca3)3 3 HCOH (B) N 2961, 2907, 2868, 1363, 1109
20348 4-C(Ca3)3 3 HCOH (B) CE
349 4-c(Ca3)3 4 C~O N 2963, 2868, 1717, 1276, 1109
350 4-C(CH3)3 4 C-O CE -
351 4-C(CH3)3 4 HCOH (A) N 2958, 2907, 2867, 1368, 1108
352 4-C(Ca3)3 4 HCOH (A) CX
25353 4-C(CH3)3 4 ~COH (B) . N
354 4-C(CH3)3 4 aCOH (B) CH
355 4-C(Ca3)3 5 C'O N 2963, 2865, 1717, 1501, 1109
356 4-C(Ca3)3 5 C-O ca
357 4-C(Ca3)3 5 HCOH (A) N 2959, 2908, 2865, 1363, 1109
30358 4-C(CH3)3 5 HCOH (A) CE
359 4-C(Ca3)3 5 HCOH (B) N 2959, 1363, 1274, 1109
360 4-C(Ca3)3 5 ~COH (B) CH
361 4-C(Ca3)3 6 C~O . N 2953, 2935, 2861, 1717, 1326
362 4-C(CH3)3 6 C~O CH
3S363 4-C(CH3)3 6 HCOH (A) N
364 4-C(Ca3)3 6 HCOH (A) CH
365 4-C(CH3)3 6 ~COH ~B) N 2958, 2907, 2867, 1363, 1108
366 4-C(CH3)3 6 aCOH (B) CH
367 4-C~Ca3)3 7 C'O N
40368 4C(Ca3)3 7 C~O CH
369 4-C(C~3)3 7 HCOH (A) N
370 4-C(CH3)3 7 HCOH (A) CH
371 4-C(CH3)3 7 HCOH (B) N
1Z74S~;~
- 17 - o. z. oo50/37398
N~. Rn m Y Z Physica~ data IR bands
372 4 - C(CE3)3 7 . HCOa (B) ca
373 4 - C(C~3)3 8 C~O N
05 374 4-C(Ca3)3 8 C~O ca
375 4 - C(CH3)3 8 ~COE (A) N
376 4-C(CH3)3 8 acoa (A) C~
377 4 - C(C~3)3 8 HCO~ (B)
378 4-C(C~3)3 8 ~COH (B) . CE
10 379 2-CF3 2 CO N 1718, 1315, 1165, 1122, 1060
380 2-CF3 2 aCOH (A) N 1315, 1165, 1123, 1060, 1040
381 2-CF3 2 HCOH (B) N 1317, 1178, 1135, 1123, 1090
382 2-Br 2 CO N 1717, 1502, 1276, 1138, 1107
383 2-Br 2 aCOH (A) N
15 384 2-Br 2 HCOH (B) N 2957, 2924, 1138, 1103, 751
385 3-F 2 CO N 2968, 1717, 1276, 1256, 1139
386 3-CH3 2 CO N
~i274521
- l& - O.Z. 0050/37398
The novel compounds have an excellent action on a
broad spectrum of plant-pathogenic fungi, especially from
the Ascomycetes and sasidiomycetes classes. Some of them
have a systemic action and may be used as soil and foliar
05 fungicides.
The fungicidal compounds are of particular interest
for combatting a large number of fungi in various crops or
their seed, especially wheat, rye, barley, oats, rice,
Indian corn, cotton, soybeans, coffee, sugarcane, fruit,
ornamentals in horticulture, and vegetables, such as cucum-
bers, beans and Cucurbitaceae.
The novel compounds are particularly suitable for
comba~ing the following diseases: Erysiphe graminis in
cereals, Erysiphe cichoriacearum in Cucurbitaceae,
Podosphaera leucotricha in apples, Uncinula necator in
grapes, Puccinia species in cereals, Rhizoctonia solani in
cotton, Ustilago species in cereals and sugarcane,
Venturia inaequalis (apple scab), Septoria nodorum in
wheat, Botrytis cinerea in grapes and strawberries,
Cercospora musae in bananas, Pseudocercosporella herpo-
trichoides in wheat and barley, Hemileia vastatrix in
coffee, Piricularia oryzae in rice, Alternaria solani in
potatoes and tomatoes, Plasmopara viticola in grapes, and
Fusarium and Verticillium species in various plants.
The compounds are applied by spraying or dusting the
plants, or treating the seed with the active ingredients.
Application may be effected before or after infection of
the plants or seed by the fungi.
The novel active ingredients can be converted into
the conventional formulations, e.g. solutions, emulsions,suspensions, dusts, powders, pastes and granules. The form
of application depends entirely on the purpose for which
the agent is to be used; at all events, it should ensure a
fine and uniform distribution of the active ingredients.
3S The formulations are prepared in the conventional manner,
for example by diluting the active ingredient with sol-
.: ' . , ' .
.
-
1;Z745Zl
- 19 - o.z. 0050/37398
vents and/or carriers, with or without the addition of
emulsifiers and dispersants and, where water is used as
the diluent, with or without an organic auxiliary solvent.
Suitable auxiliaries are, essentially, solvents, for
05 example aromatics, e.g. xylene and benzene, chloro-
aromatics, e.g. chlorobenzene, paraffins, e.g. petroleum
fractions, alcohols, e.g. methanol and butanol, amines,
e.g. ethanolamine, and dimethylformamide and water;
carriers, for example natural rock powders, e.g. kaolin,
alumina, talc and chalk, and synthetic rock powders, e.g.
highly disperse silica and silicates; emulsifiers, for
example non-ionic and anionic emulsifiers, e.g. polyoxy-
ethylene fatty alcohol ethers, alkylsulfonates and aryl-
sulfonates, and dispersants, for example lignin, sulfite
waste liquors and methylcellulose.
The fungicidal agents generally contain from 0.1 to
95, preferably from 0.5 to 90, wt% of active ingredient.
The application rates depend on the effect desired,
and range from 0.02 to 3 kg of active ingredient per hec-
tare, or more.
The novel compounds may also be used for protectingmaterials, inter alia for combatting wood-destroying fungi
such as Coniophora puteana and Polystictus versicolor. The
novel active ingredients may also be used as fungicidally
effective components of oily wood preservatives for
protecting wood against wood-discoloring fungi. The agents
are applied by treating, e.g., impregnating or painting,
the wood with them.
The agents and the ready-to-use formulations made
therefrom, e.g., solutions, emulsions, suspensions,
powders, dusts, pastes or granules, are applied in known
manner, for example by spraying, atomizing, dusting,
scattering, seed-disinfecting, or watering.
12t~4521
- 20 - O.Z. 0050/37398
Examples of formulations are given below.
I. 90 parts by weight of compound no. 11 is mixed
with 10 parts by weight of N-methyl-alpha-pyrrolidone. A
05 mixture is obtained which is suitable for application in
the form of very fine drops.
II. 20 parts by weight of compound no. 2 is dis-
solved in a mixture consisting of 80 parts by weight of
xylene, 10 parts by weight of the adduct of 8 to 10 moles
of ethylene oxide and 1 mole of oleic acid-N-monoethanol-
amide, 5 parts by weight of the calcium salt of dodecyl-
benzenesulfonic acid, and 5 parts by weight of the adduct
of 40 moles of ethylene oxide and 1 mole of castor oil. By
pouring the solution into water and uniformly distributing
it therein, an aqueous dispersion is obtained.
III. 20 parts by weight of compound no. 15 is dis-
solved in a mixture consisting of 40 parts by weight of
cyclohexanone, 30 parts by weight of isobutanol, 20 parts
by weight of the adduct of 7 moles of ethylene oxide and
1 mole of isooctylphenol, and 10 parts by weight of the
adduct of 40 moles of ethylene oxide and 1 mole of castor
oil. By pouring the solution into water and finely
distributing it therein, an aqueous dispersion is
obtained.
IV. 20 parts by weight of compound no. 87 is dis-
solved in a mixture consisting of 25 parts by weight of
cyclohexanol, 65 parts by weight of a mineral oil fraction
having a boiling point between 210 and 280C, and
10 parts by weight of the adduct of 40 moles of ethylene
oxide and 1 mole of castor oil. By pouring the solution
into water and uniformly distributing it therein, an
aqueous dispersion is obtained.
V. 80 parts by weight of compound no. 99 is well
mixed with 3 parts by weight of the sodium salt of di-
isobutylnaphthalene-alpha-sulfonic acid, 10 parts by
weight of the sodium salt of a lignin-sulfonic acid
1274S~l
- 21 - O.Z. 0050/37398
obtained from a sulfite waste liquor, and 7 parts by
weight of powdered silica gel, and triturated in a hammer
mill. By uniformly distributing the mixture in water, a
spray liquor is obtained.
05 VI. 3 parts by weight of compound no. 129 is
intimately mixed with 97 parts by weight of parti~ulate
kaolin. A dust is obtained containing 3% by weight of the
active ingredient.
VII. 30 parts by weight of compound no. 147 is
intimately mixed with a mixture consisting of 92 parts by
weight of powdered silica gel and 8 parts by weight of
paraffin oil which has been sprayed onto the surface of
this silica gel. A formulation of the active ingredient is
obtained having good adherence.
VIII. 40 parts by weight of compound no. 213 is
intimately mixed with lO parts of the sodium salt of a
phenolsulfonic acid-urea-formaldehyde condensate, 2 parts
of silica gel and 48 parts of water to give a stable
aqueous dispersion. Dilution in water gives an aqueous dis-
persion.
IX. 20 parts of compound no. 215 is intimately mixed
with 2 parts of the calcium salt of dodecylbenzenesulfonic
acid, 8 parts of a fatty alcohol polyglycol ether, 2 parts
of the sodium salt of a phenolsulfonic acid-urea-form-
aldehyde condensate and 68 parts of a paraffinic mineraloil. A stable oily dispersion is obtained.
In these application forms, the agents according to
the invention may also be mixed and applied with other
active ingredients, e.g., herbicides, insecticides, growth
regulators, other fungicides and fertilizers. When mixed
with other fungicides, the spectrum of fungicidal action
is in many cases increased.
The following list of fungicides with which the
compounds according to the invention can be combined is
intended to illustrate , and not restrict, the combination
12~7~5~1
- 2- - O.Z. 0050/37398
possibilities.
Examples of fungicides which may be combined with the
active ingredients according to the invention are as
follows:
05 sulfur
dithiocarbamates and derivatives thereof, such as
ferric dimethyldithiocarbamate
zinc dimethyldithiocarbamate
manganese ethylenebisdithiocarbamate
1~ zinc ethylenebisthiocarbamate
tetramethylthiuram disulfide
manganese-zinc ethylenediamine-bisdithiocarbamate
zinc-(N,N'-propylene-bisdithiocarbamate)
ammonia complex of zinc-(N,N'-ethylene)-bisdithiocarbamate
and
N,N'-polyethylene-bis-(thiocarbamoyl)-disulfide
ammonia complex of zinc-(N,N'-propylene-bisdithio-
carbamate)
and
N,N'-polypropylene-bis-(thiocarbamoyl)-disulfide
nitro derivatives, such as
dinitro-(l-methylheptyl)-phenylcrotonate
2-sec-butyl-4,6-dinitrophenyl-3,5-dimethylacrylate
2-sec-butyl-4,6-dinitrophenylisopropylcarbonate
diisopropyl 5-nitroisophthalate
heterocyclic structures, such as
2-heptadecyl-2-imidazoline acetate
2,4-dichloro-6-(o-chloroanilino)-s-triazine
0,0-diethylphthalimidophosphorothionate
5-amino-1-~bis-(dimethylamino)-phosphynyl]-3-phenyl-1,2,4-
-triazole
2,3-dicyano-1,4-dithiaanthraquinone
2-thio-1,3-dithio-(4,5-b)-quinoxaline
methyl l-(butylcarbamoyl)-2-benzimidazole carbamate
2-methoxycarbonylaminobenzimidazole
2-[furyl-(2)]-benzimidazole
127~5~1
- 2~ - O.Z. 0050/37398
2-~thiazolyl-(4)]-benzimidazole
N-(1,1,2,2-tetrachloroethylthio)-tetrahydrophthalimide
N-trichloromethylthiotetrahydrophthalimide
N-trichloromethylphthalimide
05 N-dichlorofluoromethylthio-N',N'-dimethyl-N-phenyl-
-sulfuric acid diamide
5-ethoxy-3-trichloromethyl-1,2,3-thiadiazole
2-thiocyanomethylthiobenzthiazole
1,4-dichloro-2,5-dimethoxybenzole
4-(2-chlorophenylhydrazono)-3-methyl-5-isoxazolone
pyridine-2-thio-1-oxide
8-hydroxyquinoline and its copper salt
2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiin
2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiin-4,4-di-
lS oxide2-methyl-5,6-dihydro-4-H-pyran-3-carboxanilide
2-methyl-furan-3-carboxanilide
2,5-dimethyl-furan-3-carboxanilide
2,4,5-trimethyl-furan-3-carboxanilide
2,5-dimethyl-furan-3-carboxylic acid cyclohexylamide
N-cyclohexyl-N-methoxy-2,5-dimethyl-furan-3-carboxamide
2-methyl-benzoic acid anilide
2-iodobenzoic anilide
N-formyl-N-morpholine-2,2,2-trichloroethylacetal
piperazine-1,4-diylbis-(1-(2,2,2-trichloroethyl)-formamide
1-(3,4-dichloroanilino)-1-formylamino-2,2,2-trichlorethane
2,6-dimethyl-N-tridecyl-morpholine and its salts
2,6-dimethyl-N-cyclododecyl-morpholine and its salts
N-[3-(p-tert.-butylphenyl)-2-methylpropyl]-cis-2,6-di-
methylmorpholineN-~3-(p-tert.-butylphenyl)-2-methylpropyl]-piperidine
1-~2-(2,4-dichlorophenyl)-4-ethyl-1,3-dioxolan-2-yl-ethyl]-
-l-H-1,2,4-triazole
1-~2-(2,4-dichlorophenyl)-4-n-propyl-1,3-dioxolan-2-yl-
-ethyl]-1-H-1,2,4-triazole
lZ7~5Zl
- 24 - O.Z. OOS0/37398
N-(n-propyl)-N-(2,4,6-trishlorophenoxyethyl)-NI-imidazol-
-ylurea
1-(4-chlorophenoxy)-3,3-dimethyl-1-(lH-1,2,4-triazol-1-yl)-
-2-butanone
05 1-(4-chlorophenoxy)-3,3-dimethyl-1-~lH-1,2,4-triazol-1-yl)-
-2-butanol
alpha-(2-chlorophenyl)-alpha-(4-chlorophenyl)-5-pyrimidine-
methanol
5-butyl-2-dimethylamino-4-hydroxy-6-methylpyrimidine
bis-(p-chlorophenyl)-3-pyridinemethanol
1,2-bis-(3-ethoxycarbonyl-2-thioureido)-benzene
1,2-bis-(3-methoxycarbonyl)-2-thioureido)-benzene
and various substances, such as
dodecylguanidine acetate
3-[2-(3,5-dimethyl-2-oxycyclohexyl)-2-hydroxyethyl]-glutar-
imide
hexachlorobenzene
D,L-methyl-N-(2,6-dimethylphenyl)-N-(2-furoyl)-alanate
methyl D,L-N-(2,6-dimethylphenyl)-N-(2-methoxyacetyl)-
-alanate
N-(2,6-dimethylphenyl)-N-chloroacetyl-D,L-2-aminobutyro-
lactone
methyl DL-N-(2,6-dimethylphenyl)-N-(phenylacetyl)-alanate
5-methyl-5-vinyl-3-(3,5-dichlorophenyl)-2,4-dioxo-1,3-oxa-
zolidine3-(3,5-dichlorophenyl)-5-methyl-5-methoxymethyl-1,3-oxa-
zolidin-2,4-dione
3-(3,5-dichlorophenyl)-1-isopropyl-carbamoylhydantoin
N-(3,5-dichlorophenyl)-1,2-dimethyl-cyclopropane-1,2-di-
carboximide2-cyano-N-(ethylaminocarbonyl-2-methoximino)-acetamide
1-(2-(2,4-dichlorophenyl)-pentyl)-lH-1,2,4-triazole
2,4-difluoro-alpha-(lH-1,2,4-triazol-1-yl-methyl)-benz-
hydryl alcohol.
,~ . .
~Z~7 ~S21
- 2~ - O.Z. 0050/37398
For the following experiments, the prior art active
ingredient 2,2-dimethyl-4-(1,2,4-triazol-1-yl)-5-phenyl-pen-
tan-3-one (A) was used for comparison purposes.
05 Use Example 1
Action on cucumber mildew
The leaves of pot-grown cucumber seedlings of the
"Chinesische Schlange" variety were sprayed at the 2-leaf
stage with a spore suspension of cucumber mildew (Erysiphe
cichoracearum). After about 20 hours, the plants were
sprayed to runoff of aqueous emulsions consisting (dry
basis) of 80~ of active ingredient and 20% of emulsifier.
After the sprayed-on layer had dried, the plants were set
up in the greenhouse at from 20 to 22C and a relative
humidity of 70 to 80%. To assess the action of the novel
compounds, the extents of fungus spread was determined
after 21 days.
The results of this experiment show that for instance
compounds nos. 2, 11, 15, 87, 99, 129, 147, 213, 215, 231,
233, 255, 339, 351, 365, 379, 380, 381~ 383 and 384,
applied as 0.025% spray liquors, had a better fungicidal
action (e.g., 100%) than prior art active ingredient A
(60%).
Use Example 2
Action on BotrY is cinerea in Pimientos
Pimiento seedlings of the "Neusiedler Ideal Elite"
variety were sprayed, after 4 to 5 leaves were well
developed, to runoff with aqueous suspensions containing
(dry basis) 80% of active ingredient and 20% of emulsi-
fier. After the sprayed-on layer had dried, the plants
were sprinkled with a conidial suspension of the fungus
Botrytis cinerea, and placed at 22 to 24C in a chamber
of high humidity. After 5 days, the disease had spread to
such a great extent on the untreated plants that the
necroses covered the major portion of the leaves.
lZ74521
- 2G - 0. z . 0050/37398
The results of this experiment show that f or instance
compounds nos. 17 t 147, 231, 233, 255 and 297, applied as
0.05~, spray liquors, had a better fungicidal action (e.g.,
97~) than active ingredient A (60~,).
05
,:
. . . .