Note: Descriptions are shown in the official language in which they were submitted.
~2~fl~
1 51,321I
COMPATIBLE SELF-CROSSLINKING POLY (AMIDE-IMIDE)
POLYEPOXIDE RESIN BLENDS AND LAMINATES MADE THER~'~7ITH
BACKGROUND OF THE INVENTION
Because they are B-s~ageable- and have very good
properties, epoxy resins are widely used in making lami-
nates. Laminates have also been made using polyimide-based
laminating resins, which have many high performance charac-
teristics not possessed by epoxy resins. However, poly-
imide resins require msre severe curing conditions than do
epoxy resins to achieve the optimum properties.
Another approach to improving laminates has been
to modify the epoxy resins with imide functional adjuvants.
For example, U.S. Patent 4,244,857 teaches the use of
certain bis amino imides as curing agents for polyepoxide
resins. Similarly, U.S. Patent 3,978,152 discloses
thermosetting compositions which are blends of certain
unsaturated bisimides with adducts possessing an amino
group formed from an epoxy resin and excess amine. U.S.
Patent 3,984,373 describes a thermosetting resin composi-
tion based on an epoxy resin incorporated with an N,
N'-unsaturated amic acid-imide containing compound.
Similarly, U.S. Patent 3,979,393 relates to the use of
anhydride containing imidyl and isoimidyl compounds as
curing agents for polyepoxide resins. In these examples,
the imide containing adjuvants are low molecular weight
material~ which are primarily utilized as polyepoxide
hardeners. Therefor , the quantity of imide containing
~27~
2 51,321I
adjuvant blended with the epoxy resin rarely exceeds that
amount which is necessary to cure the epoxy resin.
The reason the imides are added as hardeners is
that until now it has not been possible to blend or allo~
polyimide resins in any quantity with epoxy resins to
produce compatible resin blends suitable for laminating
applications. For the most part, fully imidized pol~mers
are insoluble in organic solvents, and, while some partial-
ly imidized polymers are soluble in organic solvents, the
polyimide ~omponent is often not soluble in the epoxy
component so that a compatible resin blend cannot be
formed. In those cases where a blend of epoxy and poly-
imide resins can be formed which is compatible, very large
amounts of organic solvent are required to achieve a blend
solution viscosity which is suitable for the manufacture of
prepregs. The necessity of evaporating and r~covering
these large amounts of solvents renders the process
uneconomical.
` UMMARY OF THE INVENTION
We have discovered that a particular type of
imide oligomer will form a compatible blend with an epoxy
resin, and with other types of resins, using a moderate
amount of solvent. The imide oligomer of this invention is
formed from an imide polymer which contains amic acid
groups that can be hydrolyzed so that the products of the
hydrolization become compatible with the epoxy or other
resin and serve to cure the resinous blend. By incorporat-
ing an imide oligomer into the resins, we have been able to
achieve laminates having improved properties such as a
higher glass transition temperature, better chemical
resistance, and increased toughness. Wire enamels having
good elongation and heat shock, and fast processing times
can also be made from blends containing this imide oli-
gomer. ~e have also found that a thermoplastic laminate
can be made using the imide oligomer by itself.
3 51,321I
DESCRIPTION OF THE INVENTION
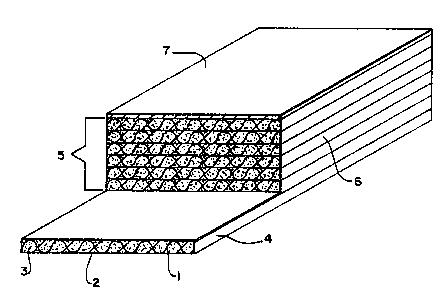
Figure 1 is an isometric view in section sf a
certain presently preferred embodiment of a laminate
according to this invention.
Figure 2 is an isometric view of a filament wound
tube, such as a launch tube, according to this invention,
partially cut away.
In the drawing, a layer 1 of a fibrous material 2
is impregnated with a resinous matrix 3 which has been
- 10 B-staged to form prepregs 4. A stack of prepregs 5 heated
under pressure to cure the resinous matrix to the C-stage
fo~ms laminate 6. A~co~per foil 7 has been bonded to one
surface of the laminate.
In Figure 2 " filaments 8, for example, of glass,
are wound and imbedded in resinous matrix 9, which has been
cure~ to the C-stage,~ orming tube 10. Filament wound
structures are made by passing roving through a resin bath
and over a mandrill, where the resin is cured.
In the first step of preparing a laminate accord-
ing to the process of this invention, a composition isprepared of a resinous component, (which can be a mixture
of resinous components), an imide polymer having amic acid
functionality, and a solvent. The resinous component, also
called a "coreactive compound," is a monomer, oligomer, or
polymer that is reactive with the imide oligomer that is
formed by hydrolyzing the imide polymer. Suitable resinous
components include bismaleimides and derivatives thereof,
polyimides, diols and diacids, diacids and diamines,
phenolic resins, capped isocyanates, and polyepcxides.
Bismaleimides are formed by reacting maleic anhydride with
an aromatic polyamin~. The polyamine is a compound having
at least two amine groups; preferably, it has exactly two
amine groups because those compounds are more readily
available. Derivatives of bismaleimides include
bismaleimides that have been partially reacted with aromat-
ic diamines or with unsaturated derivatives of bisphenol A.
4 51,321I
The reaction of the oligomer of this invention with diols
and diacids will produce a partially interpenetrating
network of a polyester-modified polyamide-imide. The
reaction of the oligomer of this invention with diacids and
diamines will produce a partially interpenetrating network
of a polyamide-imide modified polyamide~imide. Since the
reaction of the oligomer of this invention with diols and
diacids, diacids and diamines, phenolic resins, and capped
isocyanates evolves volatiles, these resin systems are
~10 limited to applications such as wire enamels and other
coating applications. A polyepoxide is any type of epoxy
resin having at le~st two epoxide groups. It may be
cycloaliphatic, novolac, an epoxy prepared from methylene
~ dianiline, an epoxy prepared from para-aminophenol, or a
brominated epoxy. The preferred epoxy resins are the
r1 diglycidyl ethers of bisphenol A, however, as they are
readily available.
The imide polymer which is hydrolyzed to produce
the imide oligomer of this invention should have suficient
~0 amic acid groups to be soluble in the solvent. However, if
too many amic acid groups are present, the product will
have deficient properties, and if too few amic acid groups
are present! the polymer will tend to be insoluble. About
5 to about 50% of the total of amic acid plus imide groups
along the polymer should be amic acid groups, and 50 to 95%
imide groups, as it is the imide groups which enhance the
properties of an impregnating resin.
The solvent should be an organic compound which
is highly aprotic in order to dissolve the imide polymer.
Suitable solvents include N-dimethyl formamide (DMF),
N-methyl-2-p~rrolidone (NMP), and dimethyl sulfoxide. The
p~eferred solvent is N~dimethyl acetamide (DMAC~ because it
is easily removed during B-staging. Mixtures of solvents
ca~ also be used. In addition, up to about 5% (all per-
centages herein are by weight based on total compositionweight, unless otherwise indicated) of a hydrocarbon
~7~
51,321I
solvent such as xylene, toluene, or Solvesso, can be added
to reduce the cost of ~he solvent. Sufficient water must
also be present to hydrolyze substantially all of the amic
acid groups in the imide polymer. Typically, the other
5 components in the composition contain sufficient water as
an impurity to perform this function, but if they do not,
additional water must b~ added so that at least about the
stoichiometric amount required for hydrolysis is present.
With some resinous components, it is also possible to
hydrolyze ~the imide polymer prior to its addition to the
composition.
The composition comprises about 5 to about 95 phr
(parts by weight per hundred parts by weight resin, where
"resin" means the resinous component plus the imide poly-
mer, based on total solids) of the imide polymer and aboutS to about 95 phr of the re~inous component. ~referably,
the composition comprises about 50 to about 80 phr of the
resinous component and about 20 to about 50 phr of the
imide polymer. The actual ratio of resinous component to
imide polymer used is determined by the desired glass
transition temperature, since a higher imide content will
result in a hiyher glass transition temperature. The total
composition is preferably about 10 to about 80% by weight
solids, the remainder being the solvent. The composition
may also include about 0.1 to about 0.7 phr of a catalyst,
if desired, to shorten the gelation time. Suitable cata-
lysts for polyepoxides and bismaleimides include tertiary
amines such as 2-methylimidazole, and benzyl dimethyl
amine; the preferred catalyst is 2-methylimidazole (2-MI).
Catalysts, if needed, for other resinous components are
well known in the art.
Other optional ingredients include about 0.1 to
about 25% (based on total solids weight) of a filler such
as alumina trihydrate. The filler performs the function of
reducing the amount of resin required, which is expensive,
and in addition, makes the composition less flammable. A
~27~
6 51,3211
final optional ingredient is about 0.1 to about 5% (based
on total solids weight) of a pigment.
In preparing the composition, the resinous com-
ponent, the imide polymer, the solvent, and the filler and
pigment, if used, are cooked until no more amic acid
functional groups are present. This can be usually accom-
plished by heating a-t about 100 to about 150C, typically
for about a half hour. Of course, the lower temperatures
are used for longer times and the higher temperatures are
used for shorter times. During hydrolysis, the water
present in the composition decomposes the amic acid func-
tional groups to form carboxylic acid and amine groups:
~NE1~3N / ~ N~-e3N~
n m
}~2--[ N~N/ ~
\C C--rNH~>NH2 1! _
L n HOOC im
The resulting imide oligomer is an aromatic
compound having a molecular weight which depends upon the
. ~,, .~
~7~
7 51,321l
number of amic acid groups in the imide polymer. The imide
oligomer necessarlly contains both amine groups and carbox-
ylic acid groups, as a result of the hydrolysis, as well as
at least one fully formed imide ring.
The carboxylic acid and amine groups in the imide
oligomer then react with groups on the resinous component
to form a new polymer containing all three groups. T~at
this occurs is shown by infrared spectrographic analyses of
sa~ples of the composition taken at 5 minute intervals as
~t is being heated. When the resinous component is a
polyepoxide, the spectrographic analysis shows the disap-
- pearance of both the amic acid peak and the epoxy peak and
the growth of a new intermediate peak~for the new polymer.
Efforts to form the same new polymer by simply mixing the
polyepoxide with the original imide polymer were not
successful because the two polymers are not compatible and
will not dissolve in each other. Nor could the new polymer
be formed by first hydrolyzing the imide polymer, then
adding the polyepoxide, because the imide portion of the
hydrolyzed imide polymer is not soluble in the mixture.
After cooling, the optional catalyst can be added if
desired. In addition, at this time, additional solvent can
be added to reduce the viscosity further, if desired.
In the next step in the process of making a
laminate according to this invention, a substrate is
impregnated with the composition containing the new poly-
mer. The substrate may be of any fibrous material includ-
ing glass, cotton, quartz, polyamide, polyaramid, paper,
graphite, carbon, or mixtures thereof and may be in many
forms including woven cloth, mat, or rovings as used in
pultrusion or filamant windings. The preferred substrate
material is, of course, dependant upon laminate end use.
The amount of resin solids impregnated into the substrate
depends upon the type of substrate used in the application.
Typically, about 20 to about 60% by weight resin solids are
impregnated with about 40 to about 80~ by weight of the
~ 2~
8 51,3~11
substrate.
In the next step in the process of making a
laminate according to this invention, the impregnated
substrate is heated to B-stage the resin. The time and
temperature required for B-staging depends upon the partic
ular resin used, but generally speaking, the impregnatPd
substrate is heated to the boiling point of the solvent or
slightly higher. This results in the evaporation of the
solvent and the advance of the resin to the B-stage, the
point at which it is non-tacky and can be handled. The
resulting article is a prepreg.
In the next step of the process of making a
laminate according to this invention, the prepregs are
stacked and heated under pressure to form a laminate.
Copper foil may be placed on either or both surfaces of the
stack to form a laminate suitable for- making printed
circuit boards. The temperature, time, and pressure used
depend upon the materials and the properties desired, but
about 150 to about 220C for one hour at 1,000 psi is
typical.
The composition can also be used to make a wire
enamel. In this case, the wire is simply run through the
composition after the imide polymer is hydrolyzed, excess
composition is removed by dies, wiping or other means, and,
in a single step, the composition is cured to the C-stage
and the solvents are evaporated. In addition, the composi-
tion can be used to make filament wound composite launch
tubes, laminates used as components for ship propulsion
room equipment, such as sub-base structures, generator
covers, and gearcase components, and laminates used as
electromagnetic launcher components.
The following is a description o the materials
used in the examples:
"Epon 829'' - a diglycidyl ether of bisphenol-A
made by Shell Chemical Co. by reacting epichlorohydrin with
bisphenol-A. In addition to the diglycidyl ether, "Epon
~2'7~
9 51,321I
829" contains an unspecified level of a proprietary pho3-
phonium halide catalyst. The material is supplied at 96 5%
solids in xylene and contains a maximum 0.03 wt.~o
hydrolyzable chlorine on an as-is basis.
~- 5 "Torlon 4000T" - a low acid value, high molecular
weight poly(amide-imide~ resin containing ~ome amic acid
functionality, supplied at 100% solids as a free flo~"ing
crumb by the Amoco Chemicals Corporation. The material ~,/as
developed primarily as a high performance injec~ion molding
resin and has the following generalized structure:
; U ~ CH2 ~ N-C ~ / N j
"Tritherm 981" - a poly(amide-imide) wire enamel
supplied at 26% total solids in 65/35 (by weight)
NMP/xylene by the P D. George Company.
- 15 "MY720" - a Ciba~Geigy high performance,
tetrafunctional epoxy resin with the following structure:
R R
N ~ CH2 ~ N < O
R R R = -CH~-CH-CH2.
The material has an epoxide equivalent weight of 125
gm/equiv and is supplied at lOO~o solids.
"PLYOPHEN 94-308" - a monomeric, aromatic
bismaleimide sold by Reichhold Chemicals Corp., made by
condensing methylene dianiline and maleic anhydride.
10 51,321I
"IM-AD94-394" - a two component polyimid iami-
nating resin sold by Reichnh1old Chemical Corp. One compo-
nent is "PLYOPHEN 94-308" and the other is an unsaturated
phenolic novolac oligomer.~K~
"COMPIMIDE 183" - a totally aromatic two compo-
nent polyimide impregnant sold by The Boots Compan~, PLC,
Nottingham, England. It is made by reacting bismaleimides
with m-aminobenzoic acid hydrazide at 100% solid~.
-~ EXAMPLE 1
~he following four resinous reactor products
based upon various polyepoxide resin/poly(amide-imide)
resin combinations were prepared:
Resin ~ A B C D
Epoxy Component(s) "Epon 829" 1'MY720"- "Epon 829" "~5Y720l'
"Epon 829"
Poly(Amide-Imide) "Torlon "Torlon"TrithPrm "Torlon
Component 4000T" 4000T" 981" 4000T"
The following is a description of the preparation of these
reactor products. A typical charge for the preparation of
20 an "Epon 829"/"Torlon 4000T" resinous reactor product
(Resin A) is presented in the following table:
Weight
ChargedWt. (gm) Composition
Material Function(gm) NV~ Equiv.(Wt. %)
25 "Epon 829" Epoxy Resin 483.3 466.4 2.48 80.0
"Torlon 4000T" Poly(amide-116 6 116.6 -- 20.0
imide)
DMAC Solvent 349.7 -- -- --
Xylene* Solvent (16-9)
30 Total Charge 949.6 583.0 2.48 100.0
*-Not charged separately - I'Epon 829" solvent
** Non-volatile.
~7~
11 51,321I
Dimethyl acetamide (349.7 gm) was charged in~o a
2,000 ml round bottom, three necked flask fitted with an
agitator, nitrogen inlet tube, thermometer, reflux condens
er and a means for heating. The flask was swept with N2
gas for 5 minutes and the flow rate adjusted to maintain a
slight positive N2 pressure in the flask throughout the
balance of the run.
The agitator was started and the DMAC was slowl~
heated to 60C wher~upon neat "Torlon 4000T" (116.6 gm) was
slowly added to the hot DMAC at such a rate as ~o prevent
clumping. After the addition of the "Torlon 4000T" was
complete, the slurry was heated to 105C to effect complete
dissolution. The uniform, smooth "Torlon 4000T"/D~AC
solution was then cooled to 100C and "Epon 829" (483.3
gm/2.48 equiv) was slowly added to the flask over a 5
minute period. After the "Epon 829" addition was complete,
a small amount of the reaction mixture was removed for Gel
Permeation Chromatographic (GPC) analysis and "Epon 829"/
"Torlon 4000T" compatibility testing. Compatibility
testing was accomplished by casting a small amount of
material on a glass slide (termed a "pill" in the following
log) and driving off the DMAC. A "hazy pill" indicates
that "Torlon 4000T" was not soluble in "Epon 829."
After the initial samples had been taken the
flask was slowly heated to 140C and held there. Sampling
was continued during the 140C hold and, when a clear pill
had been attained, the reaction was cooled and the product
set aside for evaluation. The product (Resin A) had a
solids content of 61.4% and a viscosity of 420 cps (#2 @ 20
rpm on the Brookfield Model; RVF measured @ 25C). The
significance of the GPC/IR data and other information
obtained during the run will be discussed in a later
section of this example.
The following is a log of the preparation of this
reactor product.
~7~
12 51,321I
Time Temp. (C) Remarks
11:07 RT N2, heat on DMAC, stirrer on
11:10 60 Start "Torlon 4000T" in slowly
11:15 95 "Torlon 4000T" in, hold - H20 droplets on condenser
11:16 105 Stir, hold temp. until uniform
11:27 100 Solution uniform, begin "Epon 829" addition
11:32 63 "Epon 829" in, heat to 140C, pill-l, GPC ~ample-l
11:38 75 Continue heating
11:44 100 Take pill-2 (hazy), continue heating
11:53 120 GPC sample-2, pill-3 (hazy), co~inue heating
12:05 140 Hold, GPC sample-3, pill-4 (ha~), viscosity drop
12:20 140 GPC sample-4, pill-5 (hazy), continue heating
12:35 140 GPC sample-5, pill-6 (clear), begin cooling
12:48 45 Continue cooling c
12:58 35 Pour/store for evaluation
The same equipment but slightly different proce-
dures wer~. utilized to prepare Resins B, C, and D. Pro-
cessing parameters and reactor charges for these materials
are presented in the following Tables:
Reactor Charge For Resin B
Weight
Charged Wt. (gm)Composition
Material Function(gm) NV Equiv. (Wt. %)
"Epon 829" Epoxy Resin 342.0 330.0 1.753 55.0
"MY720" Epoxy Resin 120.0 120.0 0.960 20.0
"Torlon 4000T"Poly(amide- lS0.0 150.0 -- 25.0
imide)
DMAC Solvent388.0 -- -- --
1,000.0 600.0 100.0
Processing Parameters For Resin B
Reaction Temperature - 140C
Reaction Time @ 140C - 0.5 hr
Wt. % Solids - 60.0%
Viscosity - 1,086 cps (#2 @ 20 rpm)
~'~7~
13 51,321
Reactor Charge For Resin C
Weight
Charged Composi~ion
Mat2rial Function (gm) Equiv. (Wt. %)
"Epon 829" Solids Epoxy Resin 420.0 2.Z3 70.0
"Tritherm 981"Poly(amide 180.0 -- 30.0
Solids imide)
NMP-:: Solvent 370.5 -- --
Xylenet: Solvent 214.7 -- --
10 ~1 1,185.2 100.0
::Not charged - "Epon 829"/"Tritherm 981" solvents.
Processing Parameters For Resin C
Reaction Temperatures - 140C, 160C
Reaction Times - 0.5 hr @ 1409C, 0.5 hr. @ 160C
Wt. % Solids - 50.6~
Viscosity - 1,680 cps (#2 @ 20 rpm)
Reactor Charge For Resin D
Weight
Char~ed Composition
Material Function (gm) Equiv. (Wt. %)
"MY720" Epoxy Resin 420.0 3.36 70.0
"Torlon 4000T"Poly(amide 180.0 -- 30.0
imide)
25 DMAC Solvent733.3 -- --
l,333.3 100.0
Processin~ Parameters For Resin D
Reaction Temperature - 1~0C
Reaction Time @ 140C - 0.5 hr
Wt. % Solids - 45.0%
Viscosity - 1,010 cps (~2 @ 20 rpm)
Note that a 160C processing temperature is
required to achiave compatibility between "Epon 829" and
"Tritherm 981" in Resin C. H~ating is not required to
achieve compatibility between "MN720" and "Torlon 4000T"
(Resin D) for the specified composition. However, heating
~7~
14 51,321I
at 140C is required to bring about the reduction in
viscosity necessary for laminating applications
Although no examples are provided,
tetrabrominated polyepoxides such as Do~r's "DER 542," "DER
521A75," and the like would have utility in th~ practice of
this invention. Similarly, multifunctional epoxy resins
other than "MY720" (such as epoxy novolac resins) would
have application. In like manner, nondiglycidylether
bisphenol-A resins such as Union Carbide's cycloaliphatic
epoxy resin line could be utilized in the preparation of
the disclosed amide-imide m~dified laminating resins.
EX~MPLE 2
Compositional data along with varnish properties
for a series of laminating varnishes which demonstrate the
utility of our disclosure is presented in th~ table which
follows. The varnishes were formulate~ by charging an
appropriate amount of Resin A, B, C, or D into a stainless
steel beaker fitted with a propeller type agitator. A
catalyst of 2-methylimidazole (0.28 wt.% on total resin
solids) was charged and mixed until it had dissolved.
Additional solvents, filler, pigments, and/or catalysts and
the like could have been added at this stage in the formu-
lation scheme. The varnishes were stirred 15 minutes prior
to impregnation onto style 7628 glass cloth.
25 Laminate A B C D
Resin ~ B C D
Wt.% "Epon 829" 74.77 54.83 69.80 --
Wt.% "MY720" -- 19.94 -- 69.~0
Wt.% "Torlon 4000T" 19.94 24.93 -- 29.92
Wt.% "Tritherm 981" -- -- 29.90 --
Wt.% 2-MI 0.2g 0.29 0.30 0.28
Impregnating Solids, 61.6 53.4 51.0 45.0
Wt.%
Impregnating Solvents DMAC DMAC/Methyl Xylene/NMP D~C/Xylene
Cellosolve
Varnish Viscosity, cps 350 400 1,665 1,010
Set-Time, Mins @ 153C 32.8 17.0 40.4 25.1
51,321I
Note that in the above table the indi~idual
polyepoxide/poly(amide-imide) resins are presented in terms
of composition. As an example, Laminate A c~ntains 74.77%
"Epon 829" and 19.94% "Torlon 4000T." The ratio of th se
numbers corresponds to the composition of Resin A. The
same holds true for Laminate B; i.e., the ratio of tabulat-
ed "Epon 82g"/"MY720"/"Torlon 4000T" sorresponds to the
composition of Resin B.
Style 7628 glass cloth was utiliized to prepare
15" x 15" prepregs from the varnishes outlined in the above
table. The wet prepregs were B-staged at 160C for 10
minutes. After B-staging, the prepregs were approximatel~
40% resin and 60% glass cloth.
The B-staged prepregs were cut into 7" x 7"
s~uares and press laminated by stacking nine (9) individual
pieces betw~en steel caul plates with Tedlar mold release
sheets between tha caul plates and prepreg stack. The
molding packs were loaded into a cold press with five
layers of kraft paper between the press platens and caul
plates. Individual molding packs were then heated to 180r
under 1,000 psi in 45 minutes with an hour hold at 180C.
Cooldown was accomplished under pressure.
General properties for the laminates are present-
ed in the following table.
25 Laminate A B C D
Glass Transition, 149.4 157.0 159.0 205.7
DSC, C
Z-Direction~ Expan- 60.6xlO 75.4xlO 75.1xlO 6 __
sion, In./In.tC
Solder Float, 60+ 60~ 60+ 60+
Sec. @ 525F
Acetone Resistance Excellent Excellent Excellent Excellent
DMF Resistance Excellent Excellent Excellent Excellen~
*Z-direction expansion coefficien~ measured from 40C to Tg.
From the data in the above table, it is apparent that a
16 51,321I
broad range of Tg values can be produced without the
addition of external crosslinking agents. It is further
apparent that the epoxy component as well a~ tne
poly(amide-imide) component can influence laminate Tg.
Note that the utilization of "MY720" in Lamina~e B and D
results in Tg values higher than the 149.4C shown for
Lamina'e A. Laminate C, based upon "Tritherm 981," pro-
vides a higher Tg laminate (159.0C) than Laminate A, bas~d
upon "Torlon 4~0~T" (149.4C).
10Lamina~e samples were placed in uncovered alumi-
num pans and aged in a vented, forced air oven at 225C.
We~ght losses observed during thermal aging are presented
in the following table. Note that a production G-10
~r laminate was also heat aged at 225C. NEMA grade ER-4
laminate was also aged at 225C but delaminated in 2
hour~
LAMINATE
G-10 A B C
Hrs @ Wt.%Hrs @ Wt.%Hrs @ Wt.%Hrs @ Wt.%
20225C Lo 225~C Loss225C Loss 225C Loss
24 1.2924 0.9324 1.06 24 1.08
48 1.6748 1.2048 1.44 48 1.44
72 1.9272 1.4072 1.69 72 1.70
144 2.45 96 1.5596 1.89 96 1.90
25 168 2.60168 1.99168 2.39 168 2.44
384 3.44 192 2.10192 2.53 192 2.59
672 Failed 2162.22 2162.66 216 2.72
240 2.31240 2.77 240 2.83
264 2.40264 2.86 264 2.93
336 2.65336 3.14 336 3.23
360 2.73360 3.22 360 3.32
576 3.32576 3.80 576 3.98
864 3.85864 4.27 864 4.54
Thermal aging at 225~C for Laminates A, B, and C was con~inued.
~Resin content = approximately 40%.
After 864 hours at 225C all of the polyepoxide/
17 51,321I
poly(amide-imide) based laminates were bliste~ free and
apparently retained their initial high degree of mechanical
integrity.
EXAMPLE 3
PREPARATION OF HYDROLYZED '~TORLON 4000TF"
A typical charge and accompanying log for th~
preparation of hydrolyzed "Torlon 4000T" is shown below.
MaterialWt. Charged (Gm~Composition, Wt. %
"Torlon 4000T"600.0 29.70
10 DMF 1,400.0 69.31
Deionized H2020.0 O.g9
2,020.0 100.00
TimeTemperature (C~ Remarks
8:15 RTCharge DMF/H2O Into Reactor, Heat/N2 On
15 8:20 RT Begin Charging T-4000
8:29 58 All T-4000 Charged
9:00 139Refluxing-Gardner Vis. = Y @ 24.1C
9:30 140 Gardner Viscosity = Q-R
10:00 140 Gardner Viscosity = N-O
20 10:15 140Heat off - Cool & Store (Vis. = N-O)
Dimethyl formamide (1,400.0 gm) and deionized water (20.0
gm) were charged into a 3,000 ml round bottom flask fitted
with an agitator, nitrogen inlet tube, thermometer, reflux
condenser and a means for heating. The flask was swept
with N2 gas for 5 minutes and the flow rate adjusted to
maintain a slight positive N2 pressure in the flash
throughout the balance of the run. The agitator was
started and the DMFfH20 solution heated while the "Torlon
4000T" was added to flask at such ~ rate as to prevent
30 clumping. After addition of the "Torlon 4000T" (58C~ the
slurry was heated to 140C and held until the solution
viscosity dropped from Y to N-O. The resulting hydrolyzed
product had a solids content of 29.5% solids and was
l Z 7 ~
18 ;1,321I
utilized to prepare laminate 211184-42. Further hsatiny
would not have led to a further reduction in solution
viscosity.
EXAMPLE 4
5 PREPARATION OF A LAMINATE FROM HYDROLYZED "TORLON 4000T"
This example is provided to show that the
"Torlon" hydrolysis product is resinous and can be pro-
cessed utilizing conventional techniques.
`~A 16~" x 49~2" swatch of style 7628 ~fiberglass
10 cloth was continuously impregnated with hydrol~ed "Torlon
4000T" reactor product of Example 3. The wet swatch was
then cut into three 16~" x 16~2" sec'cions and B-staged 7
minutes at 175C to produce prepreg with a resin content of
32% by weight. The prepregs were cut into 15~2!J x 7 3/4"
15strips and again impregnated with 211184-40. After
B-staging 10 minutes at 175C, the F~repregs ha~ a resin
content of 50.1% by weight.
A molding pack was constructed by stacking nine
each 7" x 7" plys of prepreg between two silicone coated
20 fiberglass bleeder plys. The molding pack was placed
between steel caul plates and the whole loaded into a cold
press with two layers of fiberglass cloth between the press
platens and caul plates. The molding pack was then heated
to 225C under 1,000 psi in one hour with an hour hold at
25 225C. Cooldown was accomplished under pressure.
After lamination under the above conditions the
resulting laminate was unitary and s~uite tough. A Tg value
of 185C was measured via Dynamic Mechanical Analysis
(DMA). The evolution of cure volatiles during press
30 lamination was not evident.
A O.5 square inch section was cut from the
laminate and placed into a sample jar containing 50 gms of
DME. After approximately one hour the resin had dissolved
thereby showing that the hydrolyzed "Torlon" remains
35 essentially a thermoplastic after lamination.
~2~
19 51,321I
EXAMPLE S
PREPARATION OF IMPREGNATING RESIN BASED UPO~T
HYDROLYZED "TORLON 4000T" AND "PLYOPHEN 94-308"
A typical charge and accompanying log for the
preparation of an impregnating resin (211184-32) based upon
hydrolyzed Torlon 4000T and Plyophen 94-308 is shown belsw.
Material Wt. (Gm) Wt, (Gm) NVComp~sition, Wt./D
Hydrolyzed "~orlon" 600.0 196.2 50.0
(ExamplP 3~
"Plyophen 94-308'1 196.2 196.2 50.0
796.2 392.4 100.0
c
TimeTem~. ( C) Remarks
1:30RT N2, Agitator and Heat On 211082-127 and 94-308
1:4090 Reaction Mixture Clear,~ ~ Reflux
15 ~ 1 54 a~42 Refluxing - Hold. Pill - Hazy
2 10142 Pill Still Hazy
2:22143 Pill - Hazy;~_ 150C
2:55150 D~ Off, Pill Slightly Hazy
3:02153 27.1 gm DMF Off - Continue H~ating
3: 30 153 Heat Off - Pill Clear - Store Resin For Evaluation
Hydrolyzed "Torlon 4000T" (600.0 gm) and "Plyophen 94-308"
(196.2 gm) were charged into a 2,000 ml round bottom, three
necked flask fitted with an agitator, nitrogen inlet tube,
thermometer, reflux condenser with Dean-Stark trap and a
means for heating. The flask was swept with N2 gas for
five minutes and the flow rate adjusted to maintain a
slight positive N2 pressure in the flask throughout the
balance of the run.
The agitator was started and the reaction mixture
slowly heated to reflux. Compatibility testing was accom-
plished by casting a small amount of material on a glass
slide (termed a "pill" in t'he log) and driving off the DMF.
A "hazy pill" indicat~s that "Plyophen 94-308" is not
soluble in the hydrolyzed "Torlon 4000T" solids. The
reaction mixture was sampled during the 140-150C hold and
51, 3211
when a clear pill had been attained, the reaction was
cooled and the product set aside for evaluation. The
product had a solids content of 51.0%, a viscosity of 460
cps at 90C and a gelation tim~ of 45 minutes at 150C.
The product was impregnated without further modification.
EXAMPLE 6
LAMINATE PREPARATION
Approximately 400 gm of the impregnant from
Example 5 was heated to 90C and utilized to impregnate a
16l~2" x 49~" swatch of style 7628 fiberglass sloth. The wet
swatch was cut into three 16~2" x 16~" sections and B-stag~d
5 minutes at 170C to produce prepreg with~ a 43.3% resin
content. A molding pack was constructed by stacking nine
each 7" x 7" plys of prepreg between two silicone coated
fiberglass cloth bleeder plys. The molding pack was placed
between steel caul plates and the whole loaded into a cold
press with two layers of fiberglass cloth between the press
platens and caul plates. The molding pack was heated to
225C under 1,000 psi in 1 hour with a 1 hour and 15 minute
hold at 225C. Cooldown was accomplished under pressure.
The resulting laminate was unitary, DMF resistant
and had a Tg of 195C as measured by DMA (Figure 2). A
portion of the laminate was post-baked 16 hours at 250C.
Post-baking resulted in a 225C Tg as measured via DMA.
EXAMPLE 7
PREPARATION OF IMPREGNATING VARNISH BASED UPON
HYDROLYZED "TORLON 4000T" AND "IM-AD 94-394"
An impregnating varnish based upon hydrolyzed
"Torlon 4000T" and "IM-AD 94-394" was prepared via a
kettling operation. The charge and accompanying log is
shown below.
r~
21 51,321I
Ma~erial Wt. (Gm) Wt. (Gm) NVCompo~ition, Wt. %
Hydrolyzed Torlon 291.7 87.5 2;.0
(Example 3)
"IM-AD 94-394"262.5 262.5 75.0
DMF 145.8
700.0 350.0 100.0
TimeTemp. (C) Remarks
8:20 RT Charge - 40+DMF, Begin IMJAD 9h-394 Addition,
N2, Heat On
8:55 60 IM-AD 94-394 Addition Complete
9:06 130 Hold, Pill Taken - Hazy
9:10 135 Mantle Down, Cool to 130 - Pill - Hazy
9:13 132 Mantle Up
9:19 130 Pill - Hazy
9:32 134 Pill - Hazy; Heat Back
9:36 130 Pill Nearly Clear
9:37 130 Gardner Viscosity - Z-l @ 25.5C, Transfer to
-~ Preparing Trough ~-
Prepregs and a single nine ply laminate w~re
prepared utilizing techniques described in Examples 3 and
4. The prepregs ware staged 4 minutes ~t 150C followed by
five additional minutes at 170C. The resulting prepreg
had a resin content of 41.6% and was laminated in a nine
ply array under 1,000 psi for 1 hour at 225C. Cooldown
was done under pressure. The resulting laminate was
unitary, DMF resistant and had a Tg value of 225C measured
via DMA. Post bake would have resulted in increased Tg.
EXAMPLE 7
PREPARATION OF IMPREGNATING VARNISH BVASED UPON
HYDROLYZED "TORLON 4000T" AND "COMPIMIDE 183"
The resin charge and accompanying log shown below
pertains to the preparation of a laminating composition
based upon hydrolyzed "Torlon 4000T" and "Compimide 183."
22 ~1,32'I
Material Wt. (Gm) Wt. (Gm) NVComposition, Wt. 7/~
"Compimide 183" 225.0 225.0 75.0
(Example 3)
Hydrolyzed 4000T 250.0 75.0 25.0
DMF 125.0
600.0 300.0 100.0
Time Temp. (C) Remarks
o:45 ~'- RT Charge - 40+DMF; Heat, N2 On; Start 183 Addition
8:50 50 "Compimide 183" Addition Complete
8:54 80 183 Dissolved,~130C
9:02 130 Hold - Pill Taken, Slightly Hazy
- 9:17 129 Slightly Hazy Pill
9:26 129 Gardner Viscosity - M ~o N (330 cps @ 25C)
9:41 130 Viscosity M-N
9:52 129 Heat Off, Cool - Hold For Evaluation
Prepreging and laminating techniques previously
described were utilized with this resin. The resin was
impregnated at 40C and staged 3 minutes at 150C followed
by 4 additional minutes B-stage time at 170C. The result-
ing prepreg had a resin content of 38.0% and was laminated
in a nine ply array at 225C for 1 hour under 1,000 psi
pressure. Cooldown was accomplished under pressure. The
resulting laminate was unitary, well cured, DMF resistant
and had a Tg of 212C via DMA.
EXAMPLE 8
- A varnish was prepared of the following
composition.
MaterialWt. (Gm) Wt. (Gm) NVGomposition, Wt. %
Hydrolyzed "Torlon 300.0 90.0 74.91
4000" (Example 3)
Epon 829 31.09 30.0 24.97
2-MI 0.14 0.14 0.12
333.23 120.14100.00
23 51,321I
The varnish was submitted for evaluation as an overcoat for
current low cost polyester coated wire and was indicted in
a pilot wire tower. The varnish had fas~ proc~ssing
characteristics, and the coating exhibited good elongation
and heat shock characteristics. The following ~able gives
the results of some of these experiments.
Sample A Sample B
Tow(Ftr/Spee) _ 28 32
.
10 Quick Jerk Passed Passed
Elongation + 1 x (%) Passed Passed
.
Electric Stress (KV) 7.9-8.0 6.5-8.3
_ _
l x heat shock 200C Passed Passed
...
220C Passed Passed
lS 240C Passed Passed
260C Passed Passed