Note: Descriptions are shown in the official language in which they were submitted.
-- 1 --
SODIUM PHOSPHATE COMPOSITION AND PROCESS
.
This invention relates to phosphate compositions, in particular ones
comprising trisodium phosphate, and processes for making them.
Trisodium phosphate c~ystalliæes ~n anhydrous and many hydrated
forms including ~he hemi-, hexa-, octa- and dodeca- hydrate forms
(see J.R. Van Wazer "Phosphorus and its Conpounds" Yol.1 page 494).
Foremost among these commercially is the dodecahydrate (Na3 P04.
12H20) but this compound, as crystallized~ usually has an analysis
showing the presence of an excess of sodium hydroxide with an Na:P
atom ratio of 3.14 or 3025:1. Mixtures of trisodium phosphate with
sodium nitrite or nitrate etc, also crystallize to give mixed salt
crystals with small extra amounts of the nitrite or nitrate, etc
anion. (see Van Wazer Phosphorus and Its Compounds Vol 1, page 494
and 495). The dodecahydrate is usually obtained by crystallization
but there have been proposals and uses many years ago of spray
drying and spray cooling to give solid dodecahydrate ~see BIOS
Report 1557, Manufac~ure of Technical Phosphates in West Germany,
1946, pages 30-33) and disclosures of flaking and also spraying into
a cooling chamber (Phosphoric Acid Phosphates and Phosphatic
Fertilizers by W.H, Waggaman published by Reinhold 1952, Second
Edition page 236). However these processes were not con~inued later
and the commercial dodesahydrate has for very many years been the
crysta11ine material w~th the excess of alkali. But this product is
well known to cake on storage and not be a free flowing powder,
making its handling more difficult.
We have now discovered a trisodium phosphate composition which can
have reduced caking properties and improved flow properties compared
to the crystalline dodecahydrate.
The present invention provides a particulate solid hydrated
phospha~e composition co0prising ~risodium phosphate and sodium
hydroxide w~th the ratio of the number of atoms to sodium to the
number of atoms of phosphorus to the number of moles of hydroxyl
group (here~nafter called the rat~o of Na:P:OH) being 3.1-3.2:1:0.1-
.27~ )7
~2--
0.2, and the overall ratio of the number of phosphorus atoms to thenumber of water molecules (hereinafter called the P:H20 ratlo) of
1:8-11, the solid composition having an outer layer comprising
trisodium phosphate hexahydrate and a core containing trisodium
phosphate dodecahydrate and sodium hydroxide, which are believed to
be present as a solid solution.
The present invention also provides a process for preparing a
particulate solid hydrated phosphate composi~ion, wherein an aqueous
solution at 90-120C of trisodium phosphate and sodium hydroxide,
wherein the atomic ratio of Na:P:OH is 3.1-3.2:1:0.1-0.2 and a
P:H20 ratio of 1:10-14 is formed into droplets and rapidly chilled
substantially out of contact with one another by counter current
contact with air to produce particles of an intermediate body with a
solid outer layer of trisodium phosphate hexahydrate enclosing a
core containing aqueous medium comprising trisodium phosphate and
sodium hydroxide and the said particles of the inter~ediate body are
cooled further to give a so7id phosphate composition of P:H20 ratio
of 1:8-11 having an outer layer comprislng trisodium phosphate
hexahydrate and a core containing trisodium phosphate dodecahydrate
and sodium hydroxide. Preferably the par~icles of the intermediate
body are separated from said counter current air before the
subsequent cooling, though at ~he separation stage the core usually
contains solid trisodium phosphate and sodium hydroxide as well as
said aqueous medium.
In the hydrated solid composition of the invention, the molar ra~io
of P to hydroxide is preferably 1:O.12-0.18 e.g. about 1:0.15. In
the hydrated solid the overall ratio of P:H20 is 1:8-11, preferably
1:9-11 such as 1:9.5-11 bu~ espec~ally 1:10-11. However the P to
water ratio is not constant throughout the solid, because it is 1:6,
or sl~ghtly above, in the outer l~yer and 1:12, or slightly below,
~n the inner core; the thickness of core and outer layer are such as
to g~ve the desired overall P to water ratio. The corelouter layer
structure may be shown by X-ray crystallographic analysis of samples
taken across a diameter of the particles~ The solid composition of
S6~7
-3-
the invention is substantially completely water soluble at 20C.
While the solid compositions usually consist essentially o~ the two
trisodium phosphates and sodium hydroxide~ they may contain small
amounts, e.g. up to 1%, especially up to 0.5% (by welght based on
the weigh~ of Na3P04) of sodium carbonate, either present as an
intentional or accidental ingredient in ~he sodium hydroxide
solution used in the production of the aqueous solution being
solidified or bec~use of absorptîon by the droplets of carbon
dioxide from the air used in the solidification process.
The solid compositions of the invention are particles obtained by
ejecting droplets of the hot aqueous solutiqn from a sprayer into a
stream of gas moving countercurrent to the droplets which
subsequently continue to move in countercurrent to the air and
essentially out of contact with each other until the intermediate
body with the solid outer layer or shell of trisodium phospha~e
hexahydrate and core containing liquid is produced. After
separa~ion i~ desired from the countercurren~ air flow, the
particles of this ~ntermediate body are allowed to cool further in
or out of contact with each other until the body solidifies
completely and its temperature is less than 30QC. The gas in~tially
contacting the droplets as they emerge from the sprayer is at a
t~mperature less than that of the droplets, preferably at less than
60ÇC e.g. at 30-~0C and that finally contacting the partly
solidified intermediate body ~ith solid shell and 1iquid or 1iqu~d/
solid core is also at less than the temperature of that body, e.g.
at 0-30C such as at 10-20C. Advantageously the droplets during
sol~dification contact gas of progressively reduced temperature e.g.
as happens when the droplets fall under gravity in countercurrent to
an upward stream of ~as e.g. air, the air being cold e.g. 0-20C at
the bottom and being warmed progressively by the molten droplets
passlng through it. This solidiP~cation may be perPormed in a prill
tower in which the hot aqueous solution is pumped to the top oP the
tower and under sufficient pressure to atomize it, the solution is
ejected from one or more, e.g. 1-6 or 2-~, nozzles in a spray head
in the ~orm of droplets wh~ch fall down the tower agains~ an upward
.L2756~7
flow of alr. The nozzles may be of the sol~d cone or the hollow
cone type and are arranged to minimize overlap between the sprays of
droplets and to m~nimize impact of the droplets on the walls of the
tower and to maximize spraying of droplets down ~he tower. The
partly solidified body with the solid shell and liquid or
liquid/solid core may be collected from the bottom of the tower and
subsequently cnoled. The ejection of hot solution into the gas
usually causes evaporation of some of the water in the solution,
thereby increasing the P:water ratio fron 1:10-14 to 1:8-11.
In the process there is initial cooling of the droplets to cause
surface solidification of the hexahydrate while at the same time
there is evaporat~on of water fram the solution to reduce its water
to P ratio. The gas initially contac~ing the hot droplets
preferably has a Relative Humidity of 10-90%. If the hot solutiGn
already has a water to P content of 9.5-11:1, e.g. 10-11:1, then the
gas may be saturated with water vapour, but advantageously hot
phosphate solutions of water to P ratio of 12-13:1 are used and the
water to P ratio is redueed in the spraying and solidification
steps; ~n order to obtain products of the desired structure it is
then essential tha~ wa~er vapour is evaporated from the droplets
before the solidl~ication of the ou~er shell is complete, as happens
when the vapour pressure of the water in the gas contacting the hot
molten droplets is less than the vapour pressure of water over the
droplets at the temperature of the droplets. When the inlet gas has
a high relatlve humidity and a higher temperature, lt is capable of
effecting less cooling and less ~ncrease of the P:water ratio of the
particles than with colder inlet gas of lower relative humidity. The
flow of droplets and the P:H20 content of the input liquid may be
ad~usted during continuous operation to compensate for varlations,
if any, in temperature and humidlty of the air and to produce a
solid product, with the higher the air inlet temperature at constant
air flow rate the lower the liquid flow rate, and the higher the
humid~ty the higher the P:H20 ratio of the input liquid. In
addition the higher the flow rate of ~he air~ the higher can ~e the
flow rate of khe liquid being solidified; there may be used ratios
~ 275iÇi~7
of the volume of liquid per hour to the volume per hour of gas, e.g.
air of not more than 1:10, e.g. not more than 1:15 or 1:20 such as
1:10-50 or 1:15-30.
The in~enmediate body has a solid shell of hexahydrate and a core
which contains a liquid phase of trisodium phosphate dodecahydrate,
and sodium hydroxide. The core usually al so contalns solid
dodecahydrate, sodium hydroxide and/or sodium phosphate hexahydrate
so the core may have partly, e.g 50-90~, solidified but not
completely solidifled. The intermediate body is usually at 30~70C,
e.g. 40-70C or 30-50C, these temperatures being the measured
average for ~he body because ~ has a relatively cold solid shell
and a relatiYely hot core containiny the crystallizing liquid phase
and usually solid phase as described above. The intermediate body
has sufficient strength that the particles do not coalesce on
cuntact wth one another.
The production of the intermediate body is usually performed in a
prill tower of height at least sufficient so that the inter~ediate
body has sufficient strength not to break when hitting the prill
tower bottom. The tower is usually 10-30M high, e.g. 12-20M. The
warm inter~ediate produ~t is then cooled further to below 35C,
e.g. to below 30C. The cool~ng may occur ~n the tower but
preferably is o~tside the tawer. Thus the intermediate body may be
cooled at the bottom of the tower by passin~ air countercurrent
through it. This cooling could occur while the particles of
lntermediate body fall under gravity out of contact w~th one another
~n a tower, e.g. with the droplets of hot liquid being sprayed down
a tower 50-70M long against a countercurrent stream of air to
produce at the bottom a completely solid composition of the
invention at below 30C. Alternati~ely a shorter tower could be
used so long as the droplets fall out of contact with one ano~her
until the intermedia~e boqy is produced and then ~he part~cles of
intenmediate boqy, a signi~icant proportion of which are ~n contact
with one another, e.g. ~n the $orm of spheroids with sem~ molten
centres, are cooled by passing air countercurrent through them,
before they lea~e the tower at the bottom a~ below 30C.
:~.2'~61~
..~
Complete cooling in the tower inevitably raises the temperature of
the air contacting the drop1ets at the top of the ~ower. Therefore
it is preferred to cool the intenmediate body outside the tower. The
intermediate body is advan~ageously separated from the
countercurrent air stream and is subsequently cooled outside the
tower~ Th~s la~er cooling can take place with at least a por~ion of
the particles in contact with one another, e~g. when air is passed
through a mass of particles o~ the intermediate body or is passed
through or over a moving optionally perforated conveyor or table on
wh i ch the intermediate body lies. However the i ntermediate body is
preferably significantly further cooled with the particles
substantlally out of contast wi~h one another. Thus the par~icles
of intermediate body may be passed from the tower~ e.g. by conveyor
or vibrating table, into a chamber where they are mixed with air,
e.g. tumbled with air and then separated in a cyclone. Most
preferably the particles of intermediate body are passed from the
tower into a second tower or conduit where they are contacted
cocurrent with air having a temperature of 0-30C e.g. by be~ng
passed into an upwardly moving air stream in which they move
cocurrently and are cooled to 15-30C, e.g. in an air lift.
If desired, before the second cooling outside the tower, any
oversize part~cles may be removed. The cooled product of the
Invention is then obtained of substantially uniform particle size
with few fine materla1s, in con~rast to the product obtained by
spray drying which gives particles with a breadth of sizes including
ma~y fines, e.g. at least S0~ of less than 0.25mm and at least 10
and often at least 2~ o~ less than 0.075mm. The process can
therefore glve a h~gher yield of product of substantially uniform
size, e.g. greater than 0.18~m, than sprqy drying.
The particles of product of the invention, which are usually
substantially spherical are preferably of 0.1-2mm, e.g. 0~25-1.5mm;
at least 95% may be of 0.18 to 1.5mm. The vast majority, e.g~ at
least 90~ by weight are usually of 0.25-lmm and a large major~ty,
e.g. at least 80% are o~ 0.35-0.7mm, e.g. about 0.5mm diameter. The
7tjÇj~7
--7--
particles of product usual7y contain less than 1% of less than
0.075mm. The particles of product of the invention are usually
obtained from hot droplets of substantially the same size sprayed
from nozzles which are 0.7~4.0mm e.g. 1.6-3.6 or about 2.5mm in
diameter.
The aqueous feed solution to the sprayer may conveniently be made by
reacting a concentrated aqueous solution containing orthophosphate
values of Na:P atom ratio less than the desired figure with the
requisite amount of concentrated sodium hydroxi~e solution to give
the solution of desired Na:P:OH ratio. The neutralization of the
sodium phosphate solution is exokhermic and the hot solution may
advantageously be used as such for the solidification process of the
1nvention, though extra heat may be provided if required in order to
keep the solution of that concentration liquid before the
solidification. Examples of solutions of orthophosphate values are
those with Na:P ratio nf 0-2.2:1, e.g~ phosphoric acid or monosodium
phosphate or mixures thereof, or disodium phosphate or mixtures
therefore with mono- or tri- sodium phosphate.
The solid phosphate c~nposit~ons of the invention have a reduced
tendency to cake, and they flow more freely than the crystalline
produc~s. They may be used in solid cleanser compositions e.g.
household cleansers.
Such cleaning composit~ons may comprise by weight 1-40%, e.g. 10-35%
of the solid composition of the invention, 40-99g, e.g. 60-~OX of
other alkaline compounds such as sodium carbonate or sesquicarbonate
sodium tripolyphosphate, or sodium sil~cate and optionally O.l-20%
of filler, e.g. sodium sulphate, and/or 0.1-2~ surfactants, e.g.
alkyl-benzene sulphonate such as dodecylbenzene sulphonate and/or 5
15X of a bleaching agent, e.g. a trichloro isoeyanurate. Abrasives
may be present or absent. These cleaning compositions may be made
by dry blending the various ingredients, e.g. by tumb1e blending.
~Z'7S~i~7
, ~
The compositions of the invention may also be used in industrial
applications, e.g. for the phosphating of steel or ~or the treatment
of boiler water.
In a modification of the composition and process of the invention at
least a portion of the sodium hydroxide content of the solid
composition and hence solution to be solidified is replaced by a
sodium salt of a monobasic inorganic acid, e.g. chloride, nitrite,
ni trate or hypochl ori te.
Thus the present invention also provides a particula~e solid
hydrated phosphate composition comprising trisodium phosphate and a
sodium c~mpound of fonmula NaX, wherein X is selected fron hydroxyl
and a monovalent inorganic anlon Z and mixtures thereof with the
a~omic ratio Na:P:X being 3.1-3.2:1:0.1-0.2, and the atomic ratio
P:Z:OH being 1: up to 0.2: up to 0.2, eOg. 1:0.01-0.2:0-0.19 and the
overall P:H20 ratio of 1:8-11, the solid composition having an
outer layer comprising trisodium phosphate hexahydrate and a core
containing trisodium phosphate dodecahydrate and the sodium compound
which is believed to be present as a solid solution.
The present invention also provides a process for preparing a
part~culate solid hydrated phosphate composition wherein an aqueous
solut~on at 90-120C of trisodium phosphate and a sodium compound
of formula NaX, where X is selected from hydroxyl and a monov21ent
inorganic anion Z and mixtures thereof with the atomic ratio of
Na:P:X be~ng 3.1-3.2:1:0.1-0.2 and ~he atomic ratio of P:Z:OH being
1: up to 0.2: up to 0.2, e.g. 1:0.01-0.2:0 0.19, and the overall
P:H20 ratio of 1:9-11 ~s ~ormed with droplets and droplets rapidly
chilled substantially out of contact with one another by
countercurrent contact with aîr to produce particles of an
intenmediate body w~th a solid outer layer of trisodlum phosphate
hexahydrate enclosfng a core oF aqueous med~um comprising trisodium
phosphate and sodium cGmpound and then said particles of
intermediate body are cooled further to give a solid phosphate
composition of P:H20 ratio oF 1:8-11 havin~ an outer layer
comprising trisodium phosphate hexahydrate and a core containing
~.~7S~)7
trisodium phosphate dodecahydrate and sodium compound. Apart fron
the change in the chemical composition of the modified solids and
the solution used to make them, the mod~,ied solids and the process
for making them are essentially the same as the solids of the
invention without the added NaZ compound, and the process for mak~ng
them.
The inorganic anion Z is monovalent from a monobasic inorganic acid
of formula HZ. The group Z may be a halide, e.g. fluoride or
chloride, nitrite, nitrate, permanganate, hypohalite, e.g.
hypochlorite or borate. Preferably the group Z is a nitrite or
chloride. The group Z may constitute the only group o~ formula X,
but there may also be present hydroxyl group, the proportion of
phosphorus ~o Z to hydroxyl being 1:0.01-0.2:0-0.19 especially
1:0.1-0.15:0-0.04, with the ra~io of P to X being prefera~ly 1:0.l-
0.18 especially 1:0.12-0.18, e.g. about 1:0.15. The modified solids
of the invention may also contain the small amounts of sod~um
carbonate as described for the solids free of ~he NaZ compound.
The aqueous solution which is converted to the modified solids of
the ~nvention~may conveniently be made by reactin~ a concentratPd
aqueous solut1On containing orthophosphate values as described above
with an Na:P ratio less than the desired ~igure with the requisite
amount of concentrated sodium hydrox~de solution in the presence of
the sodium compound NaZ to give the solut~on of desired Na:P:Z and
X:Z ratlo. If the desired product ~s to contain no excess of a1kali,
~.e. X is constituted by Z only, then enough alkali is added iust ~o
titra~e the last acidity of the disodium hydro~en phosphate, i.e. to
Na:P ratio 3:1. Examples of the solution containing orthophosphate
values are as described above.
The modified solid phosphate compositions have a reduced tendency to
cake, and hence flow more freely than the corresponding crystalline
products. Their de~ree o~ free alkalinity per uni~ volume of powder
is also reduced over the phosphate compositions without the NaZ
camponent, a benef~t in those solid cleanser compositions~ e~g.
~';75~;~7
-10-
particular household cleansers where an excess of alkali is
undesired. Furthermore the compositions comprlsing nitrite may
advantageously be used in water treabment to combine alkalinity and
nitrite content, while those with hypochlorite may be used in solid
bleaching compositions.
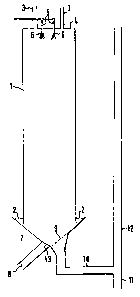
The process may be per~ormed in apparatus as illustrated in the
accompanying figure which is a schematic representation of a
prilling tower with associated pipework.
Tower 1 has inlets, 29 for air at its bottom and exits, 3, for wanm
alr in its top, 4. Also fitted in its top, 4, are two ~nlet pipes,
5, with four fixed spray nozzles, 6, through which hot liquid may be
ejected. The tower has an inverted conical bottom, 7, the inside
surfaces of which, toyether with the end of the vertical sides of
the tower, define the air inlets, 2. On one side of ~he conical
bottom is an exit, 8, for any large lumps with a movable door~ 13,
and on the other side of the conical bottom, 7, is a grating, 9,
e.g. of 30mm spacing for removal of particles of product. The
particles pass through grating, 9, down line 10 whiGh may contain
conveying means, e.~. a vibrat~ng table or screen, and are then
separated by gravity into any residual large bodies which fall down
line 11 and desired partlcles which move up line 12 carried by a
cocurrent stream of air, which enters line 11 and passes up line 12.
A suctlon f~n and cyclone (not shown) may be attached to the upper
end of line 12 ~nally to separate the particles of product from the
a~r.
In use the hot aqueous solution of sodium phosphate and sodium
hydroxide, optionally with e.g. sodium nitrite, is sprayed down the
tower 1 in countercurrent to cooler air passing up the tower from
~nlet 2 to exits 3~ The liquid is partly soli~ified to give
~ntermediate product with solid shell and partly solidified core and
the intermediate product is separated at the bottom of the tower by
the gratingJ 9, into large lumps wh~ch are retained and the rest of
the product wh~ch passes through the gratlng and is cooled and
J.i2~;~S~g~7
separated further as described above to give completely solidifed
part~culate product~
The lumps, which are agglomerations of particles and/or ma~erfal
from the tower walls, fall off the grating, 9, and periodically are
removed dcwn exlt line 8 by opening the door 13~ The air leaving
ex~ts 3 may contaln ul~ra fine dust particles of the solid, whose
phosphate values may be recovered by scrubbing (not shown).
The process Is illus~ra~ed in the ~ollowing Examples, in Example 1-6
of which the solidification apparatus was substantially as described
in and with reference to the accompanying Figure and was used in a
manner generally as described above.
488 parts of an 85% w/w aqueous phosphoric acid solution and 1135
parts of a 47% w/w aqueous sodium hydroxide solution were added
simultaneously with stirring to 248 par~s of water. The resultant
liquid of Na:P atom ratio 3.15:1 became hot (about 100C) and was
heated to 110C and its density adjusted by addition of wa~er to
1.53kg/m3 a~ 110C, corresponding ~o a P to H20 atom ratio of
1:12.8. The hot liquor was then solidified in the solidification
apparatus. The hot liquor was conveyed to the top of a tower, 1,
whfch was 4.~M diameter and 13.7M high (as shown in the accompanwing
Flgure) down which it was sprayed from four fixed atom~zation hollow
cone spr~y nozzles, 6, of 2.5mm orifice against a countercurnent
stream o~ a~r w~th a ra~io of the volume of aqueous liquor per hour
to volume of air per hour oP about 1:16.7. The temperature of the
a~r when the liqu~d from the nozzle first contacted it was 35~,
while the air entering the tower had a temperature of 18C and a
relative humidity in the range 40-90g. The process gave a semi-solid
product which substantially separated ~rom the coun~er current air
f10w which passed up the tower. The semi-solid product was sieved
by grating, 9, of 30m~ spacing to remove oversize pieces and the
particles of semi-solid product passing through the grating were
75 6l~7
-12-
passed alony line 10 by meAns of d vibrating table contained
therein. The particles, the intermediate body, had a solid outer
shell and a core of partly solidified aqueous medlum; the
temperature o~ the particles was measured as 33C. The particles
passed from the vibrating table into line 12, wherein they were
cooled further to 26C in cocurrent with an upward stream of air at
18C passing through line 11 through line 12. A ve~y small
percentage of larger product such as flakes fell down line 11. The
particles leaving line 12 at 26CO were completely solid and were
collected via a cyclone. The completely solid particles had an
analysis of 4S.6~ Na3P04 1.8X NaOH, 52.6% H20, P:H20 of 1:10.5
and Na:P of 3.15:1. The completely solid product was in the form of
substantially spherical particles with a solid shell of Na3P04
6H20 and a solid core comprising Na3P04 0.25NaOH 12H20. The
completely solid product particles had the following partic1e size
distribution: greater than 0.7mm 1.2%, 0.36-0.7mm 58.9~, 0.25-0.36mm
32.6%, 0.18-0.25mm 6.8~ and 0.07~-0.18mm 0.6~.
Example 2
In another experiment the solid particles were made as descr1bed in
Example 1.
The ease of flowing nf the solid particles was found by detennining
their angle of repose which was 20, compared to an angle of 32
for crystall~ne Na3P04 0.25NaOH 12H20. The solid particles also
were much less prone to caking compared to the crystalline Na3P04.
0.25NaOH 12H20.
~x~ples-3-6
In the same manner as described ~n Example 1 were made solid
particles having the following analyses on a weight basis:
~.2~5~
-13-
Example _ %Na3PO~ _ NaOH -- Na2~-3 P:H~O _ Na_P
3 46.5 1.7 0.1 1:10.3 3.157
4 47.5 1.7 0.2 1:9.7 3.161
44.8 1.9 0 1:10.8 3.174
6 47.4 1.6 0.2 1:9u8 3.1~2
:
In the process of Example 3 the input air had a temperature of
20.5C and a Relat~ve Humidity of 59X.
The particle size oP ~he product of Example 3 leaving line 12 was as
follows - greater than 0.7mm, 19.3%, 0.36-0.7mm ~6.~g, 0.25-0.36mm
18.0~, 0.18-0.25mm 5.1~, 0.075-0.18mm 0.6~.
The particle size oP the product of Example 6 after leaving line 12
and sievlng to remove particles larger than 0.75mm was as follows -
greater than 0.7mm 1,4%, 0.36-0.7mm 69.4%, 0.2S-0.36mm 22.6~ 0.18-
0.25mm 5.0~, 0.075-0.18mm 1.6~.
Example 7
Commercial trisodium phosphate dodecahydrate crystals were dissol~ed
hot in the m~nimum amount of water to gi~e an aqueous solution of
speclfic gravlty 1.535 at 100C. The aqueous solution was pumped at
100C through a prehea~ed hollow cone atomizing nozzle of lmm
orif~ce diameter situated at the top of a 13.7M ~ower, up which air
flowed~ The a~r en~ered the tower at ~ts bottom at ambient
temperature. The nozzle produced a spray of droplets wh~ch moved
downwards countercurent ~o the upward air and solidified to give
wanm solid particles with a partly molten core which separated from
the upward air, were collected Prom the bottcm of the tower and
allowed to cool to below 250a~ OYersized ma~erial was removed fron
a sample of the part~cles by sieving ~hrough a 1.2mm sieYe and a
sample oP the substantlally spherical particles passing through this
sieve were
s~
-14-
analyzed as containing 46.7X Na3P04 3.195:1 Na:P and P:N20 of 1:10.33. These particles were shown by Xray crystallography to have a
solid shell of Na3P04.6H20 and a solid core comprising
Na3P04Ø25NaOH 12H20. Xray crystallography on a sur~ace layer
of the solid, i.e~ its shell, showed i~ to contain Na3P04 6H20
and on a crushed sample of the solid showed It to contain both
Na3P04 6H20 and Na3P04 0.25NaOH 12H20. d lines for the
compounds in order o~ decreasing intensities were as follows:
Na3P04.6H20~ 4.283 2~.80, 2~62~ 3.29, 2.88, 2.55, 2.64, 2.70, and
for Na3P04 0.25 NaOH 1~H20, 4.34, 10.3, 2.61, 5.39, 3.32, 2.70,
2.87 and 2.86.
Exa ~ 8
678 parts of 46% w/w aqueous sodium hydroxide solution were added to
an aqueous solution of 470 parts of monosod~um phosphate and 54
parts sodium nitr~te ~n 320 parts water. The density of the hot
solution was adjusted to 1.54 kg/m3 at 105C. The hot solution
was converted to a solid product by atomization and solidification
in the manner descrlbed in Example 1. The product had an analysis
of 46.1~ Na3P04, 3.8% NaN02, 0.1X NaOI~, 0.1X Na2C03, 49.8~
H20; wi~h a P:H20 ratio of 1:9.8 and conta~ned a shell 1~yer of
Na3 P04 6H20 surroundin~ a core of Na3P04 0.25 NaN02 12H20
and NaOH~Na2C03.
A surface san~t~ng product had the ~ollow~ng compos~tion on a
we~ght basfs and was made by dry blending the ~ngredients ~n the
proportlons quoted:
Sol~d part~cles of Example 1 30X
Sod~um carbonate 40X
Pentasod~um tr~polyphosphate 20X
Tr~chloro~socyanurate sold under
the trade mark FICLOR CLEARON 10X
.~,
~75~i~7
-lS-
Example 10
A carpet cleaning formulation had the follcwing composition on a
weight bas~s and was made by dry blend~ng the ~ngredients ~n the
proportions quoted:
Solid part~cles of Example 1 25~
Pentasodium ~ripolyphosphate 25%
Tetrasodium pyrophosphate 15
Sod~um s~licate pentahydra~e
sold wnder the trade mark METSO 35%
A domest~c hard surface cleaner had the follaw~ng co~position on a
weight bas~s and was made by dry blending the fngredlents in the
proport~ons quoted:
Solld part~cles o~ Example 8 30X
Penta sodium tr~polyphospha~e 15
Sodium sesquicarbonate 54X
Surfactant dodecylbenzene
sulphonate lX