Note: Descriptions are shown in the official language in which they were submitted.
~27a~33~L
-- 1 --
SAMMARY OF THE INVENTION
This invention relates to new pyrazole derivatives
which are useful as the herbicidal agent, processes of
producing these pyrazole derivatives and also uses of
these pyrazole derivatives. More particularly, this
invention relates to a new N-arylpyrazole derivative
represented by the general formula (I) given hereinafter,
as well as a herbicidal composition comprising said N-
aryl pyrazole derivative as active ingredient. This
invention further relates to processes for the production
of said new N-arylpyrazole derivatives.
BACKGRO~ND OF THE INVENTION
Certain kinds of pyrazole derivatives are already
known to exhibit such physiological activities utilizable
as agricultural chemicals. For instance, Japanese patent
publication No. 19958/65 discloses that a class of N-
phenylpyrazole derivatives represented by the formula
R3 ~ 2
R N~N
4 Rl
wherein Rl is a hydrogen atom or phenyl group, R2 is a
hydrogen atom or a (lower)alkyl group, R3 is a hydrogen
atom, nitro group or cyano group and R4 is a (lower)alkyl
group, amino group or a (lower)alkoxy group shows herbicidal
~7~8~
-- 2
activity. sesides, Japanese patent publication No.
i4833/67 discloses that an N-(nitro--substituted phenyl)
pyrazole derivative or an N-(chloro-nitro-substituted
phenyl)pyrazole derivative represented by the general
S formula
,~
~ - N
X
CH3 CH3
wherein X is Cl or H exhibits fungicidal activity and is
useful to control fungicidal infections in crop-plants
such as tomato, rice and kidney bean. Nonetheless, such
N-phenylpyrazole derivatives of which the phenyl nucleus
is bearing halogen atoms at the 2- and 4-positions and a
hydroxy group or substituted hydroxy group at the 5-
position in accordance with this invention, as be seen
with the new compounds of this invention, have never
been known before our invention, as far as we are aware
of.
The known particular pyrazole derivatives according
to the Japanese patent publication No. 19958/65 and
No. 14833/67 can exhibit no or little herbicidal activity
and are practically not useEul as the herbicidal agent.
~Z74~33~L
-- 3 --
Accordingly, we have made our researches in an attempt
to provide such new pyrazole ~erivatives which show
remarkably higher herbicidal activities than the above-
mentioned known particular pyrazole derivatives, and which
are practicably utilizable as the herbicidal agent for
irrigated field of aquatic rice plant and also as the
herbicidal agent for farm-fields (non-irrigated) for
various kinds of crop-plants.
DETAILED DESCRIPTION OF THE INVENTION
With the above-mentioned attempt, we have synthe-
tized a number of pyrazole derivatives and researched
the utilities of the pyrazole derivatives so synthetizedO
As result, we have now found that a class of N-phenyl-
pyrazole derivatives represented by the general formula
(I) as given hereinafter is new compounds which have never
been disclosed in any literatures and that these new M-
phenyl pyrazole derivatives of the general formula (I)
exhibit significantly high herbicidal activity and are
useful as a herbicidal agent in practice.
According to a first aspect of this invention,
therefore, there is provided a pyrazole derivative
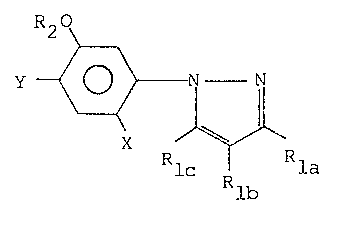
represented by the general formula
~7~33~
R20
Y~x ~
Rlc Rla
Rlb
la~ Rlb and RlC are each a hydrogen atom a
halogen atom, a nitro group, an amino group, a tlower)
alkyl group, a halo-(lower)alkyl group, a (lower)alkenyl
group, a (lower)alkynyl group, a (lower)alkylcarbonyl-
amino group, a (lower)alkoxycarbonylamino group, a mono-
(lower)alkylaminocarbonylamino group or a di-(lower)
alkylaminocarbonylamino group;
R2 is a hydrogen atom, a (lower)alkyl group, a
10 (lower)alkenyl group, a (lower)alkynyl group, a (lower)
alkoxy(lower)alkyl group, a (lower)alkylthio(lower)alkyl
group, a (lower)alkylcarbonyl(lower)alkyl group, a (lower)
alkoxycarbonyl(lower)alkyl group, a (lower)alkylthio-
carbonyl(lower)alkyl group, a cyano(lower)alkyl group,
15 a (lower)alkylsulfonyl group, phenylsulfonyl group, a
halogen-substituted phenylsulfonyl group or a (lower)
alkyl-subs-tituted phenylsulfonyl group, and
X and Y are the same or different and each are a
halogen atom.
According to a second aspect of this invention,
~791~
-- 5
there is provided a herhicidal composition comprlsing an
N-phenylpyrazole derivative of the above general formula
(I), as active ingredient, in association with a solid
or liquid carrier for the active ingredient.
According to a third aspect of this invention,
there is provided a method of inhibiting the grow-th of
unwanted weeds, which comprises applying to the weeds
or to the locus of the weeds a herbicidally effective
amount of the N-phenylpyrazole derivative of the general
formula (I) as above.
In this specification, the term "(lower)alkyl
group" appears in the terms "(lower)alkyl group",
"(lower)alkylthio(lower)alkyl group", "cyano-(lower)
alkyl group" and other terminologies which are shown
by R1a, Rlb, RlC and R2 in tihe general formula (I)
representing the new compounds of this invention. By
the term "(lower)alkyl group" is herein meant an alkyl
group of 1 to 6 carbon atoms, such as methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, sec-butyl, tert.-
butyl, pentyl, hexyl, isohexyl and the like. By the
term "(lower)alkenyl group" which is shown by Rla, Rlb
and RlC as well as by R2 in the formula (I) is an alkenyl
group containing 2 to 6 carbon atoms, such as vinyl,
allyl, l-propenyl, 2-methyl-2-propenyl, 1-methyl-2-
propenyl, 2-butenyl, 3-butenyl, 2-pentenyl, 2,4-hexadienyl
~7~34
and the like. By the term "(lower)alkynyl group" is
meant an alkynyl group containing 2 -to 6 carbon atoms,
such as ethynyl, 2-propynyl (or propargyl), 1-methyl-2-
propynyl, 2-butynyl, 1-me~hyl-2-butynyl and the like.
The halogen atom for X and Y as well as Rla, Rlb and
RlC may be Cl, Br, F and I. Thus, the substituents
Rla, Rlb, RlC, R2, X and Y in the general formula (I)
above may take any combinations of the above-mentioned
particular values of these substituents of various sorts.
. Particular examples of the new compound of the
general formula (I) according to the first aspect of this
invention are listed in Table 1 below~
~7~
-- 7
~C~
X
_
C
C r~
O ~ Ul ~D ~ o
U
S~
a) a ~: c c c c c E
ELI
X
~N m U U ~ . I u
~ ~r I
~
1:~
P~ ~ z æ
~0 ~ ~ r~
P~.
o o
oZ
~L~7~4
Ln D L~ ,_1 ~D ~1 (~ ~n 1-- L~ ~D
~) ~ O r-l ~JLn r-l D ~1 0 Ln
a~L~ L~ L~ a`~ D
.
a ~ a ~ a ~ a ~ aL~ a ~ a ~ aL~ a ~ a ~ a
C C C C C ~ C ~: C C
~)
~ l l l l l
D11 Ln ~ ~ ~ Ln
~ ~ l l l ~ l
~:: C t~ C.) C
T~
L~ I ~
~ ~ ~ O O ~ ~- I I I I
O O O Z Z:C I ~1 ~1 ~I L~
æ æ z I I z
~r ~ I I I I I
In In :4
In Ln I I I I I V
V V V V V V V V O Z Ln
L~a~ o ~ ~ ~ ~r Ln ~D 1` L
~7~3~
c~:) r- N ~ O r~ ~ ~
~D ~ r~ ~D ~ r~ r ~ o
N ~N P~ D~ N ~ N ~N ~I Q~N ~N CI N a N
C C E C C C E C C C
~1 ~I h
u ~ m
T N
1~ 3 0
I O N
~ N l :~ l ~ ~ l l l
r~ I ~ rr! ~ O I ~) N U r~ r~ l_
t) o ~ o :C ~ O ~
~ O I ~ ~ X 5: 1 1 1
''I O rr C O Z ~ ~ ) ~1 .,1 .,~
N N N N N N N ~ ~ N N
) ~ ~ u ~ v
o ~ ~ r~ o r CD a~
~1 N N N N N N N N N N
74~3~
-- 10 --
.,. ~
oa~ ~ ~ In I I1~
~ ~, .,.
~ ~ a ~ a ~ ~
s:: C C C C C C E EE E E
U ~ ~ ~ U t~ U t~
3~ C
~r
Z I I
O ~)~') O U~ N t`~
I I O O OO O
~T~ O t )11'1I:i~ ZZ Z Z Z
Z I I I :C I I II I
Z Z ~ ----` ~ C
o ~1 ~I ~ ~ In ~D 1` 00 a~ o ~1
7~334L
-- 11 --
o ~ o U~
i~ ~ O~r ~DIn ~1 0 ~ ~
Ln ~ a' ~
~1 0 00 ~ Nr~l O O ~ U~)
N ~ N a ~ ~ ~ r~, ~ ~ r~ r~ r~ ~ ~
C C, E Er . Er~ E E E E
r~r~E~,r~. r~, r~, r~, r~ C4 r ~ r.
) U ~I N 3~
U X O
~P ~U~ Il11 0 ~ O O ~ U~
zo zo o o z o o o o o o
_ _ _ _ _ _ ~ _
3~
U~
o r~LO co
1--U) ~ ' ~D
I I d 'I ~D I ~ 1 CO d~
~D t` C~ D
Q. Q~ ~N aQ,~:),Q. ~ a ~ a ~ a ~ a ~ a
E E E C E E E C.C. C C C
u ~ m o o
m m
~ I o
o I::CI I I I I I
o I O
I ~11,U~
r~
~, I X ~ ~ ~ ~ I I I I I
T! ~r
~ Nt~ ~1 N ~ O
O O O O O O O O O ~ I Z
Z Z Z Z Z Z Z Z z I In
u~
t~l O r~
N ~ r.~l~ ~ ~ ~ o Z
Z I U~
r~ ~r~ r~r~)r~ r~) r~)r~
2: ~ I
r~)r~r~ r~l ~ r~ r~ r~ r~ r~ r~
co r~ o ~ r~lr~
-
74l~
-- 13 --
Ln _
N ~ l Ln ~ -lLn
o ~ ~ ~ Ln ~ I ~ 1
Ln ~ ~ ~D ~I Ln ~I Ln~ Ln ~
Ln Ln Ln Ln ~ I Ln I . I . Ln
I ~ O ~e:r ~ o
a~ ~ ~ ~~J ~
r~, ~ ~ ~ , , ~ , , , , ~
~a ~a ~a ~a Q, Q. ~a Q, Q, ~ Q, ~a
s:: C C C E E C E E E E C
~1 ~1 ~
m
r r r r rr r r ~ r r r
o 8
~ Z ~ Z
Z o
O ~
N N ~ N I I ~ ~- O Z ~ I:
O O OC`~ N ~ ~'7 ~ ~) ~T~ O
Z Z Z Z Z Z ~ ~
N N N N I I N I I I I ~1
Ln Ln Ln Ln ^ -- Ln ^ ^ ^ ^ Ln
~ N N ~ C51 X 3:: N
Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln
Ln ~D r a: a~ o ~ ~ ~ ~ Ln ~
r r r r 1~ r r
.
33~
a) o ~ O
,~~ o~ ~ ,,
U~, ,. . .~, . .
o ~ ~~ ~ ~r~
,,o~,~
C C E E E~ E C
!r.
N N II I V~I I I I
T ~ r
~O ~~ ~ O~C 5 :c:
n ~.) In
I
N In I I I I I I I z
u ~ m m m u~ z c~
~D N r~l ~ ~n ~ c~ ~ Ln ~I r~ ~
o ~n ~ ~ o
~n 'n m Ln Ln Ln Ln ~n Ln m ~n ~n
C C ~ ~ C ~ C C C )_ C C
m m o ~ m ~ o ~
o
o
3: ~ l
~ ~ z
~ o o
I~
(~1 t~ N N ~J N ~)
T'! X 0 5:
Z Z
N ~I ~ ~ ~I N ~I
~ ~ _ _ _ _
~r) ~ ~ N
1 4 0
Z
I
n n n n Ln In Ln ~ o
~ o ~ ~ ~ ~ n ~ o
~L~74~
- 16 --
oL~ o a~
r~ ~ o u~ ~ co L ~ r
~r ~rLn q~ Ln ~ n ~
Ln LnLn Ln 1~ Ln Ln Ln Ln Ln Ln
.
N ~ ~ a ~ ~ ~ a ~ ~ a ~
C C ~: C C C CS~ E
~ m ~ v
) ~ V ~ V
11 Ln ~ ~ ~ ~ ~ ~ f~
~C ~ I I I I I I I I
3:: 1 1 1
V N ~I t~l
Ln11 V V ~J
~ ~ TL~l
~ m Ln Ln Ln
LnLn Ln~r ~ Ln Ln Ln ~ ~ ~
-I' ~ ~d' m ~ o ~ o ~1
o o o O O O O O O ~ ~
~L~7~
~D ~ ~er ~ ~f'~ ~
,~ ,-- o ,~ ~ ~ ~ ~ ~
1~ r~ r~r~ r1 r~r~ r~ r~ ~1 U')
t~ r~
n. N ClN a ~ ~~ ~ ~ ~ ~ a ~ ~ ~ ~
E C ~ 1~ C C 1~ E
r~r; r~ r~ r1
rr
t~
~r I I I I I I I
o I o
rl Z rlrlrl rlrl
Il~ I
N N!r
U) Ll~ I I~_) 5 ~
!T' !~ N I IC ~ O Ul
~'~7~
-- 18 --
U~ ~ ~
~ ~ ~ ~~ ~ ~~ a N P, Q, ~, N 1~
E E E E C E C C EE
~1 ,_1 h~1~I h ~1 ~1
m ~ m
~1 ~1 ,1 ~I h
T U
o ~ O ~I N (~ ~r
N ~ l r~
.:~... .
. . '
~74834
-- 19 --
In
~3 LnCO
~ ~In
O ~~D I I I
~ . . . . . . . . ~
N a Q~ Q, QJ Q, Q, Q, Q, Q, ~I a
C E E E E E E E El C
V ~
N
N ~
V ~ ~ ~ ~ ~ ~ X ~ O
7~3flt
-- 20 --
CO U)
oo o
u~ I I I a~ u~ U1 U~ U) U) U
~n u,
E E E E ~ ~ ~ E ~: C
O ~ V
r ~ I I I O I i I t)
O ~ O 0 1 ~ O 2
'T~ ~U ~Ul ~U~
`J N
t,) O
rr~
~r ~r ~r ~ ~ ~r ul Ll ) In u) U1
~n u) I~ OD O~ O ~ ~ ~ ~t' U
U) U~ U~
~ ~74~
-- 21 --
.
C C C C C C C E C E ~ E
~ r r~ 1- ~ r ~
U ~ V ~ ~ ~ ~ ~ 3
C C
~7~34
-- 22 --
o D~D ~ ~ O ~ U~
InInIn I I I I I LnU~U~ I
~ ~ a ~ a
C1:: C E E E E E C E E C
0 2 ~ 1 3:C 2~:
(~ ~ ~ ~ Xt~ t.) O t)
3 1 1 1 1 1 ~ ~I I I I I
!r~~T-!
~~r ~r I I I I I I I I I
~ ~ ~ I I I I I I I I I
Lt~ ~ -- -- ~ _ _ _ _ _ _
~r-- -- -- _ _ _ _ _ _ _ _
I
.
~7d~
-- 23 --
n
~r N
~ ~ I I ~ o
~1 ~1 ~ ~I In ~1 0 ~I r-l ~r o
C C E E E E C E C C E E
N I ~1
:~ I I I I ~ O
U) O V
T rT~ 0 t~ O O O
X
N N N N N N N N N N N N
Ln Ln ~n Ln n Ln Ln Ln Ln ~ Ln Ln
O ~I N1~ ~ Ln ~ 0 ~1
7~
-- 24 --
U~
In ~ o 1`u~ o ~ ~ r~ o
C ~ C C C E E C 1 E E r::
~ o
O O ~ 2:
~ 1 ~ ~ t~ In u~ ~ ~
m ~q m
In In n Ln Ln In In
Ln In In u~ In In In In In In u) In
~ ~ ~r In ~ r~ o
~ o o o o
~7~
-- 25 --
I
U~
COU~ O ~~'
U~ I I I ~ I C
~U~ ~ ~ ~r-l ~ ~2
U~ ~ ~ ~ ~ O ~
~ U
~ X
a ~ ~ a
E E E E C E
r~
V ~ t~ V ~ r~
S~ C)
O ,1
U~ ~
t ) ~ O ~ ~ ~ ~1 0
a
O
~: Q
~ r~
Q
~I ~1 a~
,C
IJ
t~l O I I
2 C ) t~ ~ ~:
:~ ~ O O O O ,~
a c
C ,~
t~
a~
~1
a)
C ~ D
3 0 a~
C
~ .
a~
m P~ m m ~ ~rl o
Q u~
C C
~I N ~1 N1~ ~1 ~ :1
.. _ ~ ~ ~ ~ O
`
~C ~ 3 X 5: ~1--- E
Q U~ o
E~ E
Lr) U~ In L~ ~ In o S-l
.. s:~ ~ O
~ D r ) ~
o o o o o o
N
~7~3~
- 26 -
Compound Numbers shown in Table 1 are referred to
hereinafter to identify the compounds as used or as
produced in the following descriptions~
Amongst the particular compounds listed in Table 1
above, the following are preferred compounds in this
invention.
(1) 1-(2-f~uoro-4-chloro-5-allyloxyphenyl)-3,5-dimethyl-
4-nitropyrazole (Compound No . 45)
(2) 1-(2-fluoro-4-chloro~5-propargyloxyphenyl)-3,5-
dimethyl-4-nitropyrazole (Compound No. 47)
(3) 1-[2-fluoro-4-chloro-5-(1-methyl-2-propynyl)oxy-
phenyl]-3,5-dimethyl-4-nitropyrazole (Compound No . 48)
(4) 1-(2-fluoro-4-chloro-5-propargyloxyphenyl)-3,4,5-
trimethylpyrazole (Compound No. 138 )
(5) 1-(2-fluoro-4-chloro-5-propargyloxyphenyl)-3,5-
dimethyl-4-chloropyrazole (Compound No. 183)
(6) 1-(2-fluoro-4-chloro-5-propargyloxyphenyl)-3,5-
dimethyl-4-bromopyrazole (Compound No . 205)
The new compounds of the formula (I) according to
the first aspect of this invention show improved herebi-
cidal activity over the known pyrazole derivatives as
disclosed in the aforesaid Japanese patent publications
No. 19958/65 and No. 14833/67. Thus, the new cornpounds
of this invention are herbicidally active against a wide
variety of unwan-ted weeds, such as barnyard grass
Dt8;~4
- 27 -
(Echinochloa crus-galli), monochoria (Monochoria
vaginalis), narrowleaf water-plantain (Alisma
canaliculatum), bulrush (Scirpus juncoides), false
pimpernel (Lindernia procumbens), and tooth cup (~otala
indica) which frequently grow in the irrigated fields
of aquatic rice plant. These sorts of weeds grown in
the irrigated fields of rice plant can be killed com-
pletely by applying the new compound at a rate of appli-
cation of 50 g or less of the compound of this invention
per 10 ares of the field. When some preferred particular
compounds are employed amongst the new compounds of
this invention, the above-mentioned sorts of weeds can
be killed completely even by application of the preferred
compound at a rate of 6.25 g or less per 10 ares. The
new compounds of this invention are also herbicidally
active against a wide variety of unwanted weeds, such as
crabgrass (Digitaria adscendens), common lambsquarters
(Chenopodium album), pigweed (Amaranthus ascendens) and
smartweed (Polygonum nodosum) which frequently grow in
the farm fields (non-irrigated) for various crop plants.
These sorts of weeds grown in the non-irrigated farm
fields can be killed completely by applying the compound
of this invention at a rate of application of 50 g or
less of the compound of this invention per 10 ares.
Application of some preferred particular compounds
~'74~;3 4
- 28 -
amongst the compounds of this invention can achieve
complete kill of the weeds grown in the non-irrigated
farm fields even at a rate o~ application of :L~.5 g or
less of the preferred compound. Further, the new com-
pounds of this invention advantageously show no signifi-
cant phytotoxicity to crop plants such as rice plant,
soy bean, sugar beet, radish, wheat, barley and maize.
Besides, it is observed that the new compounds of this
invention are neither toxic to mammalian animals nor
toxic to fishes, so that the compounds of this invention
can be applied to the weeds with safety to the crop-plants
as well as the animals.
The new compounds of the general formula (I)
according to the first aspect of this invention include
the following four particular embodiments:-
(1) According to the first embodiment, there is
provided a compound of the formula
(Ia)
Rlc Rla
Rlb
where Rla, Rlb and RlC each are a hydrogen atom, a
halogen atom, a (lower)alkyl group, a (lower)alkenyl
group or a (lower)alkynyl group,
.. ..
~74~
- 29 -
R2 is a hydrogen atom, a (lower)alkyl group, a
(lower)alkenyl group, a (lower)alkynyl group, a (lower)
alkoxy(lower)alkyl group, a (lower)alkyl-thio(lower)alkyl
group, a (lower)alkylcarbonyl(lower)alkyl group, a (lower)
alkoxycarbonyl(lower)alkyl group, a (lower)alkylthio-
carbonyl(lower)alkyl group, a cyano(lower)alkyl group,
X and Y are the same or different and each are a
halogen atom.
(2) According to the second embodiment, there is
provided a compound of the formula
la (Ib)
Rlb
where Rla, Rlb and Rlc each are a hydrogen atom, a halogen
atom, a (lower)alkyl group, a (lower~alkenyl group or a
tlower)alkynyl group,
lS R2 is a (lower)alkylsulfonyl group, phenylsulfonyl
group, a halogen-substituted phenylsulfonyl group or a
(lower)alkyl-substituted phenylsulfonyl group, and
X and Y are the same or different and each are a
halogen atom.
(3) According to the third embodiment, there is pro-
vided a compound of the formula
x~
- 30 -
R20
y ~ o ~ - !Ic)
Rlc~/ Rla
Rlb
wherein Rla, Rlb and RlC are each a hydrogen atom, a
halogen atom, a nitro group, an amino group, a (lower)
alkyl group, a halo-(lower)alkyl group, a (lower)alkenyl
group, a (lower)alkynyl group, a (lower)alkylcarbonylamino
group, a (lower)alkoxycarbonylami.no group, a mono-(lower)
alkylaminocarbonylamino group or a di-(lower)alkylamino-
carbonylamino group, provided that at least one of Rla,
Rlb and RlC is or are a halo-(lower)alkyl group, a nitro
group, an amino group, a (lower)alkylcarbonylamino group,
a (lower)alkoxycarbonylamino group, a mono-(lower)alkyl-
aminocarbonylamino group or a di-(lower)alkylaminocarbonyl-
amino group,
R2 is a hydrogen atom, a (lower)alkyl group, a
(lower)alkenyl group, a (lower)alkynyl group, a (lower)
alkoxy(lower)alkyl group, a (lower)alkylthio(lower)alkyl
group, a (lower)alkylcarbonyl(lower)alkyl group, a (lower
alkoxycarbonyl(lower)alkyl group, a (lower)alkylthio-
carbonyl(lower)alkyl yroup, a cyano(lower)alkyl group, and
X and Y are the same or different and each are a
halogen atom.
~, .. ,:
~.X7~3~
- 31 -
(4) According to the fourth embodirnent, there is
provided a compound of the forrnula
R20
Y~-- ~ ~ ~ (Id)
X Rlc Rla
Rlb
wherein Rla, Rlb and RlC are each a hydrogen atom, a
halogen atom, a nitro group, an amino group, a (lower)
alkyl group, a halo-(lower)alkyl group, a (lower)alkenyl
group, a (lowèr)alkyny]. group, a (lower)alkylcarbonylamino
group, a (lower)alkoxycarbonylamino group, a mono-(lower)
alkylaminocarbonylamino group or a di-(lower)alkylamino-
carbonylamino group, provided that at least one of Rla,
Rlb and RlC is or are a halo-(lower)alkyl group, a nitro
group, an amino group, a (lower)alkylcarbonylamino group,
a (lower)alkoxycarbonylamino group, a mono(lower)alkyl-
aminocarbonylamino group or a di-(lower)alkylaminocarbonyl-
amino group,
R2 is a (lower)alkylsulfonyl group, phenylsulfonyl
group, a halogen-substituted phenylsulfonyl group or a
(lower)alkyl-substituted phenylsulfonyl group, and
X and Y are the same or different and each are a
halogen atom.
The compound of the formula (Ia) as above according
fl
- 32 -
to the above-mentioned Eirst embodirnen-t includes, as
preferred modes, the following three types:-
(i) A compound of -the formul,a
R20
y _ ~r~) ~ e)
CH3
where R2 is a (lower)alkyl group, a (lower)alkenyl group
or a (lower)alkynyl group; and X and Y are the same or
different and each are chlorine, bromine, fluorine or
iodine atom.
(ii) A compound of the formula
2
X ~ (If)
Cl
where R2 is a (lower)alkyl group, a (lower~alkenyl group
or a (lower)alkynyl group, and X and Y are the same or
different and each are chlorine, bromine, fluorine or
iodine atom.
(iii) A compound of the formula
~74~
- 33
R20
Y ~ N _ N (I~)
CH3 CH3
Br
where R2 is a (lower)alkyl group, a (lower)alkenyl group
or a (lower)alkynyl group; and X and Y are the same or
different and each are chlorine, bromine, fluorine or
iodine atom.
The compound of the formula (Ic) as above according
to the above-mentioned third embodiment includes as a
preferred mode, the following type:-
(iv) A compound of the formula
~ ~ ~ (Ih)
X CH3 CH3
N2
where R2 is a (lower)alkyl group, a (lower)alkenyl groupor a (lower)alkynyl group; and X and Y are the same or
different and each are chlorine, bromine, ~luorine or
iodi.ne atom.
The compounds of the general formula (I) according
to the first aspect of this invention may be prepared by
a variety of processes, typically by seven processes (A)
1~7~34
- 34 -
to (G) as described below. Any of these processes (A) to
(G) may be chosen appropria-tely, depending upon the nature
of the substituents Rla~ Rlb~ Rlc and R2
compounds of the formula (I) to be produced.
S The various processes (A) to (G) of producing
diiferent types of the compounds of the formula (I) will
be described below, schematically with reference to the
reaction equations involved, where the subs-tituents Rla,
R1b and RlC are referred to merely as -(Rl)n, for sake
of simplicity of expression, and where the substituent
R2 is also simply shown as R2, though the substituents
R1 and R2 of the concerned compound as shown in the
reaction equations may have meanings which are, exactly
speaking, not the very same meanings as defined for them
with reference to the aforesaid general formula (I).
~ ~ 7
- 35 -
Process (A):
This process may be applied to the preparation of
compounds (I-A)~ i.e. those of the general formula (I)
where Rla, Rlb and RlC represent each a hydrogen, a lower
alkyl, a haloalkyl, a lower alkyl, a lower alkenyl or a
lower alkynyl, and R2 represents a group defined above t
except hydrogen, and this process (A) comprises such a
reaction between a substituted phenylhydrazine compound
of formula (II-A) and a di-carbonyl compound of formula
(III) such as acetylacetone, malonaldeh~de, 1,1,1,5,5,5,-hexafluoro-
penta-2,4-dione, 3-trifluoromethyl-penta-2,4-dione, 3-
methylpenta-2,4-dione and 4-ethylhepta-3,5-dione, with
involving the condensation and cyclization to give a
compound of formula (I-A). This may be represented by
the chemical equation:
R20
~ O O
(II-A) (III)
R20
Y--~--N~
A)
~t~483~
- 36 -
where n is 3 herein and hereinafter, unless otherwise
-
stated.
The reaction may usually be conducted in various
solvents including organic solvents such as hydrocarbons,
ethers and alcohols and water. The reaction may
proceed at room tempera~ures, but is preferably carried
out at a higher temperature up to the refluxing tempera-
ture of the solvent used, under heating. After the
completion of the reaction, the solvent may be removed
from the reaction mixture by distillation to recover the
desired reaction product. Alternatively, the desired
product may be recovered by extracting the reaction
mixture with water and an organic solvent such as
benzene, toluene, tetrahydro~uran and chloroform and
distilling off the solvent from the extract to obtain
the desired product.
Process (A) is illustrated in Examples 1 to 5
hereinlater given.
Process (B):
_ _
This process is applicable to the preparation of
compounds (I-B), i.e. those of general formula (I) where
Rla, R lb and RlC represent each a group defined above,
except a (lower)alkylcarbonylamino, a (lower)alkoxy-
carbonylamino, a mono-(lower)alkylaminocarbonylamino and
a di-(lower)alkylaminocarbonylamino, and R2 is hydrogen,
and this process (s) comprises hydrolyzing a compound of
formula (I-C) which is itself within compounds of
general formula (I) and which has as P~2 such a group
hydrolyzable to leave a hydroxyl group on the 5-position
of the benzene ring. This reaction may be represented by
the chemical equation:
R2O HO
Y --~ ) N--N --~ Y~ N~N~
(Rl)n (Rl)n
(I-C) (I-B)
The hydrolysis may be effected simply by heating
compound (I-C) together with water or in an aqueous
organic solvent, but usually carried out in the presence
of one equivalent or more of a basic compound. As the
base, there may be used typically an alkali metal
hydroxide such as sodium hydroxide, potassium hydroxide,
or an alkali metal carbonate such as sodium carbonate and
potassium carbonate. The reaction may proceed either at
room temperatures or under heating. The addition of an
alcohol such as methanol or an ether such as dioxane to
the aqueous reaction medium for forming a homogeneous
reaction phase may accelerate the reaction.
After the completion of the reaction, the reaction
- 38 -
solution is made acidic with the addition of an inorganic
acid such as hydrochloric acid and sulfuric acid and then
extracted with an organic solvent such as benzene, toluene,
tetrahyclrofuran and chloroform. The removal of solvent
from the extract by distillation yields -the desired
compound of formula (I-B).
Process (~) is illustrated hereinlater in Examples
6 to 8.
Process_(C):
This process is applicable to the preparation of
compounds (I-D) i.e. those of general formula (I) where
Rla Rlb and RlC have the same meaning as deflned above
and R2 is a group defined above except hydrogen, and
this process (C) comprises reacting a compound of
formula (I-E) where Rla, Rlb and P~lC correspond to those
of the product compound (I-D) and R2 is hydrogen, with a
halide compound of formula (IV) such as an alkyl chloride,
an alkenyl chloride and an alkylsulfonyl chloride. This
reaction may be shown by the chemical equation:
HO R20
Y ~ N + R2-Hal-~Y ~--N--N
(R )n (R )n
I-E) (IV) (I-D)
~ ~ 7
- 39 -
This substitution reaction may usuall~ be conducted
in an organic solvent in the presence of an acid binding
agent. As such organic solvent, there may be used
hydrocarbons such as benzene and toluene; ethers suc'n as
ethyl ether and tetrahydrofuran; esters such as methyl
acetate and ethyl acetate; ketones such as acetone and
methyl isobutyl ketone; nitriles such as acetoni~rile and
propionitrile amides such as dimethylformamide and
dimethylacetamide; and dimethyl sulfoxide. The acid
binding agent may be an inorganic base such as sodium
hydroxide, sodium amide and potassium carbonate or an
organic base such as triethylamine and pyridine. The
reaction may be carried out at room temperatures or
under heating to a temperature up to the refluxing tempera-
ture of the solvent used.
After the completion of the reaction, the reaction
mixture may be filtered to remove the salt(s) of the
acid binding agent as formed and then distilled to remove
the solvent used, affording the desired compound (I-D)
produced. Alternatively, the desired compound may be
isolated by adding to the reaction mixture water and an
organic solvent such as benzene, toluene, tetrahydrofuran
and chloroform to extract the compound and distilling off
the solvent from the extract to leave the compound.
Process (C) is illustrated in Examples 9 to 14.
d4~34
- 40 --
Process tD):
This process is applicable to the preparation of
compounds (I-F), i.e. those of general formula (I) where
Rla, Rlb and R1C is a halogen atom, and
this process (D) comprises reacting a halogenating agent
having a halogen atom (Z) to be introduced, with a compound
of formula (I-G) where R1a~ Rlb' P`lc and R2 co p
those of the product compound (I-F) provided that at
R1a, Rlb and RlC is a hydrogen atom, and,
for instance, all of Rla, R1b and RlC are not halogen
atoms, or one or two of Rla, Rlb and RlC are halogen atoms
other than the halogen ~Z) to be introduced, or one or two
of R1a, R1b and RlC are the same as the halogen atoms (Z)
to be introduced. This reaction may be approximately
depicted by the chemical equation:
R20~ R20
Y- ~ N - N + Halo- > Y ~ O ~ N N
Y I 1l genating y
X ~ agent X ~
(Rl)Q (Z)m ~Rl~Q
(I-G~ (I-F)
wherein Rl is as explained above but except hydrogen, and
R2, X and Y are as defined above; Q is 0, 1 or 2; Z is
each a halogen atom introduced by the halogenating agent,
m is 3, 2 or 1 and (Q ~ m) is an integer of 1 to 3.
~.~7~4
The halogenation reaction may conveniently be
effected in an organlc solvent such as haloyenaked hydro-
carbons, amides and water. Halogenaking agent which may
be used includes an elementary halogen such as chlorine
and bromine; sulfuryl halides and phosporus halides.
The reaction temperature may be appropriatel-~ chosen from
room temperature to the boiling point of the solvent used.
After the completion of the reaction, the desired
product may be isolated in many cases by distilliny off
the solvent ~rom the reaction mixture. If necessary,
however, water and an organic solvent may be added to
the reaction mixture to extract the desired compound and
the solvent may be distilled off from the extract to
afford the desired compound of formula (I-F)~
Process (D) is illustrated by way of Examples 15
to 17.
Process (E)
-
This process is applicable to the preparation of
compound (I-H), i.e. those of general formula (I) where at
la~ Rlb, RlC is nitro group, and this process
(E) comprises reacting a nitrating agent with a compound of
formula (I~I) where Rla~ Rlb' ~`lc and R2 co p
those of compound (I-H) provided that at least one of Rla~
Rlb and RlC is the hydrogen atom, and for instance, all of
Rla, Rlb and RlC are not nitro groups or one or two of P~la,
- 42 -
Rlb and RlC are nitro groups already introduced. This reac-
tion may be approximately depicted by the chemical equation:
Y ~ - ~ + Nitrating Y ~
(Rl)p (N02)q (Rl)p
(I-I) (I-H)
wherein Rl i5 as explained above but except hydrogen, and
R2, X and Y are as defined above; p is 0,1 or 2; q is 3, 2
or 1 and (p ~ q) is an integer of 1 to 3.
The nitration reaction may usually be effected in
a solvent, for example,an inorganic acid such as sulfuric
acid; an organic acid such as acetic acid; water; or a
lo mixture of an acid and water~ As the nitrating agent,
there may be used usually nitric acid and fuming nitric
acid. The reaction temperature may be appropriately
chosen from a temperature under cooling with ice-water to
the refluxing temperature of the solvent used.
After the completion of the reaction, the desired
compound may be recovered, for example, by adding water
to the reaction mixture, extracting the desired compound
with an organic solvent such as benzene toluene, tetra-
hydrofuran and chloroform and distilling off the organic
solvent from the extract to leave the desired compound.
Process (E) is illustrated by way of E~ample 18.
8~
- 43 -
Process (F):
_
This process is applicable to ~he preparation of
compounds (I-J), i.e. those of general formula (I) where
la~ Rlb and RlC is amino group, and this
process (F) comprises reducing a corresponding nitro-
substituted compound of formula (I-~I). This reaction
may be shown by the chemical equation:
R20\ R20
~X l~ 9 ( Rl ) p
(I-H) (I-J)
where Rl is as explained above but except hydrogen and R2,
X, Y, p and q are as defined above.
-
The reduction may be effected, in general, using
iron or tin or nascent hydrogen as reducing agent.
Usually, water is used as solvent. In most cases, the
addition of a small amount of hydrochloric acid to the
water can promote the reaction intended. The reaction
temperature may be appropriately chosen from a temperature
under cooling with ice-water to the refluxing temperature
of water. Warming up of the reaction mixture may promote
the reaction, shortening the reaction time.
After the completion of the reaction, the desired
~ ~7~83~
- 44 -
compound may be recovered by extracting the reaction
mixture with an organic solvent such as benzene, toluene,
tetrahydrofuran and chloroform and distilliny off the
solvent from the extract to leave the desired compound.
Process (F) is illustrated by way of Example 19.
Process ~G):
-
This process is applicable to the preparation of
acylamino-substituted compounds (I-K), i.e. those of
general formula (I) where at least one of Rla, Rlb and RlC
is an aminoalkylcarbonylamino, a lower alkoxycarbonylamino,
a mono-lower alkylaminocarbonylamino or a di-lower
alkylaminocarbonylamino group, and this process (G)
comprises acylating a corresponding amino-substituted
compound of formula (I-J). This reaction may be shown by
the chemical equation:
R20 R20
X ~ 1 p N -
(NH2)q (NH~Acyl)q
(I-J) (I-K)
where Rl, R2, X, Y, p and ~ are as defined above and
Acyl represents a lower alkylcarbonyl, a lower alkoxy-
carbonyl, a mono-lower alkylaminocarbonyl or a di-lower
alkylaminocarbonyl group.
.
" ':
~X~ 3
-- 45 --
In cases where the acyl group of the acylating
agent to be used in this process (G) i5 a lower alkyl-
carbonyl, a lower alkoxycarbonyl or a di-lower alkylamino-
carbonyl group, the acylation reaction may be carried out
using as acylating agent a lower alkanoic acid chloride
a lower alkyl chloroformate or a di-lower alky]carbamoyl
chloride, respectively, in an organic solvent such as
benzene, chloroform, tetrahydrofuran, acetone and
acetonitrile in the presence of an organic or inorganic
acid binding agent, including an organic base such as
triethylamine and pyridine and an inorganic base such as
anhydrous sodium carbonate and anhydrous potassium carbo-
nate. The reaction temperature may be properly chosen
from a temperature under ice-water cooling ko the
lS refluxing temperature of the solvent used. After the
completion of the reaction, the desired compound may be
recovered from the reaction mi~ture by adding thereto
water and an organic solvent such as benzene, toluene,
tetrahydrofuran and chloroform, separating the organic
layer thus formed and distilling off the organic solvent
to leave the desired compound.
In cases where the acyl group of the acylating
agent to be used in this process (G) is a mono-lower
alkylaminocarbonyl group, the acylation reaction may be
conducted by using a lower alkyl isocyanate in an inert
~7~
- 46 -
solven~ such as benzene, ether, tetrahydrofuran andacetone at a temperature ranging from room temperature to
the refluxing temperature of the solvent used. The
reaction may be accelerated by addiny a catalytic amount
of a tertiary amine such as triethyl amine or an organotin
compound such as dibutyltin diacetate. After the
completion of the reaction, the desired compound rnay be
isolated by distilling off the solvent used from the
reaction mixture.
Process (G) is illustrated by way of Example 20.
Now, referring back to process (A), the substituted
phenylhydrazine compounds of formula (II-A) which is to
be used as starting compound are also new compounds. The
phenylhydrazine compounds of formula (II-A) where R2 is
sulfonyl or a substituted sulfonyl group may be prepared
by process (H) given below which starts from a corres-
ponding substituted phenol of formula (V).
Process (H).
- 47 -
HO R02S-0 2
~ ~ Sulfonylation ~ ~ Nitration
y ~ , ~ _ ~ \ , Y- ~ ~ ~ 2
X X X
(V) (VI) (VII)
2 R2S-
Reduction ~ Diazotization ~--~
~0~> NH2 Y_< O ~--NH-N~2
~ Reduction ~__i
(VIII) (II-A')
where R is a substituted or unsubstituted alkyl or aryl
group.
Intermediate compounds (VI), (VII) and (VIII)
given above are also novel compounds.
In process (H), substituted phenol (V) is first
reacted with a sulfonyl halide RS02Cl or RS02Br to cause
sulfonylation of the phenolic hydroxyl group to form
sulfonyloxy compound (VI). The sulfonylation is preferably
carried out in pyridine which may serve not only as
solvent but also as the acid binding agent. Usually,
this reaction is effected in such a manner that a sulfonyl
halide is added dropwise to a mixture of a dihalophenol
and pyridine under cooling with ice-cold water, followed
by stirring the reaction mixture at room temperature. If
- ' : ' ''; ....................... .
.
'7~
- 48 -
a long time is required for the completion of the reaction,
heating to a suitable temperature which may be up to the
boiling point of pyridine makes it possible to significant-
ly shorten the reaction time. After the reaction,
pyridine salt thus deposited is filtered off and the
pyridine solvent is distilled off to leave sulfonyloxy
compound (VI). This compound may also be obtained from
the reaction mixture above by adding water and an organic
solvent such as benzene, toluene, tetrahydrofuran and
chloroform to extract the compound intended, (VI), washing
the extract with a dilute hydrochloric acid to remove
pyridine and distilling off the solvent used.
Nitro compound (VII) may be prepared by nitrating
sulfonyloxy compound (VI) with nitric acid. The nitration
may be carried out under such mixed acid condition as
usual. Thus, to a mixture of 95% sulfonic acid and
sulfonyloxy compound (VI), is added dropwise a mixture of
an equivalent amount of concentrated nitric acid and 95%
sulfuric acid under ice-water cooling,followed by stirring
~0 the reaction mixture at room temperature until the nitration
reaction becomes complete. Thereafter, the reaction mixture
is added to ice pieces for dilution, to which an oryanic
solvent such as benzene, toluene, tetrahydrofuran and
chloroform is added to extract the desired nitro compound.
Then, the solvent is distilled off from the extract to
" '` ' ` ': ' "` ' . : ' . ,
~7~
a~g --
isolate the nitro compound.
Nitro compound (VII) is then reduced by iron to
give amino compound (VIII). The reduction may usually be
conductedin water as solvent with the addition of a small
amount of hydrochloric acid to promote the reaction.
Heating the reaction mixture to a temperature of 50 - 100C
is preferred to shorten the reaction time. After the
completion of the reaction, the reaction mixture is
extracted with an organic solvent such as benzene,
toluene, tetrahydrofuran and chloroform. The removal of
the solvent from the extract by distillation yields
sulfonyloxyamino compound (VIII).
Subsequent diazotization of amino compound (VIII)
followed by reduction with stannous chloride and hydrochloric
acid results in the formation of hydrazino compound (II-A').
The diazotiazation may be effected by adding amino compound
(VIII) to aqueous hydrochloric acid, followed by adding an
equivalent amount of aqueous sodium nitrite solution
dropwise to the resulting mixture at about -20C. The
subsequent reduction may be achieved by adding the
resulting diazonium salt solution to an aqueous solution
of hydrochloric acid and stannous chloride under ice-
cooling, followed by stirring the mixture at room tempera-
ture. After the completion of the reaction, the mixture
is made weakly alkaline with the addition of an aqueous
50 -
sodium hydroxide solution, then extracted with an oryanic
solvent such as benzene, toluene, tetrahydrofuran and
chloroform. The removal of t~.e solvent from the extract
by distillation affords hydrazino compolmd (II-A').
The preparation of the hydrazine compound (II-A')
according to process (H) is illustrated later in Example 22.
Further, various members of compounds of the
aforesaid formula (I-A) where R2 is of the sulfonyl type
may be prepared in the same manner as that of the
aforesaid process (A) above, and they may be used as
starting compounds for the preparation of other compounds
of general formula (I) where ~la~ Rlb~ Rlc and R2 may
have desired meanings within the respective ranges
defined above, by utilizing any appropriate process
selected from the processes (A) to (G) described herein-
before.
- 51 -
As stated hereinbefore, any of -the processes (A)
to (G) described above may be chosen appropriately for
the production of the compounds of the general formula
tI) depending on the nature of the groups Rla, Rlb, RlC
and R2 present in the compound (I) to be produced. The
process (A) may be utilized for the production of some
members of the compounds of the general formula (I).
According to a fourth aspect of this invention, there-
fore, there is provided a process for the production of
a pyrazole derivative of the formula
R20
Y ~ ~ (I-l)
where R3, R4 and R5 each are a hydrogen atom, a (lower)
alkyl group, a halo-(lower)alkyl group, a (lower)alkenyl
group or a (lower)alkynyl group,
R2 is a (lower)alkyl group, a (lower~alkenyl
group, a (lower)alkynyl group, a (lower)alkoxy(lower)
alkyl group, a (lower)alkylthio(lower)alkyl group, a
(lower)alkylcarbonyl(lower)alkyl group, a (lower)alkoxy-
carbonyl(lower)alkyl group, a (lower)alkylthiocarbonyl
(lower)alkyl group, a cyano(lower)alkyl group, a (lower)
alkylsulfonyl group, phenylsulfonyl group, a halogen-
~7ql~
- 52 -
substituted phenylsulfonyl group or a (lower)alkyl-
substituted phenylsulfonyl group, and
X and Y are the same or different and each are a
halogen atom, which comprises reacting a hydrazine
compound of the formula
R20
Y ~ NHNH2 (II)
wherein R2, X and Y are as defined above, with a di-
carbonyl compound of the formula
O O
Il I
/C /C (III')
R5 CH R3
R4
wherein R3, R4 and R5 are as defined just above, in a
solvent in which the compound of the formula (II) and
the compound of the formula (III') are soluble and which
is unreactive with both the compounds of the formulae
(II) and (III'), at a temperature of from room temperature
to the refluxing temperasture of said solvent, to produce
the compound of the formula (I-l) above.
The reaction invoved in the above process according
to the fourth aspect of this invention may be carried out
in the same manner as described for the aforesaid process
(A).
~X7~83~
- 53 -
The process (B) as described above may be
u-tilized for the production of another some compounds
amongst the compounds of the formula (I). According to
the fifth aspect of this invention, -therefore, there is
provided a process for the produc-tion of a pyrazole
derivative of the formula
H~
Y- ~ O ~ (I-2)
where R6, R7 and R8 are each a hydrogen atom, a halogen
atom, a nitro group, an amino group, a (lower)al.kyl group,
a halo-(lower)alkyl group, a (lower)alkënyl group or a
(lower)alkynyl group, and
X and Y are the same or different and each are a
halogen atom, which comprises hydrolysing a pyrazole
compound of the formula
R2 o
Y \ O ~ ~ (I 3)
R8 R6
R7
wherein R6, R7 and R8 are as defined above,
R2 is a (lower)alkylsulfonyl group, phenylsulfonyl
.
7 ~
- 54 -
group, a halogen-substituted phenylsulfonyl group or a
(lower)alkyl-substituted phenylsulfonyl group, and X
and Y are as defined above, in an aqueous mediurn at a
temperature of from room temperature to the refluxing
temperature of the aqueous medium in the presence of a
basic compound.
The hydrolysing reaction involved in the above
process of the fifth aspect of this invention may be
carried out in the same manner as described for the
process (B) hereinbefore.
The process (C) as described above may be utilized
for the production of further some compounds amongst the
compounds of the formula (I). According to the sixth
aspect of this invention, therefore, there is provided
a process for the production of a pyrazole derivative of
the formula
R2 ~ o
4)
Rlc Rla
Rlb
where Rla, Rlb and RlC are each a hydrogen atom, a halogen
atom, a nitro group, an amino group, a (lower)alkyl group,
a halo-(lower)alkyl group, a (lower)alkenyl group, a
(lower)alkynyl group, a (lower)alkylcarbonylamino group,
~ ~7~3~
- 55 -
a (lower)alkoxycarbonylamino group, a mono-(lower)alkyl-
aminocarbonylamino group or a di-(lower)alkylaminocarbonyl-
amino group'
R2' is a (lower)alkyl group, a (lower)alkeny~ group,
a (lower)alkyn~l group, a (lower)alkoxy(lower)alkyl group,
a (lower)alkylthio(lower)alkyl group, a (lower)alkyl--
carbonyl(lower)alkyl group, a (lower)alkoxycarbonyl(lower
alkyl group, a (lower)alkylthiocarbonyl(lower)alkyl group,
a cyano(lower)alkyl group, a (lower)alkylsulfonyl group,
phenylsulfonyl group, a halogen-substituted phenylsulfonyl
group or a (lower)alkyl-substituted phenylsulfonyl group,
and X and Y are the same or different and each are a
hydrogen atom, which comprises reacting a pyrazole com-
pound of the formula
H~
-
~ (I-5)
Rlc Rla
Rlb
wherein Rla, Rlb, Rlc, X and Y are as defined above, with
a halide compound of the formula
R2'-Hal (IV)
wherein R2' is as defined above and Hal denotes a
chlorine, bromine or iodine atom, in an organic solvent
in which the compound of the formula (I-5) and the
~ ~7~34~
compound of the formula (IV) are soluble and which is
unreactive with the compounds of the formulae ~I-5) and
(IV), at a temperature of from room temperature to the
refluxing temperature of said organic solvent, in the
5 presence of an inorganic or organic acid-binding agent.
The reaction involved in this process of the
fifth aspect of this invention may be carried out in the
same manner as described for the process (C) hereinbefore.
This process is most useful to produce the new compounds
of this invention.
Tthe processes (D) and (E) as described above may
be utilized for the production of further another some
compounds amongst the compounds of the formula (I)o
According to the seventh aspect of this invention,
therefore, there is provided a process for the production
of a pyrazole derivative of -the formula
.R20
X = ~ Rg (I-6)
Rlo
where Rg, Rlo and Rll are each a halogen atom, a nitro
group, an amino group, a (lower)alkyl group, a halo-
(lower)alkyl group, a (lower)alkenyl group, a (lower)alkynyl group, a (lower)alkylcarbonylamino group, a
- 57 -
(lower)alkoxycarbonylamino grou~, a mono-(lower)alkyl-
aminocarbonylamino group or a di-(lower)alkylaminocarbonyl-
amino group, provided that one, two or three of R9, R
and Rll is or are halogen atom or nitro group,
R2 is a hydrogen atom, a (lower)alkyl group, a
(lower)alkenyl group, a (lower)alkynyl group, a (lower)
alkoxy(lower)alkyl group, a (lower)alkylthio(lower)alkyl
group, a (lower)alkylcarbonyl(lower)alkyl group, a (lower)
alkoxycarbonyl(lower)alkyl group, a (lower)alkylthio-
carbonyl(lower)alkyl group, a cyano(lower)alkyl group,
a (lower)alkylsulfonyl group, phenylsulfonyl group, a
halogen-substituted phenylsulfonyl group or a (lower)
alkyl-substituted phenylsulfonyl group, and
X and Y are the same or different and each are a
halogen atom, which comprises reacting a pyra~ole compound
of the formula
R20
X ~ ~ (I-7)
13
where R12, R13 and R14 are each a hydrogen atom, a halogen
atom, a nitro group, an amino group, a (lower)alkyl group,
a halo-(lower)alkyl group, a (lower)alkenyl group, a
(lower)alkynyl group, a (lower)alkylcarbonylamino group,
~ 4~
- 58 -
a (lower)alkoxycarbonylamino group, a mono-(lower)alkyl-
aminocarbonylamino group or a di-(lower)al~ylaminocarbonyl-
amino group' provided that one, two or three of Rl~, R13
and R14 is or are hydrogen atom and R2, X and Y are as
defined above,with a halogenating agent or a nitrating
agent in a solvent at a temperature of from 0C to the
refluxing temperature of the solvent as employed, to
convert such one or ones of the groups R12, R13 and R14
of the compound (I-7) which is or are the hydrogen atom,
into the halo group or nitro group.
The halogenation or nitration involved in this
process of the seventh aspect of this invention may be
carried out in the same manner as described for -the
process (D) or (E) hereinbefore.
The process (F) as described above may be utilized
for the production of further some compounds amongst the
compounds of the formula (I). According to the eighth
aspect of this invention, therefore, there is provided
a process for the production of a pyrazole derivative
of the formula
- 59 -
R20
Y ~ ~ (I~
R17 R15
R16
where R15, R16 and R17 each are a hydrogen atom, a halogen
atom, an amino group, a (lower)alkyl group, a halo-(lower)
alkyl group, a (lower)alkenyl group, a (lower)alkynyl gxoup,
a (lower)alkylcarbonylamino group, a (lower)alkoxycarbonyl-
amino group, a mono-(lower)alkylaminocarbonylamino group or
a di-(lower)alkylaminocarbonylamino group; provided that one,
15~ R16 and R17 is or are amino group
R2 is a hydrogen atom, a (lower)alkyl group, a (lower)
alkenyl group, a (lower)alkynyl group, a (lower)alkoxy(lower)
alkyl group, a (lower)alkylthio(lower)alkyl group, a (lower)
alkylcarbonyl(lower)alkyl group, a (lower)alkoxycarbonyl
(lower)alkyl group, a (lower)alkylthiocarbonyl(lower)alkyl
group, a cyano(lower)alkyl group, a (lower)alkylsulfonyl
group, phen~lsulfonyl group, a halogen-substituted phenyl-
sulfonyl group or a (lower)alkylsubstituted phenylsulfonyl
group, and
~ and Y are the same or different and each are a
halogen atom, which comprises reacting a pyrazcle compound
of the formula
-
~7~
- 60 -
R20
Y = ~ (I-9)
18
Rlg
where R18, Rlg and R20 each are a hydrogen atom/ a halogen
atom, an amino group, a (lower)alkyl group, a halo-(lower)
alkyl group, a (lower)alkenyl group, a (lower)alkynyl group~
S a (lower)alkylcarbonylamino group, a (lower)alkoY.ycarbonyl~
amino group, a mono-(lower)alkylaminocarbonylamino group or a
di-(lower)alkylaminocarbonylamino group; provided that one,
two or three of R18, Rlg and R20 is or are nitro group,
~ , X and Y are as defined above, with metallic iron
or tin as the reducing agent in the presence of hydrochloric
or sulfuric acid or with nascent hydrogen as formed by
reactionof metallic iron or tin with hydrochloric or sulfuric
acid, in an aqueous medium at a temperature of from O~C to
the refluxing temperature of the aqueous medium, to convert
the nitro group(s) present in the compound (I-9) into the
amino group(s).
The reduction reaction involved in this process of the
eighth aspect of this invention may be carried out in
.~ the same manner as described for the process (F) herein-
before.
,
~ 7~34
- 61 -
The process (G) as described above may be utilized
for the production of some compounds amonyst the compounds
of the formula (I). According to the ninth aspect of
this invention, therefore, there is provided a process
for the production o~ a pyrazole derivative of the formula
R20
~ "~ 10)
where R21~ R22 and R23 each are a hydrogen atom, a halogen
atom, a nitro group, a (lower)alkyl group, a halo-(lower)
alkyl group, a (lower)alkenyl group, a (lower)alkynyl group,
a (lower)alkylcarbonylamino group, a (lower)alkoxycarbonyl-
amino group, a mono-(lower)alkylaminocarbonylarnino group or
a di-(lower)alkylaminocarbonylamino group; provided that one,
21~ R22 and R23 is or are a (lower)alkyl-
carbonylamino group/ a (lower)alkoxycarbonylamino group, a
mono-(lower)alkylaminocarbonylamino group or a di-(lower)
alkylaminocarbonylamino group,
R2 is a hydrogen atom, a (lower)alkyl group, a (lower)
alkenyl group, a (lower)alkynyl group, a (lower)alkoxy(lower)
alkyl group, a (lower)alkylthio(lower)alkyl group, a (lower)
alkylcarbonyl(lower)alkyl group, a (lower)alkoxycarbonyl
~;~7~
- 62 -
(lower)alkyl group a (lower)alkylthioca~bonyl(lower)alkyl
group a cyano(lower)alkyl group a (lower)alkylsulfonyl
group, phenylsulfonyl group, a halogen-substituted phenyl-
sulfonyl group or a (lower)alkylsubstitu~ed phenylsulfonyl
group, and
X and Y are the same or different and each are a
halogen atom, which comprises xeacting a pyra~ole compouund
of the formula
R20
Y -~ C~ / ~ (I-ll)
R26 R24
~2S
where R2a~ R25 and R26 each are a hydrogen atom, a haloyen
a~om, nitro group,an a~ino group, a (lcwer)alkyl group, a halo~(lcwer)
alkyl group, a (lower)alkenyl group D a (lower)alkynyl gxoup,
a (lower)alkylcarbonylamino group, a (lower)alkoxycarbonyl-
amino group, a mono-(lower)alkylaminocarbonylamino group or a
di-(lower)alkylaminocarbonylamino group; provided that one,
two or three of R24, R25 and R26 is or are amino group~
R2, X and Y are as defined above, with a chloride
compound of the formula
Q-CO-Cl (IX)
wherein Q is a (lower)alkyl group, a (lower)alkoxy group, a
~.~7~83~L
~ 63 -
mono--(lower)alkylamino group or a di-(lower)alkylamino, in
an organic solvent in which the compounds of the formulae
(I-ll) and (IX) are soluble, at a temperature of 0C to the
refluxing temperature of said organic solvent in the presence
of an acid binding agent, or with an isocyanate compound of
the formula
Q'-N=C=O (X)
wherein Q' is a (lower)alkyl group, in an organic solvent in
which the compounds of the formulae (I-ll) and the isocyanate
compound of the formula (X) are soluble and which is un~
reactive with the isocyanate compound (VI), at a temperature
of from room temperature to the refluxing te~.perature of
said organic solvent, to acylate the amino group(s) present
in the compound of the formula (I-ll) with the acyl group
Q-CO- of the compound (IX) or with the acyl group Q'-NH~CO-
as derived from the isocyanate compound (X)~
The acylation reac-tion involved in this process of
the ninth aspect of this invention may be carried out in
the same manner as described for the process (G) herein-
before.
Now, the herbicidal composition according to -the
second aspect of this invention is described in particular.
The herbicidal composition according to the second
aspect of th.is invention may be, for example, in the form
of aqueous solution, dispersion, emulsion, dusting powder,
f~
~0
- 64 -
wettable powder, flowable powder (sol), drif-tless (Dl,-
type) powder, granules, fine granules, table-ts and others.
Any form of the composition above-mentioned rnay be pre-
pared from the compounds of formula (I) according to
conventional formulation techniques. Any desired solid
or liquid carrier or diluent may be used which has been
used conventionally in the preparation of agricultural
or horticul-tural chemical compositions.
Suitable solid carriers or diluents include
mineral powders such as kaolin, bentonite, clay, montmoril-
lonite, talc, diatomaceous earth, mica, vermlculite, gypsum,
calcium carbonate, apatite, white carbon, slaked lime,
siliceous sand, ammonium sulfate and urea; vegetable
powders such as soya bean flour, wheat flour, wood meal,
tobacco powder, starch and crystalline cellulose; macro-
molecular compounds such as petroleum resin, polyvinyl
chloride, ketone resin and dammar gum; alumina, silicates,
sugar polymers, high-dispersible silicic acid and waxes.
Suitable liquid carriers or diluents include water;
alcohols such as methanol, ethanol, n-propanol, i-propanol,
bu-tanol, ethylene glycol and benzyl alcohol, aromatic
hydrocarbons such as toluene, benzene, xylene, ethyl-
benzene, and methyl naphthalene; halogenated hydrocarbons
such as chloroform, carbon tetrachloride, dischloro-
methane, chloroethylene, monochlorobenzene, trichloro-
~X7~33~
-- 65 -
fluoromethane and dichlorodifluoromethane; ethers such as
ethylether, ethylene oxide, dioxane and tetrahydrofuran;
ketones such as acetone, methyl ethyl ke-tone, cyclohexanone,
methyl isobutyl ketone and isophorone; esters such as
ethyl acetate, butyl acetate, ethylene glycol acetate and
amyl acetate; acid amides such as dimethylformamide and
dimethylacetamide; nitrlles such as acetoni-trile, propio-
nitrile and acrylonitrile; sulfoxides such as dimethyl-
sulfoxide; alcoholethers such as ethylene glycol mono-
methylether and ethylene glycol monoethylether; aliphaticand cycloaliphatic hydrocarbons such as n-hexane and
cyclohexane; industrial gasoline such as petroleum ether
and solvent naphtha; and petroleum fractions such as
paraffins, kerosene and gas oil.
In the preparation of emulsion, dispersion, wettable
powder, flowable powder and the like, one or more surface
active agents are used for the purpose of emulsification,
dispersion, solubilization, wetting, foaming, lubrication,
spreading or the like. Surface active agents may be of
non-ionic, anionic, cationic or amphoteric type. Suitable
agents of the non-ionic type include for example polyoxy-
ethylene alkylethers, polyoxyethylene alkylesters, poly-
oxyethylenesorbitan alkylesters and sorbitan alkylesters.
Suitable agents of the anionic type include~for example,
soaps, alkylbenzene sulfonates, alkylsulfosuccinates,
~7~
- 66 -
alkyl sulphates, polyoxyethylene alkylsulfates and aryl
sulfonates. Suitable agents of the cationic type include
alkylamines such as laurylamine, stearyl trimethyl arnmonium
chloride and alkyl dimethyl benzyl ammonium chloride, and
polyoxyethylene alkylamines. Suitable agents of the
amphoteric type include carboxylic acids of betaine type
and salts of sulfuric esters.
In addition to the carriers or diluents and surface
active agents, the herbicidal composition may contain a
variety of additives, as desired. Such additives include
for example polyvinyl alcohol, carboxymethylcellulose,
gum arabic, polyvinyl acetate, gelatine, casein, sodium
alginate and tragacanth gum.
The herbicidal compositions according to this
invention may be formulated in any desired form as
exemplified above in which the compound of formula (I)
is present in an amount of 0.001 to 95% by weight, pre-
ferably 0.01 to 90% by weight. For example, the content
of the compounds of formula (I) may usually be 0.01 to
5% by weight when formulated in the form of powder, DL
powder or fine granules (F), 0.01 to 10% by weight in
the form of granules, and 1 to 75% by weight in the form
of wettable powder, aqueous emulsion or solution.
The herbicidal compositions in the form of granules,
for example, may be applied as such by dusting them
~'791~
- 67 -
on the surface of or in the soil or in aquatic medium in
an amount of about 0.3 to 300 g of the active ingredient
per 10 ares. In cases of sol powder, emulsions or
wettable powder, the compositions are usually diluted
with water or an appropriate solvent and the diluted
compositions are applied at a rate of about 0.3 to 300 g
of the active ingredient per 10 ares.
When the compounds of this invention, as such or
in the form of a composition, are used as herbicides,
they may be used, if desired, ln combination with one or
more of known herbicides, insecticides, fungicides, plant-
growth regulating agents and others for extending the
application range of the compounds with a possibility of
synergism resulting from such combinations in some cases.
Examples of other herbicides which may be used
in combination with the compounds of this invention
include the following.
Triazine herbicides
6-chloro-N-ethyl-N'-isopropyl-1,3,5-triazin-2-yl-
2,4-diamine
6-chloro-N,N'-diethyl-1,3,5 triazin-2,4-diy~-
diamine
N-ethyl-N'-isopropyl-6-methylthio-1,3,5-triazin-
2,4-diyldiamine
N-N'-di-isopropyl-6-methylthio-1,3,5-triazin-
~7~
- 68 -
2,4-diyldiamine
N,N'-diethyl-6-methylthio-1,3,5-triazin-2,4-
diyldiamine
N-(1,2-dimethylpropyl)-N'-ethyl-6-methylthio-
1,3,5-triazin-2-yl-2,4-diamine
2-(4-chloro-6-ethylamino-1,3,5-triazin-2-ylamino)-
2-methylpropionitrile
3-cyclohexyl-6-dimethylamino-1-methyl-1,3,5-
triazin-2,4(lH,3H)-dione
4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-
5(4H)-one
4-amino-3-methyl-6-phenyl-1,2,4-triazin-5t4H~-
one
Amide herbicides
2-chloro-2',6'-diethyl-N-methoxymethylacetanilide
2-chloro-6'-ethyl-N-(2-me-thoxy-1-methylethyl)acet-
ortho-toluidine
N-butoxymethyl-2-chloro-2',6'-diethylacetanilide
2-chloro-2',6'-diethyl-N-(2-propoxyethyl)ace-tanilide
2-chloro-N-isopropylacetanilide
3',4'-dichloropropionanilide
N-l-naphthylphthalamic amide
Ethyl N-benzoyl-N-(3,4-dichlorophenyl)-D,L~alaninate
N,N-dimethyldiphenylacetamide
3~
- 69 -
3,5-dichloro-N-(1,1-dimethylpropynyl)benzamide
5'-~trifluoromethansulfonamido)acet-2',4'-xylidide
N-(~,~-dimethylbenzyl)-2-bromo-3,3,-dimethyl-
butaamide
N-methyl-2-(benzthiazol-2-yloxy)acetanilide
Carbamate herbicides
S-4-chlorobenzyl diethyl(thiocarbamate)
S-ethyl azepin-l-carbothioate
S-propyl dipropyl(thiocarbamate)
S-ethyl dipropyl(thiocarbamate)
S-ethyl diisobutyl(thiocarbamate)
S-propyl butyl(ethyl)thiocarbamate
S-ethyl N-cyclohexyl-N-ethyl(thiocarbamate)
S-2,3-dichloroallyl diisopropyl(thiocarbamate)
Isopropyl carbanilate
Methyl 3,4-dichlorocarbanilate
Methyl sulfanylcarbamate
Methyl 3-(3-methylcarbanyloyloxy)carbanilate
S-isopropyl hexahydro-lH-azepin-l-carbothioate
S-3-chloropropyl 3,6-dimethylhexahydro-lH-azepin-
l-carbothioate
S-~,~-dimethylbenzyl piperidin-l-carbothioate
Urea herbicides
3-(4-chlorophenyl)-1,1-dimethylurea
3-(3,4-dichlorophenyl)-1-methoxy-1-methylurea
~7~3~
_ 70 -
3-(3,4-dichlorophenyl)-1,1-dimethylurea
1,1-dimethyl-3-(~ -trifluoro-meta-tolyl)urea
3-(4-bromo-3-chlorophenyl)-1-methoxy-1-methylurea
3-(3-chloro-4-methoxyphenyl)-1,1-dimethylurea
3-para-cumenyl-1,1-dimethylurea
3-~4-(4-chlorophenoxy)phenyl]-1,1-dimethylurea
1-(2-methylcyclohexyl)-3-phenylurea
l-benzothiazol-2-yl-3-methylurea
1-benzothiazol-2-yl-1,3-dimethylurea
1-(S-tert-butyl-1,3,4-thiadiazol-2-yl)-1,3-di-
methylurea
3-(5-tert-butyl-1,3,4-thiadiazol-2-yl)-4-hydroxy-
l-methyl-2-imidazolidone
1-(2-chlorophenylsulfonyl)-3-(4-methoxy-6-methyl-
1,3,5-triazin-2-yl)urea
Methyl 2-{[(4,6-dimethoxy-pyrimidin-2-yl)amino-
carbonyl]aminosulfonylmethyl}benzoate
l-(~,~-dimethylbenzyl)-3-(para-tolyl)urea
Toluidine herbicldes
~ -trifluoro-2,6-dinitro-N,N-dipropyl-para-
toluidine
N-(l-ethylpropyl)-2,6-dinitro-3,4-xylidine
Nl,Nl-diethyl-2,6-dinitro-4-trifluoromethyl-meta-
phenylenediamine
N-(2-chloroethyl)-~ -trifluoro-2,6-dinitro-N-
4834
_ 71 -
propyl-para-toluidine
4-methylsulfonyl-2,6-dinitro-N,N-dipropylaniline
Diazine herbicides
5-tert-butyl-3-(2,4-dichloro-5-isopropoxyphenyl)-
1,3,4-oxadiazol-2(3H)-one
2-(3,4-dichlorophenyl)-4-methyl-1,2,4-oxadia-
zolidine-3,5-dione
3-isopropyl-(lH)-2,1,3-benzothiadiazin-4(3H)-one
2,2-dioxide
4-(2,4-dichlorobenzoyl)-1,3-dimethylpyrazol-5-yl-
paratoluene sulfonate
1,3-dimethyl-4-(2,4-dichlorobenzoyl)-5-phenacyloxy-
pyrazole
Diphenylether herbicides
5-(2-chloro-~ -trifluoro-para-tolyloxy)-2-
nitrobenzoic acid
2-chloro~ 3-ethoxy-4-nitrophenoxy)-4-(trifluoro-
methyl)benzene
2,4-dichlorophenyl-3-methoxy-4'-nitrophenylether
2,4,6-trichlorophenyl-4'-nitrophenylether
Methyl 5-(2,4-dichlorophenoxy)-2-nitrobenzoate
Phenoxyaliphatic acid herbicides
2-methyl-4-chlorophenoxyacetic acid and its salts,
esters and amide derivatives
2,4-dichlorophenoxyacetic acid and its sal-ts,
3 ~74~39~
_ 72 -
esters and amide derivatives
~ -2-(4-chloro-2-methylphenoxy)propionic acid and
its salts, esters and amide derivatives
4-(4-chloro-o-tolyloxy)butyric acid and its salts,
esters and amide derivatives
S-ethyl k-chloro-o-tolyloxythioacetate
2-(2,4-dichloro-3-methylphenoxy)propionanilide
~ -(2-naphthyloxy)propionanilide
(RS)-N,N-diethyl-2-(1-naphthyloxy)propionamide
(RS)-2-~4-(2,4-dichlorophenoxy)phenoxy]propionic
acid and its ester derivatives
(RS)-2-[4-(5-trifluoromethyl-2-pyridyloxy)phenoxy]
propionic acid and its ester derivatives
(RS)-2-~4-(3-chlor-o-5-trifluoromethyl-2-pyridyloxy)
phenoxy]propionic acid and its ester derivatives
(RS)-2-[4-(3,5-dichloro-2-pyridyloxy)phenoxy]
propionic acid and its salts, esters and amide derivatives
(RS)-2-[4-(6-chloroquinoxalinyloxy)phenoxy]pro-
pionic acid and its ester derivatives
Organophosphorus herbicides
_
N-(phosphonomethyl)glycine and its salt derivatives
D,L-homoalanine-4-yl(methyl)phosphinic acid and
its salt derivatives
2-amino-4-[(hydroxy)(methyl)phosphionyl]butyryl-
alanylalanine and its salt derivatives
~74834
0-ethyl 0-6-nitro-meta-tolyl sec-bu-tylphosphoro-
amidothioate
S-4-chloro-N-isopropylcarbanyloxymethyl 0,0-
dimethylphosphorodithioate
S-2-methylpiperidinocarbonylmethyl 0,0-dipropyl-
phosphorodithioate
S-2-benzensulfonamidoethyl 0,0-di-isopropyl-
phosphorodithioate
Nitrile herbicides
2,6-dichlorobenzonitrile
2,6-dichloro(thiobenzamide)
3,5-dibromo-4-hydroxybenzonitrile
4-hydroxy-3,5-di~iodobenzonitrile
~racil herbicide_
5-bromo-3-sec-butyl-6-methyluracil
3-cyclohexyl-1,5,6,7-tetrahydrocyclopentapyrimidin-
2,4(3H)-dione
3-tert-butyl-5-chloro-6-methyluracil
Benzoic acid herbicides
3-amino-2,5-dichlorobenzoic acid and its salt
derivatives
3,6-dichloro-ortho-anisic acid and its salt
derivatives
2,3,5,6-tetrachloroterephthalic acid and its
salts, esters and amide derivatives
., . ~ .,
.. ; .
... .
~X7~33~
_ 74 -
Phenolic herbicides
2-sec-butyl-4/6-dinitrophenol and its salts and
carboxylate ester derivatives
4,6-dinitro-ortho-cresol and its salts and carbo-
S xylate ester derivatives
Quaternary ammonium herbicides
1,1'-dimethyl-4,4~-bipyridinium dichloride
1,1'-ethylene-2,2'-bipyridinium dibromide
Pyridazine herbicides
5-amino-4-chloro-2-phenylpyridazin-3(2H)-one
4-chloro-5-methylamino-2-~ -trifluoro-meta-
tolyl)pyridazin-3(2H)-one
Pyridine herbicides
4-amino-3,5,6-trichloropyridin-2-carboxylic acid
and its salt derivatives
3,5,6-trichloro-2-pyridyloxyacetic acid and its
salt and ester derivatives
l-methyl-3-phenyl-5-(~ -trifluoro-meta-tolyl)-
4-pyridone
Other herbicid_s
3-(chloro-2-amino-1,4-naphthoquinomethyl 3-[1-
(allyloxyimino)butyl]-4-hydroxy-6,6-dimethyl-2-oxo-
cyclohexa-3-encarboxylate and its salt derivatives
(~)-2-(1-ethoxyiminobutyl)-5-[2-(ethylthio)
propyl]-3-hydroxycyclohexa-2-enone
~'7~
1,2-dimethyl-3,5-diphenylpyrazoliummethylsulphate.
This invention i5 now illustrated, but not limited,
by the following examples. In Examples, all parts and
percentages are by weight, unless otherwise stated.
Example 1
Preparation of 1-(2-fluoro-4-chloro-5-methyl-
sulfonyloxyphenyl ~-3,5-di-trifluoromethylpyrazole
(Compound No. 25) (Process A)
A mixture of (2-fluoro-4-chloro-5-methylsulfonyloxy-
phenyl)-hydrazine (25.5 g), 1,1,1,5,5,5-hexafluoropenta-
2,4-dione (20.8 g), ethanol (100 ml) and water (100 ml)
was heated to reflux for one hour. After the reaction
mixture was cooled, toluene was added thereto to form two
layers. The toluene layer separated was washed with water
and dried over anhydrous sodium sulfate. Removal of the
solvent by distillation under reduced pressure gave the
titled compound (40.1 g) as a pale brown crystalline solid.
Recrystallization of this solid from a mixture of cyclo-
hexane and ethyl acetate afforded a white crystalline solid.
m.p. 87-89.5C.
Example 2
Preparation of 1-(2,4-dichloro-5-isopropoxyphenyl)-
3,5-di-trifluoromethylpyrazole (Compound No. 27) (Process A)
(2,4-dichloro-5-isopropoxyphenyl)hydrazine (23.5 g)
was admixed with 1,1,1,5,5,5-hexafluoropenta-2,4-dione
~;~7~
- 76 -
(20.8 g) and the resultant adrnixture was slowly warmed
and maintained at 120C for one hour. After the reaction
mixture was cooled, toluene was added thereto and the
resulting admixture was washed with an aqueous dilute
hydrochloric acid solution and then with water and dried
over anhydrous sodium sulfate. Removal of the solven~ by
distillation under reduced pressure gave the titled com-
pound (37.4 g) as a pale yellow oil. Purification of this
oil by silica gel column chromatography yielded a color-
less oil. nD3 = 1.5478.
Example 3
Preparation of 1-(2-fluoro-4-chloro-5-methyl-
sulfonyloxyphenyl)-3,4,5-trimethylpyrazole (Compound NoO
148) (Process A)
A mixture of (2-fluoro-4-chloro-5-methylsulfonyloxy-
phenyl)hydrazine (25.5 g) of the formula:
CH3S02~ 0
Cl ~ NH-NH2
3-methylpenta-2,5-dione (CH3COCH(CH3)COCH3) (11.4 g),
ethanol (100 ml) and water (100 ml) was heated to reflux
for one hour. After the reaction mixture was cooled,
toluene was added thereto to form two layers. The toluene
layer separated was washed with water and dried over
~'~74~3~
- 77 -
anhydrous sodium sulfate. ~emoval of the solvent by
distillation in vacuo gave the titled compound (2903 g)
as a pale yellow crystalline solid. Recrystallization
of this solid from a mixture of n-hexane and ethyl acetate
afforded a white crystalline solid. m.p. 123-125C.
Example 4
Preparation of 1-(2-fluoro-4-chloro-5-phenyl-
sulfonyloxyphenyl)-3,4,5-trimethylpyrazole (Compound
No. 150) (Process A)
A mixture of (2-fluoro-4-chloro-5-phenylsulfonyloxy-
phenyl)-hydrazine (31.7 g), 3-methylpenta-2,4-dione (11.4 g)
and water (150 ml) was stirred at 70C for one hour~
After the reaction mixture was cooled, toluene was added
thereto whereby to form two layers. The toluene layer
thus separated was washed with water and dried over an-
hydrous sodium sulfate. Removal of the solvent by
distillation in vacuo gave the titled compound (37~5 g)
as a pale brown crystalline solid. Recrystallization of
this solid from carbon tetrachloride afforded a white
crystalline solid. nD3 = 1.5492.
Example 5
Preparation of 1-(2~fluoro-4-chloro-5-propargyloxy-
phenyl)-3,4,5-triethylpyrazole (Compound No. 167) (Process
A)
(2 -fluoro-4-chloro 5-propargyloxyphenyl)hydrazine
- 78 -
(21.5 g) and 4-ethylhepta-3,5-dione (15.6 g) were reacted
with each other and processed in the same manner as in
Example 1 to afford the titled compound (30.1 g) as a
pale yellow oi~. Purification of this oil by silica gel
column chromatography gave a white crystalline solid.
m.p. 62-65C.
Example 6
Preparation of 1-(2-fluoro-4-chloro-5-hydroxyphenyl)-
3,5-dimethyl-4-nitropyrazole (Compound No. 37) (Process B)
A mixture of 1-(2-fluoro-4-chloro-5-methylsulfonyloxy-
phenyl)-3,5-dimethyl-4-nitropyrazole (36.5 g), 2N aqueous
sodium hydroxide solution (100 ml) and ethanol (50 ml) was
stirred at 40C for 30 minutes. After cooling, the re-
action mixture was acidified with an aqueous hydrochloric
acid solution and extracted with toluene. The toluene
layer thus obtained was washed with water and dried over
anhydrous sodium sulfate. Removal of the solvent by
distillation in vacuo gave the titled compound (28.1 g)
as a pale yellow crystalline solid. Recrystallization of
this solid from a mixture of n-hexane and ethyl acetate
afforded a white crystalline solid. m.p. 157-158C.
Example 7
Preparation of 1-(2-fluoro-4-chloro-5-hydroxyphenyl)-
3,4,5-trimethylpyrazole (Compound No. 122) (Process B)
A mixture of 1-(2-fluoro-4-chloro-5-methyl~
~ ~7~3~
- 79 -
sulfonyloxyphenyl)-3,4,5-trimethylpyrazole (33.3 g), 2N
aqueous sodium hydroxide (100 ml) and ethanol (50 ml) was
stirred at 40C for 30 minutes. After cooling, the re-
action mixture was acidified with aqueous hydrochloric
acid and then extracted wth toluene. The toluene layer
thus obtained was washed with water and dried over an-
hydrous sodium sulfate. Removal of the solvent by
distillation in vacuo gave the titled compound (24.2 g)
as a pale yellow crystalline solid. Recrystallization
of this solid from a mixture of n-hexane/ethyl acetate
yielded a white crystalline solid. m.p. 158-160C.
Example 8
Preparation of 1-(2,4~dichloro-5-hydroxyphenyl)-
3,5-di-methyl-4-chloropyrazole (Compound No. 173)
(Process B)
A mixture of 1-(2,4-dichloro-5-methylsulfonyloxy-
phenyl)-3,5-dimethyl-4-chloropyrazole (37.0 g) and 2N
aqueous sodium hydroxide was stirred at 50C for 30 minutes.
After cooling, the reaction mixture was acidified with
aqueous hydrochloric acid and then extracted with toluene.
The resulting toluene layer was washed with water and
dried over anhydrous sodium sulfate. Removal of the
solvent by distillation in vacuo gave the titled com-
pound (26.8 g) as a pale yellow crystalline solid.
Recrystallization of this solid from a mixture of
~L~'74~33~
- 80 -
n-hexane/ethyl acetate afforded a white crystalline solid.
m.p. 172-174C.
Example 9
Preparation of 1-(2-fluoro-4-chloro-5-propargyloxy-
phenyl)-3,5-dimethyl-4-nitropyrazole (Compound NoO 47)
~process C)
A mixture of 1-(2-fluoro-4-chloro-S-hydroxyphenyl)-
3,5-dimethyl-4-nitropyrazole (28.7 g), propargyl bromide
(13.1 g), anhydrous potassium carbonate (15.2 g) and
acetonitrile (200 ml) was stirred at 60C for 2 hours.
After cooling, the reaction mixture was filtered through
a filter to remove crystalline solid matters and the
filtrate was concentrated to a small volume under reduced
pressure. The concentrated solution was admixed with
toluene and water to form two layers. The toluene layer
formed (the extract) was separated, washed with water and
then dried over anhydrous sodium sulfate. Removal of the
solvent by distillation in vacuo gave the titled compound
~31.2 g) as a pale yellow crystalline solid. Recrystal-
lization of this solid from a mixture of n-hexane/ethyl
acetate yielded a white crystalline solid. m.p. 125-126.5C.
Example 10
Preparation of 1-(2-fluoro-4-chloro-S-isopropyloxy-
phenyl)-3,4,5-trimethylpyrazole (Compound No. 129)
(Process C)
~7~
- 81 -
A mixture of 1-(2-fluoro-4-chloro-5-hydroxyphenyl)-
3,4,5-trimethylpyrazole ~25.5 g), isopropyl iodide (18O7 g),
anhydrous potassium carbonate (14.5 g) and dimethylsulfoxide
(200 ml) was stirred at 80C for one hour. After cooling,
the reaction mixture was admixed with water and toluene to
form two layers. The toluene layer formed was separated,
washed with water and then dried over anhydrous sodium
sulfate. Removal of the solvent by distillation in vacuo
gave the titled compound (31.1 g) as a pale yellow oil,
which was then purified through silica gel column chromato-
graphy to yield a colorless oil. n2D3 = 1.5406.
Example 11
Preparation of 1-(2-fluoro-4-chloro-5-allyloxy-
phenyl)-3,4,5-trimethylpyrazole (Compound No. 136)
(Process C)
A mixture of 1-(2-fluoro-4-chloro-5-hydroxyphenyl)-
3,4,5-trimethylpyrazole (25.5 g), allyl bromlde (13.3 g),
anhydrous potassium carbonate (14.5 g) and acetonitrile
(150 ml) was heated under reflux, with stirring for 3
hours. After cooling, the reaction mixture was filtered
through a filter to remove the inorganic salts therefrom
and the filtrate was concentrated to a small volume under
reduced pressure, affording the titled compound (28.7 g)
as a pale brown crystalline solid. Recrystallization of
this solid from cyclohexane yielded a white crystalline
~7L~4
- 82 -
solid. m.p. 97-99C.
Example 12
Preparation of 1-(2-fluoro-4-chloro-5-ethoxy-
carbonylmethyloxyphenyl)-3,5-dimethyl-4-chloropyrazole
~Compound No. 187) (Process C)
1-(2-fluoro~4-chloro-5-hydroxyphenylJ-3,5-dirnethyl-
4-chloropyrazole (27.5 g) was dissolved in tetrahydrofuran
(200 ml), and to the resultant solution was added sodiurn
amide (4.0 g). When the evolution of ammonia gas from the
reaction mixture ceased, ethyl monochloroacetate (12.3 g)
was added dropwise to the reaction mixture. After the com-
pletion of addition, the reaction mixture was heated under
reflux, with stirring for further 3 hours. To the reaction
mixture, after cooling, were added toluene and water to
form two layers. The organic layer as separated was
washed with water and dried over anhydrous sodium sulfate.
Removal of the solvent by distillation in vacuo gave the
titled compound (33.9 g) as a pale brown crystalline solid.
Recrystallization of this solid from cyclohexane afforded
a white crystalline solid. m.p. 101-104 C.
Example 13
Preparation of 1-(2, 4 -dichloro-5-isopropoxyphenyl)-
3 r4 ,5-trimethylpyrazole (Compound No. 132) (Process C)
A mixture of 1-(2,4-dichloro-5-hydroxyphenyl)-
3,4,5-trimethylpyrazole (27.1 g), isopropyl bromide (12.3 g),
74~
aqueous sodium hydroxide (4.2 g) and dimethylformamide
(150 ml) was stirred at 70C for 2 hours. To the reaction
mixture, after cooling, were added water and toluene, to
form two layers. The toluene layer as separated was
washed with water and then dried over anhydrous sodium
sulfate. Removal of the toluene by distillation in vacuo
gave the titled compound (27.9 g) as a pale yellow oil~
which was then purified through silica gel column chromato-
graphy to afford a white crys-talline solid. m.p. 56-58Co
Example 14
Preparation of 1-(2,4-dichloro-5-propargyloxyphenyl)-
3,5-dimethyl-4-chloropyrazole (Compound No. 140) (Process C)
1-(2,4-dichloro-S-hydroxyphenyl)-3,5-dimethyl-4-
chloropyrazole (29.2 g) and propargyl bromide (12.0 g)
lS were added to a solution of sodium methoxide which was
previously prepared from metallic sodium (2.3 g) and
methanol (200 ml). The resulting mixture was heated under
reflux, with stirring for 3 hours. To the reaction mixture,
after cooling, were added water and toluene to form two
~0 layers. The toluene layer as formed was separated, washed
with water and then dried over anhydrous sodium sulfateO
Removal of the solvent by distillation in vacuo gave the
titled compound (30.1 g) as a pale brown crystalline solid.
Recrystallization of this solid from a mixture of cyclo-
hexane/acetone afforded a white crystalline solid.
~x~
- 84 -
m.p. 156.5-158C.
Example 15
Preparation of 1-(2-fluoro-4-chloro-5-methyl-
sulfonyloxyphenyl)-3-trifluoromethyl-4-chloro-5-methyl-
pyrazole (Compound No. 81) (Process D)
Into a solution of 1-(2-fluoro-4-chloro-5-methyl-
sulfonyloxyphenyl)-3-trifluoromethyl-5-methylpyrazole
(37.3 g) in chloroform (200 ml) was introduced chlorine
gas (7.1 g) at 60C for 20 minutes. Removal of the
solvent from the reaction solution by distillation in
vacuo gave the titled compound (40.2 g) as a pale yellow
oil which soon crystallized on standing at room tem-
perature. Recrystallization from a mixture of n-hexane/
benzene yielded a white crystalline solid. m.p. 72-74.5C.
Example 16
Preparation of 1-(2-fluoro-4-chloro-5-methyl-
sulfonyloxyphenyl)-3,5-dimethyl-4-chloropyrazole (Compound
No. 189) (Process D)
Into a solution of 1-(2-fluoro-4-chloro-5-methyl-
sulfonyloxyphenyl)-3,5-dimethylpyrazole (31.9 g) in chloro-
form (200 ml) was introduced chlorine gas (7.1 g) for 10
minutes under water-cooling, and then the reaction mixture
was stirred for further 30 minutes to effect the chlorin-
ation reaction. Removal of the solvent from the reaction
solution by distillation in vacuo gave the titled compound
~;~7a~
- 85 -
~35.2 g) as a pale brown oil. Purification of this oil by
silica gel column chromatography yielded a colorless oil.
n~3 = 1.5431.
Example 17
Preparation of 1-(2-fluoro-4-chloro-5-ethoxyphenyl)-
3,5-dimethyl-4-chloropyrazole (Compound No. 175) (Process D)
To a solution of 1-(2-fluoro-4-chloro-5-ethoxyphenyl~-
3,5-dimethylpyrazole (26.9 g) in chloroform (200 ml) was
added dropwise sulfuryl chloride (13.5 g) for 10 minutes
under water-cooling, and the reaction mixture was stirred
for further 30 minutes to effect the chlorination reaction.
Removal of the solvent from the reaction solution by dis-
tillation in vacuo gave the titled compound (30.0 g) as a
pale yellow crystalline solid. After recrystallization of
the solid from a mixture of n-hexane/ethyl acetate, the
resulting white crystalline product showed the melting
point of 106-107.5C.
Example 18
Preparation of 1-(2-fluoro-4-chloro-5-methyl-
sulfonyloxyphenyl)-3,5-dimethyl-4-nitropyrazole (Compound
No. 52) (Process E)
To 1-(2-fluoro-4-chloro-5-methylsulfonyloxyphenyl)-
3,5-dimethylpyrazole (31.9 g) was added 95% sulfuric acid
(35 ml) and then added dropwise an acid mixture of 95%
sulfuric acid (7.5 ml) and 61% nitric acid ~10.3 g) under
33~
- 86 -
cooling with iced water, and the reaction mixture was
stirred at room temperature Eor further one hour, and was
then poured into iced water. The reaction mixture was
extracted with toluene and the resulting toluene layer
was washed with lN aqueous sodium hydroxide solution and
then with water and dried over anhydrous sodium sulfate.
Removal of the solvent from the extract in toluene by
distillation in vacuo gave the titled compound (33.1 g)
as a yellowish brown crystalline solid. Recrystallization
of the solid from a mixture of cyclohexane/acetone yielded
a pale yellow crystalline solid. m.p. 150.5-152.5C.
Example 19
Preparation of 1-(2-fluoro-4-chloro-5-isopropoxy-
phenyl)-3,5-dimethyl-4-aminopyrazole (Compound No. 69)
(Process F~
1-(2-fluoro-4-chloro-5-isopropoxyphenyl)-3,5-di-
methyl-4-nitropyrazole (32.8 gj and metallic iron (33.5 g)
wère suspended in water (140 ml), and the resultant sus-
pension was heated at 80C, to which 35% aqueous hydro-
chloric acid (3.2 g) was then added dropwise. Afterthe completion of addition, the reaction mixture was
stirred for further 30 minutes at 80C. Subsequent to
cooling, the reaction mi~ture was brought to pH 9 with
aqueous sodium hydroxide solution, followed by addition
of toluene to form two layers. The organic layer as
4~34
- 87 -
separated was washed with water and dried over anhydrous
sodium sulfate. Removal of the solvent by distilla~ion
in vacuo gave the titled compound (27.7 g) as a pale
yellow oil which soon crystallized on standing at room
temperature. Recrystallization from a mixture of n-
hexane/ethyl acetate yielded a white crystalline solid.
m.p. 94-96.5C.
Example 20
Preparation of 1-(2-fluoro-4-chloro-5-isopropoxy-
phenyl)-3,5-dimethyl-4-methylaminocarbonylaminopyrazole
(Compound No. 74) (Process G)
To a solution of 1-(2-fluoro-4-chloro-5-iso-
propoxyphenyl)-3,5-dimethyl-4-aminopyrazole (29.8 g)
in tetrahydrofuran (100 ml) was added one drop of triethyl-
amine and then added dropwise a solution of methyl iso-
cyanate (6.0 g) in tetrahydrofuran (50 ml ? . After the
completion of addition, the reaction mixture was left
to stand at room temperature for one hour. Removal of
the solvent from the reaction solution by distillation in
vacuo gave the titled compound (34.7 g) as a white
crystalline solid. Recrystallization of the solid from
a mixture of cyclohexane/acetone provided a white
crystalline solid. m.p. 184-185C.
8;~4L
- 88 -
Example 21
Preparation of l-(2-~luoro-4-chloro-5-
methylsulfonyloxyphenyl)-3,5-
dimethylpyrazole (Compound r~o. 112)
~Process A]
A mixture o~ 2-fluoro-4-chloro-5-methylsulfonyloxy-
phenylhydrazine (25.5 g), acetylacetone (10.0 g),
ethanol (lO0 ml) and water (lO0 ml) was refluxed for 2
hours. After cooling, toluene was added to the reaction
mixture to separate layers. The toluene layer as
separated was washed with water and then subjected to
distillation under a reduced pressure to remove the
toluene, yielding the titled compound (29.3 g) as pale
yellow crystals. Recrystallization from a mixture of
hexane/ethyl acetate gave colorless crystals with melting
point 77.5 - 79C.
Example 22
(a) Preparation of 2-chloro-4-fluorophenyl methanesulfonate
To a mixture of 2-chloro-4-fluorophenol (14.7 g)
and methylsulfonyl chloride (11.5 g), under ice-cooling,
pyridine (30 g) was added dropwise. The resulting
mixture was stirred at room temperature for 30 minutes to
complete the methylsulfonylation, after which the
pyridine was distilled off under xeduced pressure.
Toluene and water were added to the residue and the
~-~8~ -
mixtuxe was washed~i~l a dilute aqueous hydrochloric acid,
then with water. The toluene layer as separated was
dried over anhydrous sodium sulfate and then the toluene
was distilled off to afford the titled compound (20.8 g)
as a pale yellow oil. After purification by silica gel
chromatography, the oil was made colorless with a
refractive index nD3 = 1.5117.
(b) Preparation of 2-chloro-4-fluoro-5-nitrophenyl
methanesulfonate
A 95~ sulfuric acid (35 ml) was added to 2-chloro-
4-fluorophenyl methanesulfonate (22.5 g), to which was
then added dropwise a mixture of 95~ sulfuric acid (7.5 ml)
and 61% nitric acid (10.3 g) under ice-water cooling and
stirring to effect nitration reaction. After the completion
of the addition, the reaction mixture was stirred for
further 30 minutes at room temperature and then poured
onto ice pieces and extracted with toluene. The toluene
extract as separated was washed with lN aqueous sodium
hydroxide solution and then with water and dried over
anhydrous sodium sulfate. The toluene was distilled
off to afford the titled compound (25.9 g) as pale yellow
crystals with melting point 64 - 65~5C.
(c) Preparation of 2-chloro-4-fluoro-5-aminophenyl
methanesulfonate
A suspension o~ 2-chloro-4-fluoro-5-nitrophenyl
~7~&~
- 90 -
methanesulfonate (27.0 g) and iron (33.5 y) in water
(140 ml) was heated for reduction of the nitro group
into amino group. When the temperature of the reaction
mixture reached 80C, 35% hydrochloric acid (3.2 g) was
added thereto. Upon cooling to room temperature, the pH
of the reaction mixture was adjusted to 9.0 with the
addition of an aqueous sodium hydroxide solution. The
reactionmixture was then extracted with toluene and
tetrahydrofuranO The organic layer so separated was
washed with water, dried over anhydrous sodium sulfate
and then distilled under reduced pressure to remove the
solvent, affording the titled compound (22.3 g) as a
pale yellow oil which was then purified by silica gel
chromatography to give colorless crystals with melting
point 79 - 80C.
(d) Preparation of 2-chloro-4-fluoro-5-hydrazino-
phenyl methanesulfonate
A mixture of 2-chloro-4-fluoro-5-aminophenyl
methanesulfonate (24.0 g)~ 35% hydrochloric acid (63 g)
and water (4~ ml) was stirred at 60C for 30 ~.inutes.
Then, a solution of sodium nitrite (9.1 g) in water
(55 ml) was added dropwise to the resulting mixture at
-20C to effect diazotization reaction. The resulting
mixture was added under stirring to a pre~ormed and
cooled (-20C) solution of 35% hydrochloric acid (52 ml)
~.~74~
-- 91 --
and stannous chloride hydrate t58 g) in water (100 ml)
and the stirring was continued for 30 minutes under cooling.
Then, the reaction solution was brought to room tempera~
ture and the pH of the solution was adjusted to 8.0
with an a~ueous sodium hydroxide solution. Then, the
reaction solution was extracted with toluene and tetra~
hydrofuran and the extract was washed with water, dried
over anhydrous sodium sulfate and subjected to distilla-
tion under a reduced pressure to rernove the solvent
used, affording the titled compound (21.9 g) of the
formula:
CH3SO2-O
Cl~ NHNH2
as yellow crystals. Recrystalli~ation from cyclohexane
gave pale yellow crystals having melting point 75 -
77C.
Examples 23 to 30 illustrate some e~odiments forformulating compounds of the general formula (I) as the
herbicidal compositions. Compounds Nos. correspond to
those given in Table 1 above. Of course, this invention
is not limited to these Examples but may include various
~x~
- 92 -
!
modifications. Thus, the herbicidal compositions
according to this invention may contain various other
additives in any appropriate proportions and may com-
prise one ormore further herbicidal compounds in any
5 appropriate proportions. In the following Examples, all
parts are by weight.
Example 23
Preparation of granules
Compound No. 20 ( 1 part), lauryl sulfate (1 part)~
lQ calcium lignosulfonate (1 part), bentonite (30 parts~ and
clay (67 parts) were mixed together. Water (15 parts)
was added to the mixture and the whole mixture was
kneaded in a kneader, then granulated in a granulator
and dried in a fluidized drier to obtain granules
containing 1% of the active compound.
Example 24
Prèparation of granules
According to the method same as that shown in
Example 23, a granule preparation containing 1% of acitve
ingradient was formulated from Compound No. 34 ( 1 part),
lauryl sulfate ( 1 part), calcium lignosulfonate ( 1
part), bentonite (30 parts), clay (67 parts) and water
(15 parts).
Example 25
Pre~aration of granules
7~
- 93 -
The procedure of Example 23 was rep~ated except
that Compound No. 47 was used in place of Compound No. 20,
whereby to formulate a granule preparation conta1ning 1%
of the active compound.
Example 26
Preparation of wettable powder
Compound No. 41 (15 parts), white carbon (15
parts), calcium lignosulfonate ( 3 parts), polyoxyethylene
nonylphenol ether (2 parts), diatomaceous earth ( 5
parts) and clay (60 parts) were ground and homogeneously
mixed together in a mill to obtain a wettable powder
containing 15% of the active compound.
Exam~le 27
The procedure of Example 26 was repeated except
that Compound No. 138 was used in place of Compound No.
41, whereby to form a wettable powder containing 15~ of
t~e active compound.
Example 28
Preparation of an emulsifiable concentrate
Compound No. 47 (20 parts), Sorpor 700 H (an
emulsifier produced and sold by Toho Chemical Industry
Company) (20 parts) and xylene (60 parts) were mixed
together to obtain an emulsifiable concentrate containiny
20~ of the active compound.
- 94 -
Ex mple 29
Preparation of an emulsifiable concentrate
The procedure of Example 28 was repeated except
that Compound No. 140 was used in place of Compound No .
47 to obtain an emulsifiable concentrate containing 20%
of the active compound.
Example 30
Preparation of a dusting powder
Compound No. 183 (0.5 parts), silicic acid
anhydride in the form of fine powder (0.5 parts), calcium
stearate (0.5 parts), clay (50 parts) and talc (48.5
parts) were ground and homogeneously mixed together to
obtain a dusting powder containing 0.5% of the active
compound.
The following Test Examples 1 - 4 were conducted
to demonstrate the herbicidal activity of the new
compounds according to this invention.
Test Example l
This test was to evaluate the herbicidal effects
of the compound under test on the weeds predominant in
the irrigated field of aquatic rice plant, as well as
the phytotoxicity of the compounds under test to aquatic
rice plants as transplanted.
- 95 -
Each of biscuit pots having a top surface area
of 1/5000 ares was packed with a farm soil (alluvial soil).
In the surface layer of the soil in the pots were sown
- uniformly 50 seeds each of barnyard grass, bulrush, narrow-
S leaf water-plantain, monochoria, false pimpernel and tooth
cup. One day after the sowing, the pots were irrigated
with water and the depth of the irrigating wa-ter on -the
soil surface in the pot was kept at 2 cm. Three days
after the sowing, stocks each comprising two aquatic rice
plants of 4-leaved stage were transplanted in each pot at
the rate of three stocks of the rice plants per pot.
One day after the transplantation of the rice plant, the
emulsifiable concentrate as formulated according to the
procedure of Example 28 above was diluted with water and
the aqueous emulsion preparations so made was dropwise
added to the irrigating water in the pot at the rate of
application of 10 ml of said aqueous emulsion preparation
(corresponding to the rate of 50 g of the active lngredient
per 10 ares) for the pre-emergence herbicidal treatment.
The test was conducted with two replicates. Thirty days
after the treatment, the herbicidal activity of each com-
pound under test as well as the phytotoxicity of the
compound to aquatic rice plant were assessed according
to the following gradings. The test results obtained
are shown in Table 2 below.
7~8~3~
- 96 -
Gradings for evaluation of the Rate (percentages) of
herbicidal effects ob~ained kill of weeds
.
100%
4 80% to less than 100%
3 60% to less than 80%
2 40% to less than 60%
1 20% to less than 40%
0 Less than 20%
Gradings for evaluation of the Observed degrees of
phytotoxicity to rice plant damage of rice plant
Complete kill
4 Great damage
3 Medium damage
2 Low damage
1 Very low damage
0 Substantially null
`` 1.;~74~ 4
- 97 ~
Table 2
Herbicidal Effect
Test Narrow- Phyto-
Com- Barn- leaf False toxicity
pound yard Bul- water Mono- pimper- Tooth to aquatic
No. ~rass rush plantain choria nel cut rl plant
1 5 4 S 5 5 5 0
2 5 4 5 5 5 5 0
3 4 4 5 4 5 S 0
4 4 4 4 4 5 5 0
4 5 5 5 5 0
6 5 5 5 5 5 5 0
7 4 4 5 5 5 5 0
8 4 5 5 5 5 5 0
9 4 ~ S 4 5 5 0
4 5 5 5 5 0
11 5 4 5 4 5 5 0
12 4 4 5 5 5 5
13 4 4 4 4 5 5 0
14 5 4 5 5 5 5 0
lS 5 5 5 4 5 5 0
16 5 5 5 4 5 5
17 5 5 5 5 5 5 0
18 4 4 5 4 5 5 0
19 5 5 5 5 5 S 0
- 20 5 5 5 5 5 5 0
~7~
- 98 -
21 5 5 5 5 5 5
22 4 4 5 4 5 5
23 5 4 5 5 5 5 0
24 4 4 5 5 5 5 0
4 5 5 5 5 0
26 4 4 4 5 5 5
27 5 5 5 5 5 5 0
28 4 4 5 4 5 4 0
29 4 4 5 5 4 5 0
4 5 5 5 5 0
31 4 4 4 4 5 5 0
32 4 4 4 4 5 4 0
33 5 5 5 5 5 5 0
34 5 5 5 5 5 5 0
0
36 4 4 4 4 5 5 0
37 5 4 5 5 5 5 0
38 5 5 5 5 5 5 0
39 5 5 5 5 5 5 0
0
41 5 5 5 5 5 5 0
42 5 4 5 5 5 5 0
43 5 5 5 5 5 5 0
44 5 4 5 5 5 5 0
0
7~334
46 5 5 5 5 5 5 0
47 5 5 5 5 5 5 0
48 5 5 5 5 5 5 0
49 5 4 5 5 5 5 0
4 4 5 5 5 5 0
51 4 4 4 5 5 5 0
52 5 5 5 5 5 5 0
53 4 4 4 4 5 5 0
54 5 4 4 5 5 5 0
4 4 5 5 0
56 5 4 5 5 5 5 0
57 5 5 5 5 5 5 0
58 4 4 4 5 4 5 0
59 4 4 4 5 4 5 0
4 5 4 5 5
61 5 4 4 4 5 5
62 4 4 4 5 5 5
63 5 4 4 4 5 4 0
64 5 4 5 5 5 5 0
0
66 5 4 5 5 5 5
67 5 4 4 5 5 5 0
68 4 4 4 5 4 5 0
69 4 4 4 4 4 5 0
0
~L~7~
-- 100 --
71 S 4 4 4 5 5 0
72 4 4 4 4 5 4 0
73 5 4 5 5 4 5 0
74 5 4 5 5 5 5 0
4 4 4 5 5 0
76 4 4 4 5 5 5 0
77 5 5 5 5 5 5 0
78 5 4 5 5 5 5 0
79 5 5 5 5 5 5 0
0
81 5 4 5 5 5 5
82 5 4 5 5 5 5
83 5 5 5 5 5 5 0
84 4 5 4 4 5 5 0
4 4 4 4 5 5 0
86 5 4 5 5 5 5
87 5 4 5 5 5 5 0
88 4 4 4 4 5 5
89 5 5 5 5 5 5 0
4 5 5 5 5 0
91 5 4 4 5 5 5 0
92 4 4 4 4 4 5 0
93 5 4 5 5 5 5 0
94 4 4 4 5 5 5 0
4 4 4 5 5 5 0
:` . j,
~4~33~
-- 101 --
96 5 4 5 5 5 5 0
97 4 4 5 5 5 4
98 4 4 5 5 4 5 0
99 4 5 4 5 5 5 0
100 4 4 4 5 5 5 o
101 5 4 5 5 5 5 o
102 5 4 5 5 5 5 o
103 4 5 4 4 5 5 o
104 5 4 5 5 5 5 o
105 5 5 4 5 5 5 o
106 5 5 4 5 5 5 o
107 4 4 5 4 5 5 o
108 5 4 5 5 5 5 o
109 4 4 4 5 5 5 o
110 5 4 5 5 5 5 o
111 4 4 4 5 5 5 o
112 4 4 5 5 5 5 o
113 4 5 4 5 5 5 o
114 4 4 4 5 5 5 o
115 5 4 5 5 5 5 0
116 4 4 4 4 5 5 o
117 4 5 4 4 5 5 0
118 4 4 5 4 5 5 0
119 4 4 5 4 4 5 0
,, ,,~
83~
- 102 -
120 4 5 5 5 5 5 0
121 4 4 4 4 5 5 0
122 5 5 4 5 5 5 0
123 4 5 4 5 5 5 0
124 5 5 5 5 5 5 0
125 5 4 5 5 5 5 0
126 5 5 5 5 5 5 0
127 5 4 5 5 5 5
128 5 5 5 5 5 5 0
129 5 5 5 5 5 5 0
130 5 4 5 5 5 5 0
131 4 5 5 5 5 5 0
132 5 5 5 5 5 5 0
133 5 4 5 5 5 5 0
134 4 5 5 5 5 5 0
135 4 5 5 5 5 5 0
136 5 5 5 5 5 5 0
137 5 4 5 5 5 5 0
138 5 5 5 5 5 5 o
139 4 5 5 5 5 5 0
140 5 5 5 5 5 5 0
141 5 5 5 5 5 5 o
142 4 4 5 5 5 5
143 4 5 5 5 5 5 0
144 4 4 4 4 5 5 0
~ 7 ~
- 103 -
145 4 5 4 5 5 5 0
146 4 4 4 5 4 5
147 5 5 5 5 5 5 0
148 5 5 5 5 5 5 o
149 4 4 5 4 5 4 0
150 4 4 4 5 4 5 o
151 5 4 5 5 5 5
152 5 5 5 5 5 5 o
153 4 4 5 5 5 5 0
154 5 4 5 5 5 5 0
155 4 4 5 5 5 5 0
156 5 4 5 5 5 5 o
157 4 4 5 5 5 5 0
158 4 5 5 5 5 5 o
159 4 5 5 5 5 5 0
160 5 4 4 5 5 5 o
161 5 4 4 5 5 5
162 5 4 5 5 5 5 0
163 5 5 5 5 5 5 0
164 4 5 4 4 5 5
165 4 4 5 4 5 5 0
166 5 4 5 5 5 5 o
167- 5 5 5 5 5 5
168 4 4 5 5 5 5
169 4 4 4 5 5 5 0
3~
- 104 -
170 4 5 4 5 5 5 o
171 5 4 5 5 5 5 o
172 4 4 5 5 5 5
173 4 4 5 5 5 5 0
174 5 4 5 5 5 5 0
175 5 4 5 5 5 5 0
176 5 5 5 5 5 5 o
177 4 5 5 5 5 5 0
178 5 5 5 5 5 5 o
179 5 5 5 5 5 5 0
180 4 4 4 4 5 5 o
181 4 5 5 5 5 5 o
182 5 5 5 5 5 5 o
183 5 5 5 5 5 5 0
184 5 5 5 5 5 5 0
185 5 5 5 5 5 5 o
186 4 4 5 5 5 5 o
187 4 5 5 5 5 5 0
188 4 4 4 5 5 5 o
189 4 5 5 5 5 5 o
190 4 4 4 5 5 5 o
191 4 4 4 4 5 5 o
192 4 4 5 4 5 5 o
193 4 4 5 5 5 5 0
194 4 4 5 5 5 5 0
- 105 -
195 4 5 5 5 5 5 0
196 4 4 4 5 5 5 o
197 5 5 5 5 5 5 0
198 4 4 5 5 5 5 o
199 4 5 5 5 5 5 0
200 4 4 4 4 4 5 o
201 4 5 4 5 5 5
202 4 4 4 5 5 5 0
203 5 5 5 5 5 5 o
204 4 5 4 5 5 5 o
205 5 5 5 5 5 5 0
206 4 4 5 4 4 4 0
207 4 5 5 5 5 5 o
208 4 4 5 5 5 5 0
209 4 5 4 5 5 5 0
_ _ _ __ _ _ _ . _
Cbmpara-
tive
~mpound
A 1 0 1 2 3 2 0
B 2 1 2 3 3 3
C O O 0 1 0 0 0
D 0 0 0 1 1 0 0
E 3 0 3 2 3 3
__ , . . . . _ _ _ _ _
8~
- 106 -
Notes:-
In Table 2 above,
Comparative compound A:
<~ N -- N
CH3 CH3
(a compound as disclosed in Japanese patent publicationNo. 19958/65).
Comparative compound B:
~} N-- N
CH3 CH3
Cl
(a compound as disclosed in Japanese patent publication
No. 19958/65).
Comparative compound C:
i <N02
<~ N-- N
CH3 CH3
33~
- 107 -
(a compound as disclosed in Japanese patent publication
No. 14833/67).
Comparative compound D:
NO ~ N N
CH3 CH3
(a compound as disclosed in Japanese patent publication
No. 14833/67).
Comparative compound E:
Cl ~ NO2
("Chlornitrophene, commercially available herbicide).
Test Example 2
Thi.s test was to evaluate the herbicidal effects
of the compounds under test on the weeds predominant in
the irrigated field of aquatic rice plant, as well as the
phytotoxicity of the compounds under test to the aquatic
rice plant when the compounds were applied at low rates
of application of 25, 12.5 or 6.25 g of the active in-
- gredient per 10 ares.
334~
- 108 -
The procedure of Test Example 1 above was repeated,
except that the herbicidal treatment of the weeds was
conducted by applying the test compounds at 1ow rates.
The herbicidal effects on the weeds and the phytotoxicity
to aquatic rice plant were assessed in the same way as in
Test Example 1 above. The test results obtained are
summarized in Table 3 below.
7~3~
-- 109 --
~d. o o o o o o o o o o o o
s
) 8
~ u) un n 1~ un un un un un In In un In In un
R ~ ~ un In ~r un un u) u~ un un u) u) u) un un u~
~ u~
un u ~ un n r un un un un un ~ un un un
o ~ O u) un un un un
E ~1 un ~ o un ~ ~o un ~ ~D un ~ ~D un N u:~
~ 8 z ~ ,~ o ~
~.~748~3~
-- 110 --
ooo ooo ooo ooo ooo ooo ooo
u~ In Ll~ ~ In U~ In u~ ~ u~ u~ u~ l u
n ~ ~ ul u~ ~ U~ ~ In u~ m ~ u~
u~ U) u~ u~ ~ ~ Ln n ~ u~ L~ u~ Ln u~
.
r u~ n u~ U) ~ U~ L~ n u~
u~ u~ L~ u~ u~ r U) In ~
r In In n In Ln ~ ~n In U~ U~ ul In u~ In U~ ~r
L~ U~ ~ In ~ In
Ul ~ U) ~ Ir) ~ 1~1 N U1 t~l In ~1 1
1~1 N ~O Ul t~l ~D U~ D In ~1 ~ 11~ ~1 ~ U 1 ~ D Ul
~ ~1 ~I .-1 ~ 1 ~ 1 ~ J
I~ ~ ~ U~ C~ O~ O
~ fr~ 0 ~1 ~ ~r
~'7~8~l~
!
000 000 000 000 000 000 000
n n In U~ U~ Ln U~ In ~ ~ ~ L~ u~
In U~ ~ U~ r L~ In ~ Ln ul u~ In ~ ~ U~
Ln Ln u~ u~ In In ~ r In u~ n Ll~ LO ul Ln ~
In 11') ~r In It7 ~ Il') Il~ Ul IJ') U~ 1-') L~ Il'~ Il') ~r
n ~ r ~ ~ n ~ L~Ull
~ u~ ~ u~ u~ u~
n u~ ~ u~ Ln ~ u~
11~ N 111 N ~rl N 11'1 N ~1 N LO N U~ N
Ln ~1 ~D 11~ N lD 11~ t~l U >11~ 15~ ~1 ~ In ~1 ~ U~ N ~D
N r-l N ~1 N ~1 N ~1 N r~ t~ 1 N ~/
r-l t~ U7 ~O 1- CO (:~1
~r ~r ~r ~ ~ ~ u~
~'~7~334
- 112 --
I
o o o ~o o o ~o o o~o o ~,~o o o~o o ~o o o
u)u)u~ U~ u~ u~ u~In u~ u~
u~ In In U~ In ~ ~ In In In In U~ In L~ In u~
r) u~ u~ u~ In ~ er ~ In ~ U~ Ul U~ er
~ ~ In ~ ~P U~ n u~ l Ln ul u
r In U~ ~r In ~ ~ U~ ~ ~ U~ n u~ In In U~
n In Ih In U~ r ~ r In U~ Ul U~ U~ Ul ~
L~ ~ ~ U~ U~ U~ In
) N ~ l It) t~l 1~1 Ll'~ ~1 11~ t~l U~
U-) N ~D n ~1 ~ 1~') N ~0 n NU~ It~ N ~D InN ~) Itl ~1
N ~--1 N ~ N ~i t~l ~1 N ~1 ~ 1 ~
U~ ~D I~ t` r~ ~ oo
-
~;~7~
_ 113 --
I
ooo ooo ooo ooo ooo ooo ~
u~ ~n ~ u~ In Lr In In Ul Ul ~ U~ ~ ~ In ~ u~ In u) u~ u~
n Ul ~ n In In u~ In ~ u~ ul ~ u~ u~
u) u~ In u~ u) u~ ul u~ U) u~ Ln In U~ m u~ I
In In ~ In U~ n u~ In ~ ~ ~ ~n In In ~ U~ In ~ u~
In ~ UlIn m ~ In ~ Ln ~ u~ ~ n u~ ~ ~r In U~ In
u~ u~ ~ ~ In ~r u~ In ~ m n ~r ~ ~ In Ln
U~ U~ ~ U) U~ In U~
U~ N U') N Ir) N U) N Ul N 1~ N Il-) N
U) N ~0 U~ N ID 1~ N ~ 1~ N ~ In N ~ U`) N U~ 111 N U:)
N ~1 N r--l N ~1 N ~ N 1--l N ~ N r--l
a~ ~r ~D a:~ o~ t~1 u)
CO N N N N r~l ~
~1 ~1 ~ ~1 -1 r~l
7~34
-- 114 --
ooo ooo ooo ooo ooo ooo ooo
~ Ln u) u~ Lf~ Ln L~ Ln Ln Lr~ ~ In ~ LO u~
Ln In U~ U~ ~ ~r u~ n u~ LO U~ In L~ Lr) ~ u~
In In U~ U~ r u~ ul u~ Ln In Ln ~r u~ u~ ~ u~
U~ Ul In ~ ~ In U~ U~ In U~ ~ ~ r In U) ~r ~ In u~
Ul In ~ ~r Ln ~ In U~ ~r In ~r ~r u~ r u~
n In In ~ u~ r u~ rIn U~ U~
L~ U~ In Ln ~U~
Il') ~ 117 t~l 111 N Lt~ ~1 Lll ~1 In N In ~1
D It~ N ~O 10 t~l ~ Il') t~ ~D u'l ~ 0 Lf) t~l ~ LO t~
N ~ ~I ~1 N ~1 ~1 ~1 ~I .--1 N ~1
CO O ~1 1~ ~ 1~1 r~l
~ ~ ~ ~ ~ In ~O
~ ~ ~ ~ ~1 ~
~4~3~
-- 115 --
ooo ooo ooo ooo ooo ooo ooo
In n In U~ In Ul ~ ~ U~ Ln In ~r u~ Ln ~ u1 In ~ m
n In U~ U~ ~ ~ In n u~ In Ln U~ Ln In U~ U~
Ul Ln ~ In U~ ~ U~ ~ U~ ~ U~ ~ U~ In u~
r u~ n In U~ n u) ~ In ~r In In U~ ~ Lr ~ In L~
rLr n u~ u~ n In ~ U~ U~ In u~ u~
In U~ ~r u~ r u~ r ~ In U~ Lr) In I
U) In ~ n u~ u~ u~
10 N In ~ 1/~ ~1 11~ ~1 11 ) t~l 111 N U~
11~ ~ ~ Ul 1~1~D U ) N ~ Ir~ N ~ 1~ N ~ 11~ ~ ~ 1~ N ~0
~ ~1 ~ 1-1 ~ l ~ 1 ~ 1 ~ t~
.
I~ ~ co a~ ~ ~
I_ ~ ~J ~1
3~
- 116 -
ooo ooo ooo ooo ooo ooo ooo
~ In Lt~ ~ In ~~ O O ~ ~ O O O O
ul n u) u) It~ LO U~ ~ ~ ~-1 0 ~ ~O O O O O
U'l U~ ~) Lr) ~ U~U') LO U'l~ o o ~ o o o o
u) u~ 1~) Ln u~ In ~ Lf~ O O O ~1 ~1 0 O O O
r u~ ~ 1 u~ In InO O O~1 0 0 O O O
u~ r Ln~ Ir~u~u) InU~U~ OOO ~100 OOO
U~) 15~ Itl Ltl In LO U'')
111 N Ll~ ~1 Ln ~1 L~ l11~ t~ IJ~ ~1 u~) ~
1 ~ 1~ ~I W ~ Itl S~l ~DU~ ~1 ~ Itl ~I U) Irl 1~1 ~)
t~ ~ 1 t~l ~1 ~ ~1t~J ~J (~
In I~ ~ ~ ~ _
3 E
'7~
- 117 -
o o o o o o E
o o o ~ ~1 o 3
~1
O
o o o ~ ~ o O
~0 ~
E
o o o ,1 ,1 o ~0 Q
E~
~ O
O O O ol N O E ~1
t,4~
a~ a
Ooo ooo ~.
o o o ~ ~1 o Q
~ O
If ~ It)
L~l N Lo ~1
n ~ ~ ~ D
1 ~1
a~, ~ :~:
~ ~ _
8~
- 118 -
Test Example 3
This test was to evaluate the herbicidal activit~
of the compounds under test on the weeds predominant in
the non-irrigated farm field of various crop plants, as
well as the phytotoxicity of the compounds to crop plants.
The test of evaluating the herbicidal effects of
the test compounds on the weeds was conducted according
to the following procedure. Thus, each of biscuit pots
having a top surface area of 1/5000 ares was packed with
lQ a farm soil (alluvial soil). Seeds of crabgrass, common
lambsquarters, pigweed and smartweed were sown by mixing
50 seeds of each sort of weed uniformly with the soil
present in the 1 cm deep surface layer of the soil in
each pot and then lightly pressing on the top surface
of the soil in the pot. Two days after the sowing, the
top surface of the soil in the pots was herbicidally
treated by spraying thereon such an aqueous emulsion
preparation containing the compound under test which
had been prepared by diluting with water the emulsifiable
concentrate as formulated by the procedure of Example 28
above, at a rate of application oE 100 ~ per 10 ares of
the field (corresponding to the rate of application of
100 g of the active ingredient per 10 ares). The test
was conducted with two replicates. Thirty days after
the sowing, the herbicidal effects were assessed with
~7~34
-- 119
the same gradings and in the same way as described in
Tast Example 1 above~
The test of evaluating the phytotoxicity to crop
plants was performed according to the following procedure.
Thus, each of buiscuit pots having a top surface area of
1/10,000 ares was packed with a farm soil (alluvial soil).
Seeds of various crop plants, namely 5 seeds of soy bean,
10 seeds of sugar beet, 10 seeds of radish, 10 seeds of
wheat or 5 seeds of maize were sown on the soil surface
in the separate pots, followed by lightly pressing on the
top surface of the soil. One day after the sowing, the
soil surface was treated by spraying thereon an aqueous
emulsion preparation containing the test compound which
had been prepared by diluting with water the emulsifiable
concentrate as made in Example 28 above, at a rate of
application of 100 Q of the emulsion preparation per
10 ares of the field (corresponding to the rate of appli-
cation of 100 g of the active ingredient per 10 ares).
The test was conducted with two duplicates. Thirty days
~0 after the treatment with the test compounds, the phyto-
toxicity of the tested compounds to germinated crop plants
was assesssed in- the same way and with the same gradings
as set out in Test Example 1 above. The results obtained
are summarized in Table 4 below.
1~74B~
-- 120 --
.N¦ooooooooooooo
-~1 o o c~ o o o o o
~n
,
o
::~ ;~ooooooooooooo
~ t)
o ~ ~
. ~ o o o o o o o o o o o o o
æ~l o O O O O O O O O O O O
~,
I ~ In Ln
~ ~ ~ U~
~7~
-- 121 --
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
~ ~7~
-- 122 --
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
-
~7~ 34
-- 123 --
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
GO a~ o ~ ~ ~ ~ In ~ I~ o~ cn o ~ ~ ~ ~
~ 3~
-- 124 --
I
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
8~
-- 125 --
o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o
o C) o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o
~ ~7~33~
- 126 --
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
o ~1 ~ ~ ~r ~ ~ ~ co a~ o ~ ~ ~ ~r
O O O O O O O O O O r--l r~
:
-
~;~7~8;~4
- 127 -
ooooooooooooooooo
ooooooo~o~ooooooo
ooooooooooo~ooooo
ooooooooooooooooo
ooooooooooooooooo
r co ~ o ~ ~ ~ ~ n ~D r co a~ o ~ ~ ~
~ ~7~8;~
-- 128 --
I
oooo~oooooooooooo
oooo~oooooooooooo
oooo~oooooooooooo
ooooooooooooooooo
ooooooooooooooooo
~ ~ ~ r~ co o~ o ~ ~ ~ ~ u~ r oo a~ o
-- 129 --
ooooooooooooooooo
o o o o o o o o o o o o C~ o o o o
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
~74~
- 130 -
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
0 a~ o ~ ~ ~ ~r ~ ~ r~ co o~ o ,~
-
7483~
-- 131 --
I
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
ooooooooooooooooo
7~
-- 132 --
!
O O O O O O O O ~1 0 0 0 N
O O O O O O O O ~ ~J O O N
OOOOOOOO OOO0~1
OOOOOOOO OOOO~J
OOOOOOOO OOOO~
u~ In ~ o o o ~
~r Ln Ll'l In ul ~ ~ ~r ~Y~ ~) o o ~r
~c m t~ ~ ~
oooooooo ~
8' ~
-- 133 --
E
a
U~
a)
a
o
Q
~r rl
U
~ o ~ ~
Q ~ ~ S~
3 \ /
O ~ o ~O
::
O ~ ~
L, h
O ~
s:: ~ C E
E
O rl O O
Q~ ~ ~ U
E rl E
O o a
a
.,~ .
a
S~ O
r
~ ~ ~ rl
E E
o u~ o E~
.. ..
a~ ~
O O
Z Z
- 134 ~
Test Example 4
This test was to evaluate the herbicidal effects
of the compounds under test on -the weeds predominant in
the non-irrigated farm field of various crop plants, as
well as the phytotoxicity of the compounds to crop plant
when the compounds were applied at low rates of application
of 50, 25 or 12.5 g of the active compound per 10 ares.
The procedure of Test Example 3 above was repeated,
except that the herbicidal treatment of the weeds pre-
dominant in the farm field (non-irrigated) was effected
by applying the test compounds at low rates. The herbi-
cidal effects on the weeds and the phytotoxicity to crop
plants were assessed in -~he same way as in Test Example 3
and with the same gradings as set out in Test Example 1
above. The test results obtained are shown in Table 5
below.
- 13S -
-~ ¦
~Dl o o o O o o o o o o o o o o o
. ~ ~ o o o o o o o o o o o o o o o
.~1
1
c ~ ~ $ ~ ~ ~ ~ u~ u~ ~ In u~ ~r In u~ In In I
U '1 -~31 m U 'r "~ r ~ m ~ u~
,Q ~ C
E~ ~ ~ ~ ~ u~ u~ In U7 In u~ u~
r 3 ~
c ~ ~ r u~ ~ ~ u~ In In Ln Ul Ul
~ U~ U~ U~ In
O U U ~ 1~ ~ O U7 ~ O L~ 1 O U~ t~l O U7 ~1
0 ~ ~ U~ t~l ~1 U~ t~ ~1 U'~ ~J ~1 In N --1 Ll) N ~1
lX7~3~
- 136 -
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o s~ o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
In In Ul In U~ In ~ r u~ r ~ ~ u~
u~ r Lr~ r In U~ In u~ Ln I
u~ Ln Ln In U~ ~r u~ r In In ~ In U~ U~ ~
Ln n Ln u~ ~ ~ ~ r u~ In In U~ Ul In ~r
Lrl In u~ ~ n u~
o ~ I o ~ I o ~ I o u~ ~I o 1~ N O L~l N O
U~ ~ ~1 Il~ J It~ Ll') ~ 1 1~ I ~ Ll') ~ ~_
~O ~` CO ~1
ro ro ~ ~
-
~ ~7~8~
- 137 -
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
ooo ooo ooo ooo ooo ooo ooo
o o o o o o o o o o o o o o o o o o o o o
ooo ooo ooo ooo ooo ooo ooo
u~ u~ ~ In ~ In Ln In In In In ~r m In ~r u~ m
n u~ u~ Ul In In In In In u~ In ~ u~ Ln m In m u~ In ~
u~ ~ In In ul In u~ u~ Ln In In ~r In ~r ~r In u~ ul u~ In ~r
~n u~ Ln ~ ~ u~ n ~ m u~ u~ u~ m In
L~ u~In In ~ u~ u~
o In ~ o ~ ~ o In ~ o In ~ o u~ ~ o u~ ~ o u~
IS) ~ In ~ /1~ 1 11~ N ~ In ~ ~1 Il~
t`'l ~D U'l O
n ~ lo r~
~4~
-- 138 --
ooo oo o o oo o o o o o o o oo o o o
o o o o o o o o o o o o o o o o o o o o o
ooo ooo ooo ooo ooo ooo ooo
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o
:
r ~ ~ Lr)U~ u~ r ~r mu~ut
In ~r ~ n ~ In In In U~ In ~ ~r In U~ ~r
r u~ u~ In U~ In u~ ~ ~r In ~ ~r ~ r
u~ ~ ~ u~ ~ n u~ ~ ~ Ln In ~ U~
U~ U~ In U~ ~ Ln U~
O 1-~ ~ o In ~I O ul t~l O It~ N O U-) ~ O ~ ~ O IS~
~ ~1 Ir) ~ ~ 1~1 ~J r-l Ir~ N ~ Ir~ ~J ,J, Ir) N ~1 1
~r I_ a~ o ~ o~
r~ I_ r~ oo o~ ~o
~ X7~
-- 139 --
~ o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln ~r Ln Ln Ln
.
n Ln ~r Ln Ln ~ n Ln Ln Ln Ln Ln Ln Ln Ln Ln ~r ~r Ln Ln ~r
Ln n Ln Ln Ln Ln Ln Ln Ln Ln Ln Ln "~ Ln Ln Ln ~ ~r Ln Ln Ln
Ln Ln ~ Ln Ln ~ Ln Ln Ln Ln Ln ~r Ln Ln Ln Ln ~ ~r Ln Ln Ln
t Ln Ln Ln Ln Ln Ln Ln
o Ln ~ o Ln ~ o Ln ~ o Ln ~ o Ln ~ o Ln N o Ln ~
Ln ~ ~ Ln N ~ Ln ~ ~ In ~ ~ Ln ~ ~ Ln ~ ~1 Ln ~ ~1
~t OD O~ ~ ~0 a~ o
N ~I ~ ~1 ~If
,: ";.
~ ~7~F~3~
-- ].40 --
o o o o o o o o o o o o o o o o o o o o o
ooo ooo ooo ooo ooo ooo ooo
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
In U~U~ U~ r u~ u~ r ~ u~ r
InIn ~r u~ ~ ~ ~ r u~ r In U~r LnIn ~ Ln
r Ln ~r ~ u~ u~ r In U~ ~r Ln ~r~r u~
r u~ ~ u~ r Ln u~ ~r Ln~n ~r u~
U~ U~ ~ U) In ~ U~
O U~ ~ O In ~ O 11~ N O Ltl ~1 O 1S l ~ O u-) ~ O 1~
1~ 1 U') 1~ll~ l 1~ 1 U~ 1
~1 t~l ~ ~I ~ ~r u~
~r ~ u~ u~ t- r~
~ ~1 ~J ~ ~ ~ r-l
`
-- 141 --
ooo ooo ooo ooo ooo ooo ooo
o o o o o o o o o o o o o o o ~ o o ~ o o
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
o o o o o o o o o o o o o o o o o o o o o
InU~U~ ~ u~ n ~ ul~In ooo ooo
Ir) Ir) Ir) Ir'l 10 ~ Ir) Ir~ ~ U) U1 Ir1 Irl Lrl ~ N O O O O O
~ n U~ ~ ~ ~ U) ~ r) ~ N O O N ~1 0
1 10 In Ir~ U1 In ~) Ir~ 1~ 1~) In ~ N ~ O N ~1 0
L~ Il~ In It~ U~ U~ Lt')
O Ir) N O Ir) N O Ir) N O Ir1 N O ~1 N O Ul N 0 117 N
Irl N ~1 ~) N ~1 1~1 N ~1 In N_I In N ~ U) N ~ 1~) N ~1
.~
U~ ~ U~ ~D ~ :q
N N N ~ 8
. ' ', ~,, '' ' ':: ' '
3~
-- 142 --
o o o o o o ~1 o o
. a)~rl
o o o o o o ~ 1 o O
0u
O o o O O o o o o O :~
O ~ :~
~ a o
o o oo o oO o o E
0
o o o o o ~ o o E~ C E~
.,
o o oo o ot~l o o o R
~1 o.~
O O OO O O N --1 0 O ~I C
U~
~ C ~:
~ O O O O O ~ ~ O ~ ~
U
. a) ~ 0
OOO OOO ~O .
h ~ 0 ~
m
. E E -1
. o 111
It')11~ 1~ O U) li~
O 1~1 ~ O In N O n ~
n N ~1Itl t~l ~1 . .
~V
~ V zo
1~ U _