Note: Descriptions are shown in the official language in which they were submitted.
UNIT DOSAGE DELIVERY DE~iICE
INTRODUCTION
The manufacture of typical solid unit dosage compositions,
such as tablets and capsules containing one or more active
ingredients, requires sophisticated equipment operating at
high speed, as well as substantial capital investment. In
addition, various excipients are incorporated with the
active ingredient in order to increase the volume of a
unit dose composition to a manageable size, and/or provide
other properties to the composition such as binding,
lubricating, disintegrating, dissolution rate enhancing,
and flow character. Because each active ingredient has
its own particular physical and chemical properties,
dosage formulations and manufacturing techniques must be
developed for each active ingredient. The development of
dosage formulations and rela~ed manufacturing processes is
a major expenditure of pharmaceutlcal manufacturers and
research organizations. For exampie, in order to develop
a tablet formulation, many tedious processes, such as
milling, sizing, micronizing, and granulating, are often
required to pre-treat raw materials and powder mixes. An
acceptable tablet formulation must produce tablets having
acceptable stability, content uniformity, disintegration
and dissolution rates. Also the powder mix must have
specific physical properties in order to process the
formulation using high speed tableting equipment. In
" ~:
-2-
addition, the use of e.~cipients reduces stabillt~,
bioavailability and dissolution rate of drugs.
It is an object of the present invention to provide a unit
dose device for dispensing an excipient-free comestible
product into a liquid.
SUMMARY OF THE INVENTION
The present invention relates to a device for dispensing a
comestible product into a liquid, said device comprising
dispensing means having an e~terior wall and an interior
wall and at least one opening and dispersed within said
dispensing means is a comestible product having a porous
( lattice structure, said structure having a shape defined
by the interior wall of said dispensing means, with at
least a part of said porous lat~ice structure being
communicable with the liquid in which said device is
placed.
,
~he present invention further relates to a method for
preparing a device for dispensing a comestible product
comprising dissolving a comestible product in a suitable
solvent an~ crystallizing the comestible product in a
dispensing means having an e~;terior wall and interior wall
and at least one opening, allowing the comestible product
to crystallize within the dispensing means in the form of
-3-
~7~
a Ol'O-lS lattice structure ha~ring a shape defined by the
interior wall of said dispensing means.
' (
The unit dose delivery device of ~he present inventioll is
particularly useful for providing measure~ auantitieS o
an active ingredient to a solutioll.
DETAILED DESCRIPTION OF THE INVENTION
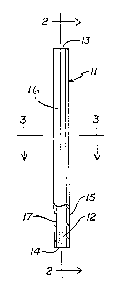
Figure 1 is an elevational view of a cylindrical u11it dose
delivery device of a construction in accordance wi'h ~he
present invention;
Figure 2 is a central sectional elevation or the device of
( Figure 1, taken along plane 2-2 of Figure l;
Figure 3 is a hori~ontal sectional view of the device of
Figure 1, taken along plane ~-.3 of Figure 1.
Figure 4 is an elevational view of a further embodiment of
a unit dose delivery device of the present invention.
.
Eigure 5 is a central sectional view of the device of
Figure 4, taken along plane 5-5 OI Figure 4.
Figure 6 is a horizontal sectional view of the device of
Figure 4, taken along plan 6-6 of Figure 4.
(20
-4-
-
~7~
Figures 7-9 are elevational views of ~uLt11er embodimen~s
of unit dose devices of the preseIlt in~elltion.
(
In FI~. 1, numeral (11) represents a device capable of
dispensing a comestible product into a liauid. In ~his
preferred embodiment, the device (11) comprises a
dispensing means (16) having an e.~terior iall (15) and an
interior wall (17) and two openiIlgs (13, 1?) and disposed
within the dispensing means (16) is a comestible product
(12) in the form of a porous lattice structure having a
shape defined by the interior wall (17). The dispensing
means (16) may be made of paper or synthetic organic
plastic or other suitable material. The shape of the
dispensing means (16) is not overly critical, although it
( is preferred to employ a dispensing means (16) having a
cylindrical shape. The dispensing means (16) has an
exterior wall (15) and interior wall (17) in communication
with two openings ((13) and (1~)). The comestible prod~lct
(12) disposed within the dispensing means (16) has a
porous lattice structure defined by tlle interior wall (17)
of the dispensing means (16). The comestible ma~erial may
be dispensed into a liquid into which the device (11) of
- the present invention is placed by stirring the device
(11), or allowing liquid to penetrate the porous lattice
structure o~ the comestible material, and the like.
The devices of the present invention preferrably comprise
( a dispensing means having at least two openings wherein at
least one of the openings beincJ communicable uitl~ the
liquid in which said device is placed.
~ .
In one embodiment of the present invention the de~ce mav
be prepared by:
i) preparing a solution of a comest~ble pL-~duct ~n
an appropriate solvent at a temperattlre
sufficient to allow the comestible prod~lct to
dissolve in the solvent;
ii) placing a measured amount of the solution in~o a
dispensing means comprising an exterior wall and
an interior wall and having at least one opening;
iii) allowing the solution to cool in the dispensing
means at a rate sufflcient to allow
( crystallization of the comestible produc~ withi
the dispensing means in a porous lattice
structure deîined by the interior walls OL the
dispensing means;
iv) removing excess solvent from the porous lattice
structure disposed within the dispensing means.
The comestible products employed herein includes for
- example edible foods or food additives or pharmaceutical
agents which crystallizes in essential a pure porous
lattice structured form. Illustrative of edible foods or
food additives include sweeteners such as
aspartyl-L-phenylalanine methyl ester and salts thereof,
( saccharin, cyclamate, acesulfam K and the like.
~27~
Represelltative of the phaL-maceutical agents incl-l~e for
example, aspirin, spironolactone, vita~nir.s ancl the like.
(
The comestible product must be com?atible with a sol;ent
wherein the comestible product will essentially dissol.e
and subsequently crystallize in a poro~l3 lattice
structure. In one embodiment, the comestible ~rc~uct is
dissolved in a solvent wherein the comestible product is
insoluble at lower temperatures, but soluble at elevated
temperatures. Therefore, heating a solution contailling
the comestible product in such a solvent system ~.~ill
dissolve the~comestible product in the solvent system.
Subsequent cooling of the solution within a dispensing
means will permit the comestible produc' to crvstalli-e in
( the form of a porous lattice structure having a shape
defined by the interior wall of the dispensing means. It
should be noted that the porous iattice structure Ihus
formed contains essentially p~re~comes~lble product. The
excess solvent is removed ~ conventional techniques.
Thus, the preparation of the devices of the present
invention may be utilized as the final pu~ification step
in the preparation of a comestible product.
The solvent or mixtures of solvents employed as the
solvent system utilized to prepare the devices of the
present invention vary with each active ingredient and are
readily ascertained by one of ordinary skill in the art.
( For example, the preferred solvent for preparing devices
-7-
~7~7
containil1g aspartame is hot water or a ~10t wa-~e~^-alcohol
solution, such as a hot water-meth~nol solut on. The
( preferred solvent for preparin~ dev~ Ces cont2ini.1g
aspartame sulfate is a water-alcol1ol solution col1tainin~ a
ratio of water/alcohol in the range of from about l:25 to
l:5. The preferred solvent for preparing devices
containing sodium saccharin is a mixture of
water:methanol:isopro anol in a ratio of from about 2:2:l0
to ~:5:~0.
In addition, the devices of the present invention may be
prepared utilizing a two solvent system. The comestible
product is dissolved in the first solvent. To the
resulting solution is added a second solvent which is
( miscible with the first solvent but wherein the comeslible
product is insoluble. The resulting mi~ture is placed
within a suitable dispensing means and the comestible
product will crystallize in~he--~orm of a porous lattice
structure having a shape deined;by the interior wall of
the dispensing means.
In accordance with the present invention solutions
- containing from 4 to 40% by weight of a comestible product
in a solvent system may be employed.
The devices of the present invention may be utilized to
dispense one or more comestible products into a liquid.
( Therefore, included within the scope of the present
~79Li3~3~
invention are de~ices wherein one or more comes~ible
products in a porous lattice structure disposed witl~in the
dispensin~ means. The preparation of a device o~^ ~.Q
present invention containing two or more cor,)est ble
products is accomplished using techni~-les such as, ~or
e~ample, co-precipitation, entrapment of a second
comestible product within the porous lattice st~.-uc~uLe of
the first comestible product or adc~tioll of a solut-oll of
the second comestible product to the porous lattice
structure formed by the first comestible product follo:~ed
by removal of the solvent. The term "co-precipi~atlon" as
used herein includes dissolving multiple comestible
products in a solvent system and then allowing the
comestible products to simultaneously form a porous
(15 lat'ice structure within the dispensing means. Entrapmen~
of a second comestible product within the porous lattice
structure formed by a first comes~ible product is
accomplished by adding the s~ecDnd comestibie producl to a
solution comprising the first cQmestible product dissolved
in a solvent system wherein the second comestible product
is insoluble. Therefore, as the ~irst comestible p-oduct
crystallizes, the second comestible product which is
- insoluble in the solvent becomes entrapped within the
resulting porous lattice structure. A third method for
incorporating a second comestible product within the
device of the present invention, is to add a solution
containing the second comestible product in a solvent
wherein the first comestible product is insoluble, to the
o - -
~L~7~ 7
porous lattice structure formed by the first comesti~le
product and subsequeIItly remoVinCJ the solvent.
The porous lattice structure characteristics of the
comestible product disposed withill the dispensing means is
utilized to replace the bul~ing effect of e.~ci?ients. For
example, the density of aspartame and asDar~ame sulrate in
a porous lattice structure âS in t~e presellt in~-ention is
within a range of from about 0.07-0.2g/cc.. Th~ls the
porous lattice structures utilized in the present
invention are about 5 to 30 times bul~ier than
conventional compressed tablets containing aspartame or
as~artame sulfate whose densities are often greater than
l.Og/cc.
A comestible product having a porous lattice struc~ure has
an increased surface area and dissolution rate when
compared to a comestible product in a conventional unit
dose îorm, such as tablets. For examvle, aspartame
sulfate in the form of a porous lattice structure
dissolves extremely fast with respect to conventional
compressed tablets containing aspartame sulfate.
An additional advantage of the new dosage forms of the
present invention is that the dosage forms are essentially
excipient free. This provides significant advantages over
conventional dosage forms and in particular allows the new
~5
~10-
5~--
~z~
dosagQ ~orms to be utili_ed in fo~mu1atio~ls req~ g hicJi~
dissol-ltioll rate, stability or illc~easeà ~ioavai1ab~ y.
In addition, the comestible product i~ ~he LO``..` 0- ';
porous lattice structure may be removed ~-o~ ~'e
dispensing means and utilized âS a unit dose ~ t or -'1~'
like. If employed in this manner, it is pleferred _o
remove the porous lattice structure ?rior to rell1ovln~
e~cess solvent. If a pure form of a comestible product as
a porous lattice structure is too fragile ~or l1all~
surface spraying the porous lattice structule tJith a
binder solution, such as a 5% PVP alcohol solution, may
reduce this problem.
( As used herein, the term "dispensing means" rerers to a
structure capable of dispensing or delivering a comQstible
lS product disposed therein in a liquid. Various
modifications of the illustrated structures in thQ above
FIGURES may be made within the scope of this invelltiol1.
The cross-sections of the dispensing means may be modi~icd
so as to be of different geometric shapes, e.g.,
hexagonal, fluted, triangular, star-shaped, etc. The
- dispensing means, if tubular in shape, may have plugs
i inserted, molded or pressed in to block off the comestible
product from the ends of the dispensing means. In
addition the comestible product may be covered over with
sealing means which do no-t cover the entire device, e.g.,
pressure sensitive tapes, to further protect them.
~7~
The preferred materials of constructioll of the dispeIlsing
means are plastics. These may be mold_d or e~ ded ~n
the shapes illustrated, with perfol-ations, crimps,
projections, grooves, slots, and undercuts, or ma~ be
produced in tubular or rod fo-~m and the desired
configurations may be subseaue!ltly made by ~ier_irg,
perforating, cutting, routiny, etc. Also modi ica~ions o~
the illustrated structures can be employed. However, fol-
ease of manufacture and cost effectiveness a cylindl-icaL
form is pre,erred. Although paper may be used, when
employed it is preferably treated with plastic or other
material to improve wet strength of the device. Almost
all non-toxic thermoplastic or thermosetting synthetic
( organic polymers are useful, but, as a practical ;natter,
with respect to ease of manufacture and economic
considerations, the most important of these are
polypropylene and polyethylene, both of which can be made
with and processed by high speed extrusion, perforating
and filling equipment. Such plastics may be made
transparent or translucent and the user of the device is
able to detect the extent to which active ingredient had
- been consumed.
The following examples are provided to further demonstrate
the principles and the applications of this invention.
-12
~7~ 7
E~..A~IPLE 1. Aspa~-tame Sulfate Stirrer
( 0ne end oî commercial plas~ic st~^aws (10" long ~; 0.~5" d~a.~ were heat-sealed at a ~'5 angle and cut open a small hole at
the tip. An aspartame sulfate solu~ion was prepared by
dissolving 10.0 g of aspartame (~96' pure) in a warm solution
containing 1.667 g sulfuric acid (~6' pure) in 10 ml distilled
water. To the resulting soluton was added 100 0 ml of cold
ethanol and the resulting solution was mixed. The straws were
immersed into the solution. Aspartame sulfate be~an to
c-ystallize within each straw within 30 seconds. After about
30 minutes, the straws were removed from the solution and
placed on absorbent paper to drain the solveIlt and ~llow the
aspartame sulfate which had crystalli-ed in the îorm of a
( porous lattice structure to dry. The de~rices thus prepared
were effective in delivering aspar~ame sulfate into cold
solutions.
EXAMPLE 2. Preparation of Aspartame Sulfate "Tablets"
."
Plastic straws, (10" long x 0.25" diameter), were used. AI1
aspartame sulfate solution was prepared using the procedure
-20 given in Example 1. The straws were bound together with two
rubber bands and immersed into an aspartame sulfate solution as
soon as the solution is prepared. After 30 minutes the straws
were removed from the solution and place on absorbent paper to
remove part of the solvent. The aspartame sulfate that had
~25 crystallized in the form of a porous lattice structure was
-13-
` @_
pressed to desired thicl;ness, puslled o~lt of the st~aw ~Isin~J a
plunger. The wet "tablet" tllus prepared ;as dried on an
absorben~ paper. A bincer soiu~io2l, 5o ?`.~ 'n alcohol, was
sprayed on tlle tablets and the tablets were allowed to air
dry. The tablets thus obtained dissol~-e rapidlv ~n cold
be~era~es.
EXAMPLE 3. Preparation of Aspartame "Tablets"
~, .
The procedure of Example 2 is followed except tllat an aSDartame
solution, prepared by dissolving 7.0 g of aspartame in 100 ml
hot distilled water, was used in lieu of aspartame sulfate
solution. The aspartame crystallized in the form of a porous
latt_ce structure within the straws in app.o.~imately two (2)
( hours. In cold water the aspartame tablets thus produced
disintegrate quickly upon stirring and dissolve faster IAan
lS powder aspartame.
EXAMPLE 4. The Density of Unit Dose "Tablets"
, . . .
1) Aspartame Sulfate Tablet - A pure aspartame sulfate
tablet obtained from E~ample 2, neither compressed nor
coated with PVP, has a size of 11 mm lon~ Y~ 5.5 mm
diameter and weighs 23 mg. Therefore its density is
0.088g/cc. An aspartame sulfate tablet coated with PVP
has a size of 9 mm long x 5.5 mm diameter and wei~hs 25
mg. Therefore its density is 0.12g/cc.
c
-14-
~27~
2) Aspartame Tablet - A pULe aspart-~e table~ obtained from
Example 3, neither compressed nor coated witlI P~P, has a
size of 10 mm long ~ m~ di_ne~e~ -n~ gh ~ . al`~
a density of 0.093g,~cc.
EXA~IPLE 5. Sodium Saccharin Stirrer
Sodium saccharin, (4.0 g), was dissolved in -l.0 ml o~ d st;llea
water and 3.0 ml of methanol. The resulting solutioIl was
heated to about 60C. To the solution was added
isopropylalcohol, preheated to about 65C, until a volume of
60 ml was attained. The resulting solution was mixed and a
total of lO0 straws, 130mm long x 3mm diameter, were inserted
into the solution. Upon cooling, sodium saccharin crystallizes
within the straws in the form OI fluffy needle crystals having
a porous lattice structure. After the solution was cooled to
room temperature, the straws were removed and placed on an
absorbent paper to drain the solvent. The straws were air
dried overnight. The sodium saccharin crystallized within the
straws dissolves ins!antly in cold water.
EXAMPLE 6. Aspartame Stirrer
lO.Og of aspartame was dissolved in lOOml of hot water.
Plastic straws (4mm i.d.) held together with rubber bands were
dipped into the aspartame solution as soon as aspartame starts
to crystallize. Crystallization of the aspartame within the
C straws in the form of a porous latice structure was completed
-15-
~2~ 7
by cooling the solution to abo~lt 10C alld tllell to 5C. Tlle
straws were removed from the solution and vertically placed on
absorbent paper to drain l:emai~ g so~ ioIl. T}le ~-t stra~.~s
~ere then dried in a vacuum o~n at 50C ovelni~'lt
In hot drinks such as cor^fee and tea tl~e stirre~ st~-a~
containing aspartame released aspartame rapidly.
E~AMPLE 7. Flavored Aspartame And Aspartame Sulfate Stirrers
The following solutions were prepared:
1. 2.50g citric acid and 0.50g artificial lemon-lime flavor
~576 (Felton) were dissolved in 22.0g of absolute e~hanol.
(
2. 2.50q citric acid and 0.50g artificial orange oil
California type ~430 (Felton) were dissolved iIl 2.0g of
absolute ethanol.
3. 2.50g citric acid and 0.50g artificial cherly îlavor t'306
~Felton) were dissolved in 22.0g absolute etl~anol.
~ Six drops of one of the above solutions were added to an
aspartame tablet prepared according to Example 3 or an
aspartame sulfate tablet prepared according to ExaMple 2. The
tablet was placed on a metal screen. Alternatively the above
solutions may be added to aspartame sulfate stirrer of Example
C 1 through the empty end of the stirrer. The wet dosage forms
-16-
thus prepared were then ci~^iQd in a vacuuM oven. i1hQII these
flavored dosaae forms were dissolved in cold wa~er, the
rQsultillg solu~ion had a sweet, flavorQd _ st-.
E~AMPLE 8: Aspartame and Sacch-r~n Com~ination StirrQr
Sodium sacchariIl ( .Oa) was d~ssolv-d in a mi.ture o ~.Oml
methaIlol and 4.0ml of water. Aspartame powder (5.0a) was added
to this solution and the resultinc~ solutioIl was mixed. A
boiling solution of isopropanol (80ml) was added to the
solution and the resulting mixture was agitated to dispense
undissolved aspartame into a homogeneous suspension. About lOO
plastic drinking straws of 5 l/2 inches long x -~mm inside
diameter bonded by a rubber band were inserted into the
mixture. The mixture containing the straws was cooled to room
temperature and then to below 0C. The solvent wl~hin the
lS straws was removed by vacuum filtration. A 15o P'~'P isopropanol
solution was sprayed on the end oî the straw whereiIl the
aspartame and saccharin had crystaIlize in the form of a po-ous
latice structure and the straws were air dried. The
aspartame:saccharin product thus prepared dis301ves auickly in
cold water.
EXAMPLE 9: Calcium Cyclamate And Sodium Cyclamate Combination
Stirrer
The stirrers of this example were prepared in accordance with
the same procedure of Example lO except that:
~5
~7~7
l. A solution contaiin~ 20.0g OL calcium cyclamate,
dissolved in a hot so~ ioll con~-ill-ng ^0 Qml w~.t2r and
SOml of ethanol was utilized.
2. A solution containing 20.0g of sodlum cyclam~te dissol~ved
in a hot solution contai~ling 30ml wa~er alld ~Cml ~r
ethanol.
The solutions were to cool to a temperatures below O~C _o
precipi~ate the sweeteners in stirrers. Tlle sol~-ents weLe tllen
removed by vacuum filtration.
EXAMPLE 10: Sodium Cyclamate And Asp2l-~ame Combinallon Sti~--er
(
The same procedure used in Example 8 was utili~ed employillq a
solution containing 5.0g of sodium cyclamate and ~.Og o~
aspartame dissolved in a hot solution containillg 20ml wa~er and
80ml ethanol. The sodium cyclamate and aspartame were
co-precipitated within a straw at a temperatul-e OI less than
0C, and the remaining solvent was removed by vacuum filtration.
- EXAMPLE 11. Content Uniformity of A Pure Aspartame Sulfate Unit
~ose
Empty capsule and tablet unit dose blisters having five small
holes drilled on both the top and bottom of each blister were
utilized as the dispensing means. An aspartame sulfate
-18-
solution was prepared by dissolving 1.6G g of s~ ric ~cid
(96/o) and 10 0 g of aspa~tame (96o pure) in 20 ml ~arm
distilled .~ater. Tlle a~ueous so utiO.l ;.as added to ~50 ml of
ethanol preheated to 60 C.
The blisters were completely immersed ~itl~in ~1le llct as~artame
sulfate solution. Upon coolin~ of the solutioll, as~artame
sul,^ate crystallizes in the form of a porous lattice
structure. Following crystallization of the as~artame s~llfate
in the form of a porous lattice structure, the blisters ~er-
recovered, cleaned, and the solution remaining wi-thin the
blisters was removed. The blisters were then air dried. Each
blister was analyzed to determine as~artame content ("~PM
Content") and content uniformit-~. The results obtained are
illustrated in Table I.
Table I
~PM CONTE~lT
SAM~LE I. D. (m~,~dose)
Tablet blister 1 2~ 8
" " 2 25.~
" " 3 2S.0
" " 4 24.6
23.8
" " ~ . 23.8
,l ~- 7 26.1
- " " 8 26.1
9 24.5
" " lO 24.8
" " 11 23.3
" " 12 23.6
" " 13 24.3
" " 14 24.0
25.5
-19-
Capsule Blister l -~2 7
-L1 7
" " 3 42 g
f 4 ~l~L 1
-1 2
" " 6 ~1 7
E~AMPLE 12. Content Uniformity of Pure Sp ronolactone
Containiny Unit Dose Devices
Spironolactone (10.0 g.) was dissolved in 300 ml of hot
methanol at a temperature of appro.Yimately 65C. A total Or 10
unit dose capsule blisters with small holes were immersed in
the hot methanol solution allowing the capsule blisters to fill
with the hot solution. On cooling, spironolactone
crystallizatized in the form of long needle crystals.
~ollowing crystallization of the s~ironolactone the soiution
( remaining within the blisters was removed and the blisters were
dried. The spironolactone content uniformity of the blisters
~as determined and results are illustrated in the following
Table:
.. ..
Table II
SPIRO~IOLACTONE
CONTENT
SAMPLE ~my/dose)
1. 64.8
2. 65.5
3 63.6
4. 64.3
5. 63.4
6. 63.2
7. 65.0
~. 63.5
9. 63.9
10. 63.8
-20-
- ` ~
~ær~ 7
~ltllou~h this in~elltioll has been descriked :~ith respect 'o
specific modification, the detai~s thereof are not to be
constr~l-d ~s limitatiolls, for it ;.i1l be a~arent ~ha~ arious
equi-~-alents, chan~es and modilication may be resor~ed to
wi~hout departin~ from the spirit and scope ~llereof and it is
understood that such equi~-alent embodiments are intended to be
includad therein
lQ
-21-