Note: Descriptions are shown in the official language in which they were submitted.
-- 1 --
AGENTS
In the field of chemical hybridizing agents
l-aryl-1,4-dihydro-~4-oxo-6-alkylpyridazine-3-carboxylic
acids are highly active pyridazine type gametocides.
Although t-hese compounds are highl.y effective as chemical
hybridizi~g agents they do possess the disadvantage of
producing plant injury-and poor seed quality when
overdo~ed sli~h~ly above their e~fective dosage rate.
The 5~carboxypyridazines of the present invention are
unexpectedly sa~er ohemical hybridizing agents since ~hey
do not have any adverse effects as :regards the quality of
seed or amount of plan~ injury at d~sage rates far above
wha~ is required to produ~e maximum male sterility which
allows for higher percent yields of hybrid :seed produced.
S~MMARY OF T~E IN~ENTION
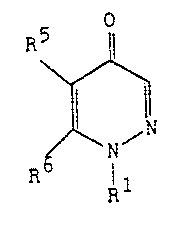
This invention relates to 1-aryl-1,4 dihydro-4-o~o~
5-carboxypyridazines of the formula
,1
~` :iL27~V~8
wherein Rl is an aryl or alkyl group;
R5 is a carboxy (COOH) group or the alkaline metal salt
thereof~ a carbalkoxy (COOR) group or a carboxamide (CONRR)
group wherein R ls an alkyl group and the agronomically
acceptable acld addition salts thereof, and
R6 is an alkyl or aryl group.
The pyrldazines of this type offer the advantage of causing
less in~ury to treated plants while inducing male sterility
thereby making them excellent plant growth regulators for use as
cereal hybridizing agents.
As utilized in the present specification and claims, the
term l'aryl" is meant to include phenyl or naphthyl groups or
phenyl or naphthyl groups substituted with up to three sub-
stituents selected from the group consisting of halogen, nitro,
trihalomethyl, (Cl-C4)alkoxy, (Cl-C~)alkyl, and cyano. The term
alkyl as utilized in the present specification and claims is
meant to include alkyl groups of up to 4 carbon atoms which may
be straight or branched chain alkyl groups.
A preferred embodiment of this invention relates to
compounds of ~ormula (I) wherein
Rl is an aryl group substituted with up to three sub-
stltuents selected from the group consistlng of halogen~ nitro,
trihalomethyl, (Cl-C4)alkoxy, (Cl-C4)alkyl, and cyano; and
R6 is a (Cl-C4)alkyl group.
A more preferred embodiment of this invention relates to
compounds of ~ormula I wherein
R5 is a carboxy (COOH) group or an alkali metal salt
thereof, or a carbalkoxy (COOR) group wherein the group
R is an alkyl group o~ up to 4 carbon atoms and the
agronomically acceptable acid additlon salts thereof.
A most preferred embodiment of this invention relates to
compounds according to Formula I wherein
Rl is a phenyl group substituted with up to two
substituents selected from the group consisting of halogen,
nitro, trifluoromethyl, methoxy, methyl, and cyano, and
R6 is a methyl group or ethyl group.
- ~7s(3~a
Typical compounds whi~h are encompassed by this
invention include:
l-phenyl-1,4-dihydro~4-oxo-6-methylpyriclazine-5--carboxylic
acid
1-phenyl-1,4-dihydro~4-oxo~6 ethylpyridazine-S-carboxylic
acid
l-phenyl-1,4-dihydro-4-oxo-6-propylpyriclazine ~-carboxylic
acid
1-phenyl-1,4-dihvdro-4-ox~-6-butylpyridazine-5-carboxylic -
acid
l-phenyl-1,4-dihydro-4-oxo-6-benzylpyridazine-5-carboxylic
acid
1 phenyl-1,4-dihydro 4-oxo-6-phenylpyridazine-5-carboxylic
acid
1-(4-chlorophenyl)-1,4-dihydro-4-oxo-6-methylpyridazine-5-
carboxylic acid
1-(4-bromophe~yl)-1,4-dihydr~-4-oxo-6-ethylpyridazine-5-
carboxylic acid
1-t3,4-dichlorophenyl)-1,4-dihydro-4-oxo-6-propylpyridazi~e
~5-carboxylic acid
1-(4-iodophenyl)-1 ~4r dihydro-4-oxo-6-butylpyrida%ine-5-
carboxylic acid
1-(4-fluorophenyl)-1,4-dihydro-4-oxo-6-benzylpyridazine-~-
carboxylic acid
1-(4-chlorophenyl)-1l4-dihydro 4-oxo-6-phenylpyridazine-5-
car~oxylic aci~
1-(3-chlorophenyl)-1,4-dihydro-4-oxo~6-methylpyridazi~e-5-
~arboxylic acid
1-(2-chlorophenyl)-1,4-dihydro~4-oxo-6-ethylpyridazine-5-
carboxylic acid
1-(3-bromophenyl)-1,4-dihydro 4-oxo-6-propylpyridazine-5-
carboxylic acid
1-(2-bromophenyl)-1,4-dihydro-4-o~o-6-butylpyridazine-5-
car~oxylic acid
" ~ ~7~098
1-(2,4,6-trichlorophenyl)-1,4-dihydro-4-oxo-6-benzyl-
pyridazine-5-carboxylic acid
1 (4-metbylphenyl)-1,4-dihydro-4-oxo-6-phenylpyridazine-5-
carboxylic acid
1-(4-trifluoro~ethylphenyl)-1,4-dihydro-4 ox~-6-methyl-
pyridazinew5-carboxylic aci~
1-(3 ethoxyphenyl)-1,4-dihydro-4-oxo-6-ethylpyridazine-5-
carboxylic acid
1-(4 methylthiophenyl)-1,4-dihydrG-4-oxo-6-propyl-
~yridazine-5-carboxylic acid
1-(3-cyanophenyl)-1,4-dihydro-4-oxo-6-butylpyridazine-5-
carboxylic acid
1-(2-chloro-4-methylphenyl)-1,4~dihydro-4-oxo-6-benzyl-
pyridazine-5-carboxylic acid
1-(2-tr;fluoromethyl-4-chlorophenyl)-1,4-dihydro-4-oxo--6-
methylpyridazine-5-carboxylic acid
1-(2-trifluoromethyl-4-bromophenyl)-1,4 dihydro-4-oxo-6-
ethylpyridazine-5 carboxylic acid
1-(2-chloro-5-trifluoromethylphenyl) 1,4-dihydro-4-ox~-6-
ethylpyridazine-5-carboxyli~ acid
1-(2-naphthyl)-1,4-dihydro-4-oxo-~ butylpyridazine-5-
carboxylic acid
and the agronomically acceptable alkali metal and acid
addition salts thereof.
The ollowing is a sequence utilized to prepare the
compounds of the present invention.
.
7S~9~3
--5--
t)2~3 ----~ C~2C~, __~
CO2C~ ,COCl C2C~I~ xyle:~e C~g `t~
.. ,,.. _,
III
~D~ ~ ~ >
TV CB,OE
.~
'
so4 a~
~ > C~,~
--CD2 ~5
. .
Vl:
;
The synthetic sequence outlined ab~ve is unique in
its ability to produce (V) and (VI). No directed syntheses of
either of these classes of c~mpounds has been reported in the
literature.
... . .
The compounds of Formula (V) are the subJect Or a copending
Canadian Patent Applicati.on 386~273 filed September 21, 1981 by
Dennis R. Patterson, which is assigned to a common assignee.
In the above reaction sequence a 3-oxoglutarate is :first
reacted with sodlum hydride thereby replaclng a hydrogen atom
~rom the active mekhylene group followed by reactlon with acetyl
chlorlde to .~orm the intermediate of Formula II which then
rearranges under acld conditions to form the pyrone of Formula
III. The pyrone ls then reacted wlth a d:Lazonlum salt to form a
hydrazone o~ Formula IV. The hydrazone is then rearranged ln
the presence o~ a base to form the dicarboxypyridazine of
Formula V which i.s then decarboxylated under acidic conditions
to form the 5-carboxypyrldazine of Formula VI.
A more preferred synthetlc route to the compounds of the
present lnvention is outlined below.
o ~
3C~, ~3 CO,c~ 2CX3
~ 80Z
C~l
Cl CH,COCl C~,o ~ c~,o ~ o,a
> C~ W~ N~08 ~ ~
1~ o~,~,o
Cl C~, Cl
-VIII 42~ IX lOOZ
v~
i,,~
~27~
In this approach the 3-oxoglutarate i5 first reacted with a
diazonium salt to form the hydrazone of Formula VII which is then
reacted with isopropyl magnesium chloride followed by reaction
with acetyl chloride to form the dicarboxypyridazine ester of
Formula VIII which is then hydrolyzed to the corresponding acid
of Formula IX. Decarboxylation under strongly acidic conditions
as above will again form the monocarboxy pyridazine of Formula
VI.
The following examples are provided to illustrate the
processes for preparing the compounds of the present invention.
These examples are not to be considered in any way as being
limitations on the breadth and scope of the present invention.
Process A-
Synthesis of 6-methyl-5-carbomethoxy-4-hydroxy-2-pyrone
Via dimethyl-2-acet~1-3-oxoglutarate
A 3-liter, three necked, round bottomed flask was equipped
with addition funnel, paddle stirrer and thermometer. The Elask
was charged with 300 ml dry toluene and sodium hydride (50% as a
dispersion in mineral oil, 82.8 g, 1.72 moles). The addition
funnel was charged with dimethyl~3~oxoglutarate (dimethyl ester
of acetone-1,3-dicarboxylic acid, 300 g, 1.72 moles). The flask
was cooled to 5 in an ice-water bath. The diester was added
dropwise to the sodium hydride slurry, not allowing the reaction
temperature to exceed 10. Complete addition required 3 hrs.
The resulting mixture was stirred 30 min. at 5. Acetyl chloriae
(135 g, 1.72 moles) was then added dropwise through the addit;on
funnel, being careful to maintain the pot temperature at 5-10.
After complete addition, the resulting slurry was stirred a
further 30 min., then poured slowly into 500 ml water saturated
with ammonium chloride. The resulting mixture showed a pH of 6.
The layers were separated.
." 1 ~
.. ~.. i ~" ~
\ ~`~
The aqueous phase was extracted with m~thylene chloride
(3 x 100 ml). The combined organic layers were taken to
dryness in vscuo to leave a yellow oil. ~acuum
distillation of this oil (at O . 5 mm ~g) gave fractions
boiling ~rom 50-1~0. ~ major fraction, bp 85-110 (97g)
contained the desired ~c~tylated diester, along with some
s~arting material, as inferred by NM~.
The impure acetylated diester (97 g) obtained above,
was dissol~ed in 300 ml dry ~ylene, along with p-toluene-
sulfonic acid (100 mg). This mixture was refluxed into a
Dean-Stark trap for 12 hr. ~he resulting dark solution
was cooled in an ice-water ~ath. The desired pyrone
crystalli~es out as fine needles (28.1 9, 10% yield based
on dimethyl 3-oxoglutarate). An analytical sample was
crystallized ~rom ethyl acetate. ~R (CDC13): 5.6 ppm
(S, 1~); 4.1 (S, 3H1; 2.7 (S, 3~). IR (C 2C12):
~75h, 5.95, 6.90, 9.10. mp 104-106.
Elemental AnalYsis
Expe~ted: C: 52.:18; ~: 4..38
Pound: C: 52.30; ~: 4.44
Syntùesis of l-(p-chlorophenyl)-l~4-~ih~r~-4-ox~-3
carboxy-5-carbomethoxy ~6-methylpyridazlne
A 250 ml, three necked, round bottomed flask was
e~uipped with addition ~unnel, paddle stirrer, and
thermometer. The .flask was charged with ~0 ml ~ethanol,
sodium acetate (16.0 9, 0.198 mole), and pyrone (8.0
g, 0.043 mole). p-Chlorobenzenediazonium chloride ~as
prepared on the side by th~ dropwise addition of ssdium
nitrite (3.3 g, 0.047 m~le) in 10 ml water to a cooled
(5) slurry containing p-chloroaniline (5.6 g, 0.043
mole) in aqueous hydrochloric acid (16.5 ml 12N ~C1
~0.198 mole] plus 10 ml water). The diaz~nium chloride
solution was added dropwi~e to the solution containing
the pyr~ne. This addition wa~ carried out during 10 min.
~7~
with no noticeable exotherm. After complete addition, the
resulting orange slurry was stirred for 40 min. at room
temperature. Suction filtration gave an orange filter cake which
was washed repeatedly with water, then sucked dry during 2 hr.
The filter cake thus obtained was placed back into the three
necked flask used aboveO Methanol (200 ml) was added to glve a
slurry. Morpholine ~10.0 g., 00115 mole~ was added in one
portion, A mildly exothermic reaction occurred, and a dark,
homogeneous solution was obtained. After stirring 10 min~, the
solution was poured into 300 ml water. This was extracted with
methylene chloride (3 x 100 ml). The combined organic extracts
were extracted repeatedly with dilute aqueous sodium hydroxide
(pH 8). The combined aqueous basic layers were acidified with 6N
hydrochloric acid. With cooling and scratching, a solid
crystallized from solution. Suction filtration gave the product
a light brown powder (8.2 g, 60~ yield based on the pyrone NMR
(CDC13): 7.5 ppm (multiplet, 4H); 4.0 (S, 3~) 2.3 (S, 3H). IR
(CH2C12): 5.75 , 6.22, 6.90. mp 203-204 (dec.). An analytical
sample was crystallized from methanol.
Elemental Analysis
Expected: C: 52.10; H: 3.44; N: 8.63
Found: C: 52.06; H: 3.43; N: 8089
Synthesis of l-(p-Chlorophenyl)-1,4-dihydro-4-oxo-5-carboxy-6-
5 methylpyridazinel-(p-chlorophenyl)-1,4-dihydro-4-oxo-3-carboxy-5-
carbomethoxy-6-methylpyridazine (2.0 g, 6.2 mmoles) was dissolved
in 10 ml, concentrated sulfuric acid, in an atmosphere of dry
nitrogenO This was warmed rapidly to 200 and maintained there
for 40 min. The resulting dark solution was cooled, then poured
into cold water (50 ml). A brown precipitate formed immediately.
Suction filtration and thorough washing with water gave the
desired acid tl.0 g 63% yield), pure by NMR.
, .
.. - ~;f~75~
.
--1.0--
NRM (CDC13): 8..5 ppm (S, 1~); 7.~ tAB quarte~, 4~); 2.8
ppm (S, 3~). IR (Nujol): ~.81~ . mp 207-208 (dec.).
~ n analytical sample was obtained by ~rystallization
from-methanol.
E:~ l~s
Expected: C: 54.45; ~: 3.43; ~: 10.59
Found: C: 54.45; ~: 3OS2; N: 10.16
Pr~cess B:
_Ae r~ ~ b-dr~z~7e
A 10-liter widemouthed polyethylene container -was
fitted with a s~irrer paddle and addition funnel. This
container was charged with dimethyl-3-oxoglutarate (1 kg,
5.75 ~oles), methanol (1.5R.), and sodium acetate (1 kg,
12.. 19 moles). p-Chlorobenzenedi~zonium chloride (5.75
moles) was generated on ~he side in seven equal portions,
by combining p-chloroaniline (7 x 104 g, 5.75 moles),
hydrochloric acid (7 x 314 ml ~2Nr ~6 moles), .water (7
200 ml) and sodium nitrate (7 x 65..6 g in .100 ml ~2~
6.66 moles). The diazonium salt was added dropwi~e,
rapid-y to the reaction kettle. The p~ was monitored
periodically and maintained at 5 during the course o~ the
rea~tion by adding sodium acetate vla spatula. At the
end of the procedure, another 800 g of sodium acetate bad
been added. The resulting mixture was allowed t~ stand
ov~rnight, then suction filtered and the filter cake
w~shed thoroughly with water.. ~he brick-red filter cake
was air dried to give 1.4 kg of the desired p-oduct (I)
(80~ yield). NMR tCDC13): 7.5 ppm (S, 4~); 3.9 ppm (S,
6~); 3.7 ppm (S, 2~. This compound is known to the
literature. See: Bulow and ~opfner, Berichte, 34, 71
(1901); ibid, 44, 2835 (1911).
~2~b~ .
~ dry l-liter, four-necked roun~ bottomed flask wa~
~L27~ 8
fitted with stirrer, thermometer, nitrogen inlet, and
rubber septum. The flask was charged with dimethyl-2,3-
dioxyglutarate, 2~p-chlorophenyl hydraæone (50 g, 0.16 mole)
in dry tetrahydrofuran (170 ml). This solution was maintained
under an atmosphere of dry nitrogen while cooling to ~ C.
Isopropyl magnesium chloride (72 ml, 2.25 N in ethyl ether,
0.16 mole) was added dropwise vla syringe, maintaining the pot
temperature at 5-10C. After complete addition, the mixture was
stirred for lS min. in the cold, then acetyl ohloride (12 ml,
13.0 9, 0.16 mole) was added dropwise, rapidly, keeping the
temperature of the reaction mixture below 1~ C. The resulting
dark ~olu~ion was allowed to warm to room temperature during
2 hrs. Water (200 ml) was added. This mixture was stirred for
30- min., then extracted with ethyl ace~ate. The extracts wexe
dried over MgSO4, then filtered and reduced in volume ln
vacuo. The resulting dark oil was dissolved in -et~yl
ether, and cooled in an ice bath. The desired diester
~rystallized out to give 22.1 g yellow powder (42%
yield), ~p 153-54~C. NMR (CDC13): 7.6 ppm (multiplet,
4H); 4.0 ppm (S, 6~); 2.3 ppm (S, 3
I~ (C~2C12): 5.75~ , 6.12 , 9.15
Table I below sives the structure, melting poin~ and
elemental analysis for some of the more representative
compounds encompassed by the present invention which were
prepared by the processes discussed above.
TABLE I
~0~
R6 N ,N
~X
9.~750913
'
C ~ u u~ ~ ~ e c~ u7 ~ 1~ ~ r~ m ~ _~
_~Oj ~ ~ ~ 0 ~
~ ~ r- ~ ~ u ~ ,n c~ r~ ~ u~ u7 0
~r ~r ~ ~ r~ ~ e~ rl ~ ~ ~ ~r ~r ~r ~ r~
E ~ _1 c~ ~ ~.D t`l ~ o ~ ~ ~ ~ O u er 1`
I`C~ er~ C~ C~ 00~ ~1~ ~DO ~ 0~0
u7u U~U~ 0~ ~D~ Ul~ C~ ~D~O 1`1` ~-
3 _
~ r ~ _ ~, ~
J" ;_ ~
~ ¦ Z ~ Z l Z ~ Z ~ Z ~ ~ Z I ' a
xlu ~~U ~U~ I= ~U ~5~ U jl-
ZXj~ ~ r~ ~r In ~O r~ cl c~ j
3L275V~38
u~ c~ r ~ ~ ~ ~ ~o ~ ~o r~ ~D c~ ~ ~ r~
o o ~ t- o ~ ~ ~; ~ ~ u~ r ~ ~ o ~r
0 o o ~ ~r ~ ~ o o a~ a~ ~ a~ r r o o r~
~u~ ~ ~_ _~_1 c
~: c:~ ~.o a~ ~'J ~ ~ t~ ~ ~ Q ~ t~ c~ t~
_I Z t~ o ~ o~ ~ ~r In cn r-~ ~ ~ o a~ c~
.n ~r
~ CD CD ~ ~ r u~~ r~l ~ a~ u~ c~
E _ o r- ~ l u~ ~-- :r ~ t` ~D ~--I ~) ~ O a~
_1 ~ a~ r- u~ ~O ~r ~rco c) I` r- ~ ~ ~ .0
~u~ ~ ~P~ u~u~ u~u~ u~ ~ u7u~
O O
C ~.~ . _ O cl
O O O` $ O O _~ O 0~0 O u~
o ~ o r~ a~ a~ ' u~
~:r _l O O ~ _l c~ r~
wl ~ ~ c~ ~ ~ ., ~
el ~ ~ . ;
Z Z 117 .:J Qc~ jZ __
XIU (J C j~ Y ~ l U~ o~
~ i JJ
-Zl ~ o
Xlo ~ ~` ~ 1~' ~ ~c ~
~;~7509~3
- 13a -
The compounds of the invention are particularly useful as
chemical hybridization agents in cereal crops, such as wheat,
barley, corn, rice sorghum, millets, oats, rye, triticale, forage
crops and the like. When used as chemical hybridization agents,
the compounds effectively induce a high degree of selective male
sterility, without also inducing significant female sterility, in
the treated plants and without causing significant growth
inhibition of the treated plants. ~s used herein, the term male
sterility includes both actual male sterility, as evidenced by a
lack of ma]e flower parts or by sterile pollen, and functional
male sterility, in which the male flower parts are unable -to
cause pollination. The compounds of the invention also cause
other plant growth regulatory responses, such as, for example,
control of flowering, control of fruiting and inhibition of seed
formation in non-cereal species and other related growth
regulatory responses.
750~8
--14--
When used as plant growth regulators, the compounds of the
invention are applied in any amount which will be sufficient to
effect the desired plant response without causing any undesirable
or phytotoxic response. For example, when the compounds oP the
invention are used as chemical hybridization agents, they are
generally applied to the crops to be treated at a rate of about
1/32 to about 20 pounds per acre and preferably about 1/8 to
about 10 pounds per acre. The rate of application will vary
depending on the crop being treated, the compound being used Eor
treatment, and related factors.
To obtain hybrid seed, the following procedure is generally
employed. The two parents to be crossed are planted in alternate
strips. The female parent is treated with a compound of the
invention. The male-sterile female parent thus produced will be
pollinated by pollen Erom the other, male-fertile, male parent,
and the seed produced by the female parent will be hybrid seed
which can then be harvested by conventional means.
A preferred method of applying a compound of the invention
as a chemical hybridization agent is by foliar application. When
this method is employed, selective male sterility is most
effectively induced when the compound is applied bet-~een flower-
initation and meiosis. The compounds of the inventions may also
be applied as a seed treatment by soaking the seed in a liquid
formulation containing the active compound or by coating the seed
with the compound. In seed treatment applications, the compounds
of the invention will generally he applied at a rate oE about 1/4
to about 10 pounds per hundred weight of seed. The compounds of
the invention are also effective when applied to the soil or to
the water surEace in rice crops.
I i'
~2~
The compounds of the invention can be used as plant growth
regulators either individually or in mixtures. For example, they
can be used in combination with other plant ~rowth regulators;
such as auxins~ gibberellins, ethylene-releasing agents such as
ethephon, pyridones, cytokinins, maleic hydrazide, succinic acid
2,2-dimethylhydrazide, choline and its saltE;, (2-chloromethyl)
trimethyl-ammonium chloride, triiodobenzoic acid, tributyl-2,4
dichlorobenzylphosphonium chloride, polymeric ~-vinyl-2-
oxazolidinones, tri(dimethylaminoethyl) phosphate and its salts,and N-dimethylamino-1,2,3,6-tetrahydrophthalamic acid and its
salts, and the like, and under some conditions may be used
advantageously with other agricultural chemicals such as
herbicides, fungicides, insecticides, and plant bactericides.
A compound of the invention can be applied to the growth
medium or to plants to be treated either by itself or, as is
generally done, as a component in a growth regulant composition
or formulation which also comprises an agronomically acceptable
carrier. By "agronomically acceptable carrier" is meant any
substance which can be used to dissolve, disperse, or dif~use a
compound in the composition without impairing the effectiveness
of the compound and which by itself has no significant
detrimental effect on the soil~ equipment, crops, or agronomic
environment Mixtures of the compounds of the invention may also
be used in any of these formulations. The compositions of the
invention can be either solid or liquid formulations or
solutions. For example, the compounds can be formulated as
wettable powders, emulsifiable concentrates, dusts, granular
formulations, aerosols, or flowable emulsion concentrates. In
such formulations, the compounds are extended with a liquid or
solid carrier and, when desired suitable surfactants are
incorporated.
,
!
~275~98
It is usually desirable,-particularly in fGliar
applications, to include adjuvants, such 2S wetting agents,
spreading agents, dispersing agents, stickers, adhesives,
and the like, in accordance with agricultural prac~ices.
~xamples of adju~ants which are ~mmonly used in the art
can be found in the John W. ~cCutcheon, Inc. publication
~Detergents and Emulsifiers Annual.~ -
The compounds of the invention can be dissolved in any
appropriate solvent. ~xamples o~ .solvents which are useful
in the practioe of this invention include water, al~ohols,
ketones, aromatic hydrocarbons, halogenated hydrocar~ons,
dimethyl~ormamide, dioxane, dimethyl sulfoxide, an~ the
like.. Mixtures of these .solvents can also be used. The
concentration of the solution can vary from about 2% to
.15 about 98% by weight with a prefer.red range being about .20%
to about 75%.
~or the preparation o~ emulsifiable concentrates, the
compound can be dissolved :in organic sol~en~s,.such as
benzene, toluene, ~ylene, methyla~ed naphthalene, corn oil,
.20 pine oil, o-dichlorvbenzene, i~ophoronel cyclohexanone,
methyl oleate, and the like, or in mixtures of these-
solvents~ together with .an emulsifying agent or surfa~tant
which -p~rmits dispersion in water. Suitable emulsifiers
in~lude, for exEmple, the ethylene oxide derivatives of
alkylphenols or lc)ng-chain alcohDls, mercaptans, carboxylio
a~ids, and reactive amines and partially esterified
polyhydri~ alcohols. .Solvent-soluble sulfates or
sulfonates, such as the alkaline earth-salts or amine sal~s
of alkylbenzenesulfonates and ~he ~atty alcohol ~odium
sulfates, having surf aoe-~ctive properties can be used as
emulsifiers either alone or in conjunction with an ethylene
oxi~e reaction produ~t. Flowable emulsion concentr~tes are
formulated similarly to the emulsifiable concentrates and
in~lude, in addition to the above components, water and a
stabilizing agent such as a water-soluble cellulose
.. . .. . . . . .. . .
~ ~7509~
der ivative or a water-soluble salt of a polyacrylic acid.
The concentration of the active ingredient in emulsifiable
concentrates of usually about 10% to 60!~ by we ight and in
flowable emulsion concentrates, this can be as higb as
about 75%.
Wettable powders suitable for spraying, can be
prepared by admixing the compound -with i~ finely divided
solid, such ~s clays, inorganic silicates and carbonates,
and silicas and incorporating wetting agents, sticking
agents, a~d/or dispersing agents in such mixtures. The
conoentration of active ingr~dients in.such formulations is
usually in ~he range of abou~ 20% to 98~ by weight,
preferably about 40% to 75~. A dispersing a~ent may
generally constitute about 0.5% to about 3% by weigh. of
~he composition, and a wetting agent may generally
constitute from about 0.1% to about 5% by weight of the
composition.
Dusts can be prepared by mixing the compounds of the
invention with finely divided iner* solids which may be
organic or inorganic in nature. Materials used for this
purpose include,.for-example, botanical flours, silicas,
silic~tes, carbonates and clays. One convenient method of
preparing a dust is to dilut a wettable powder with a
finely divided carrier. Dust concentrates ~on~aining abou~
20~ to 80~ o~ the acti~e ingredient are commonly made and
are subsequently diluted ~o about 1~ to 10~ by weight use
ooncentrations.
Granular formulations can be prepared by impregnating
a solid such as granular ~uller's ear~h, vermiculite,
yround corn cobs r seed~ ls,-~ncl-ùaing br~n or oth~r grain
hulls, or similar ma~erial. A ~soluti:on of one or more of
the compounds in a volatile oxganic solv~n~ can be sprayed
or mixed with the granular -solid and the solvent then
removed by evaporation. ~he granular material can have any
suitable size, with a preferably size range of 16 to 60
~75~
-18-
mesh. The active compound will usually comprise about 2 to 15%
by weight of the granular formulation.
Salts of the compounds of the invention can be formulated
and applied as aqueous solutions. The salt will typically
comprise about 0.05 to about 50% by weight, preferably about 0.1
to about 10%, of the solutionO These compositions can also be
further diluted with water if desired prior to actual
application, In some applications, the activity of these
compositions can be enhanced by incorporating into the
composition an adjuvant such as glycerin, methylethylcellulose,
hydroxyethylcellulose, polyoxyethylenesorbitan monooleate,
polypropylene glycol, polyacrylic acid, polyethylene sodium
malate, polyethylene oxide, or the like~ The adjuvant will
generally comprise about 0.1 to about 5% by weight, preferably
about 0.5 to about 2%, of the composition. Such compositions can
also optionally include an agronomically-acceptable surfactant.
The compounds of the invention can be applied as sprays by
methods commonly employed, such as conventional hydraulic sprays,
aerial sprays, and dustso For low-volume applications a solution
of the compound is usually used. The dilution and volume of
application will usually depend upon such factors as the type of
equipment employed, the method of application, the area to be
treated and the type and stage of development of the crop being
treated.
The following examples will further illustrate the growth
regulatory activity of the compounds of the invention but are not
intended to limit the invention in any way.
EXAMPLE 18
Chemical Hybridization Activity
The following procedures are used to evaluate the activity
of the compounds of the invention for inducing male sterility in
cereals.
An awned variety (Fielder) and an awnless variety
- -
7S09~3
.
~19-
(May-64) o~ spring wheat are planted at the rate of 6 to 8
seed~ per 6 incb pot containiDg a sterile-medium of 3
parts soil and 1 part humus. The plants are grown under
~hort-day (9 hour) conditions for the first 4 weeks to
obtain good vegatative growth before flower initiation.
The plants are then mo~ed to long-day (16 hour) conditions
which are provided by high intensity lights in the green
hou~e. The plants are fertilized at 2, 4, and 8 weeks
zfter planting with a water soluble fertilizer (16-25-16)
at the rate of 1 tsp/gal of water, and are frequently
sprayed with isot~x for aphid cont~ol and dusted with
sulfur for powdery mildew contr~l.
Test ~ompounds are foliarly applied to the awned
female plan~ when ~hese plants reach the flag leaf
emergence stage (stage 8 on Feekes' scale). All compounds
are applied in a carri-er volume of SQ ~al~A cDntaining a
surfactant, uch as Triton~ X-100 surfactant at the rate
o. 2 oz~50 gal.
After spike emergence but before anthesis, 4 to 6
spikes per pot are bagged to prevent ou~crossing. At the
firs~ signs of flower opening, two spikes per ~ot are cross
pollinated, using the approach method, with the awnless
male parent. As soon as the seeds become plainly visible,
spike length is measured and seeds per spikelet counted in
both bagged and crossed spikes. Male sterility can then be
calculated as percent inhibition of seed set in bagged
spikes of treated plants, and female fertility in cros~ed
spikes can be calculated as percent of control seed set.
After maturity the seed on crossed spikes are planted for
determination of percent hybridization.
Percent sterility, percent fertility, and percent
height inhibition are calculated from the following
formulas:
a. % Sterility = (Sc ~ St/Sc~ x 100
S~ - seeds/spikelet in bagged spikes o~ control
plants.
~7S(~8
-2.0-;
S~ = seeds/spikelet in bagged spikes of treated
plan~s,
b. % Fertility = (Ft/FC) x 100
~t = seeds/spikelet in approach ~rossed spikes
of treated plants
Fc = seeds~spikelet in unbagged spikes of
control plants
c. % ~eight inhibition = (}~c ~ ~t/E
Ec = ~eight of control plants
Et = ~eight of treated plants
Table II summarizes typical results obtained in the
eval~ation of compounds of the invention. ~ da~h indicates
that no determina~ion of value was made.
TABL~ II
Yo~
R ~ ~ ~
~X
)9~
--21--
Ex. Rate (~Acre) ~ ~,~,
/2 74 2 0
91.0 o
2 g9.1
2 1/8 29 . 4 O
1/2 99 1 0
:~ 100 o
2 ~57 9 0
8 "100 0
2 3 1 0
8 5~.3 0
1~ 77 0 0
2 100 . 0 0
8 100 . O ~
6 1~8 82.0 0
a loo . o O
7 1~8 28.-0 0
2 100 . 0 0
8 100 . 0 0
8 1 4 O
4 5.0 0
R 3.0 0
9 1 72 0 0
4 100. 0 0
8 100 . 0 0
--22--
TABL13 II ( continued )
Rate (il!Acre)SterilitY 5; ~ (o-9)
1 10 . O o
2 3.0 0
4 8.0 o
8 8.0 o
11 1/~ 2.0 o
0 0
2 0 0
4 16.0 o
12 1/2 1. 0 o
0 0
2 0 0
4 57.0 0
13 1 99.0 o
2 100 . 0 o
4 100 . 0 o
8 100 O 0 o
14 1/4 0 . 8 o
.1/2 0 o
0 0
2 6.0 o
1~ 1/410 ~ 8 o
1/239 . 4 o
~0.8 o
2100 . 0
16 1/419 . 9 o
1/241 . 2 o
90.5 0
2 93.2 o
17 1/8 4~.2 o
1/2 7.0 o
2 27.1
8 14.0 o
-Z3-
The 5-carbc xypyr i daz i nones of the present invention
exhibit an improved margin of safety while maintaining high
levels of activ~ty .as cDmpared to the 3-carboxypyridazones.
Greenhouse data on the l-aryl 1,4-dihydro-4-oxo-6-
alkylpyridazine--5-carboxylic acids of the present invention as
compared to l(p-chlorophenyl)-1,4 dihydro-4-oxo-6-methyl
pyridazone-3-carboxylic acid, a kn~wn compound, are presented
in Table III.
TABLE III
. Na~
;750
--24--
TABLE III ~continu~d)
X R Dosage (t~/acre)Sterility Injury
H CH3CH2- 1/8 3.1~ 0
1/2 26 . 6% 0
2 46.9~ 0
8 55.3~ 0
3,4-diClCH3CH2- 1/8 5~7~ 0
1/2 57O9~ 0
2 99 . 1% 0
8 100 . 0% 0
4-ClCH3CH2- 1/8 77 . 0~ ~ 0
1/2 98.0% 0
2 100 . 0% 0
8 100 . 0% 0
4-BrCH3CH2- 1/8 20 . 0% 0
1/2 82 . 0% 0
2 99 . 0% 0
8 100.0% 0
H CH3 1/8
1/2 28 . 0% 0
2 100.0% 0
8 100 . 0~ 0
COa,Na
1/4 7~ . 0~ 0
~B3 ~ 1/2 96.0%
r O l 1 100.0~ 1
2 100 . 0% 5
~1
75~
-25-
The improved safety margin of the 5-carboxypyridazinones
relative to the known l-(p-chlorophenyl)-1,4~dihydro-4-oxo-6-
methylpyrldazine-3-carboxyllc acid also makes them effective
cereal breedlng tools when applied directly to the seed of the ~emale parent before planting.
lt ls to be understood that changes and variations of the
subject matter of this invention may be made without department
from the spirit of the invention as defined by the appended
claims.
..~j,~. .,