Note: Descriptions are shown in the official language in which they were submitted.
4~
The present invention concerns the domain
of paper-making, and relates more particularly
to a novel unEalsifiable safety paper comprising
an aromatic product, a product for rendering a
paper unfalsifiable and an aqueous or organic compo-
sition useful, in particular, for rendering a paper
unfalsifiable.
So-called "safety" papers, used in particu-
lar for manufacturing handwritten pieces for payment
and official do-uments, such as cheques, travellers'
cheques, etc..., must be protected against any
attempt at falsifying the writing or stamps borne
on the papers by means of any chemical reagent
or modern process, such as ink erazer pencil. Such
erazer pencils enable the coloured inks presently
used for handwriting or printing by inking pads
to be cleanly eliminated.
However, the majority of safety papers
presently available on the market react only insuffi-
ciently to the attempts at falsiEying with ink
erazer pencils. This often provokes the appearance
of a fluorescent yellow colour visible only with
difficulty by the naked eye and, moreover, possibly
detrimental for certain uses.
French Patent No. 2 365 656 describes
a safety paper comprising a chemical sensibilizing
composition based on an acid-base indicator, very
sensitive to pH variations. The indicator is selec-
ted, in particular, from the phthaleine or sulfo-
phthaleine group. If the pH rises (due to the action
of the erazer pencil), the paper develops a colou-
ring. However, all the products described are deli-
cate to employ in paper-making, mainly due to their
conditions of solubilization, the pH of use or
~2764~
reversibility or stability.
French Patent No. 2 399 505 and its Certifi-
cate of Addition No. 2 402 739 describe a safety
paper comprising a chemical sensibilizing composition
based on a salt of oxypyrene tricarboxylic acid,
called Pyranine. The action of an erazer pencil
on such papers develops a fluorescent yellow colou-
ring.
French Patents Nos. 2 406 027, 2 427
426 and its Certificate of Addition No. 2 432 576
describe a safety paper comprlsing dinitrophenols
which, under the action of an erazer pencil, become
non-fluorescent yellow in colour.
French Patent No. 2 410 702 describes
a safety paper comprising a chemical sensibilizing
composition composed of Pyranine associated with
an optical brightener and with various other com-
pounds. The action of an erazer felt pen on such
a paper leads to a fluorescent yellow colouring.
It is an object of the invention to propose
a compound which, associated with a paper, renders
the latter unfalsifiable, even by erazer pencils,
by instantaneously developing a colouring clearly
visible to the naked eye.
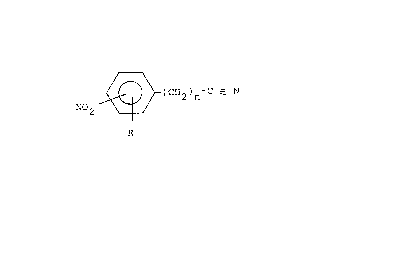
The paper according to the invention
is characterized in that it comprises, on its surface
and~or in its mass, at least one compound responding
to formula:
(I) o~ ~ ( 2 n
in which the substituents R and/or NO2 may be in
~2'7~L14
--3--
orthô, me-ta or para position with respect to (CH2)n
- CN;
- R corresponds to hydrogen, to an alkyl group
of Cl to C8, substituted or not, to a non-substituted,
N-substituted or N,N-substituted amine group,
to NO2, to a halogen;
- n is equal to 0 or 1.
The invention also concerns a process
for rendering a paper unfalsifiable, characterized
in that at least one compound of formula I is associa-
ted therewith.
The paper may be of any fibrous constitu-
tion, purely cellulosic or partly synthetic, to
which may be added the additives conventional in
paper-making, namely inorganic fillers, various
resistance agents, binding agents, resins, shading
dyes, neutral, acid or basic sizing products, alumina
sulfate for acid sizing or adjustment o~ the pH,
etc...
Numerous compounds may suit for the pur-
poses of the present invention.
Among these, preference will be given
to those responding to the following two sub-
structures:
NO2 \
(I) R - ~ C - N
in which the substituent R may be in ortho, meta
or para position with respect to CN, with R = H,
substituted or non-substituted alkyl, NH2, NHR',
NR'R" (R' and R" being various aryl groups, in
particular substituted by CN and/or NO2 groups
P~7~ 4
~4.
or alkyl groups), halogen, N02.
For example:
- metabenzonitrile : NO2
~ ~ CN
- amino-2 nitro-5 benzonitrile : NO2
~ N
- dinitro-3,5 benzonitrile : H2 NO2
~ N
- chloro-2 nitro-5 benzonitrile : 2~ No2
/ ~ N
_ N-N di (cyano-2 nitro-4 phenyl) amine :
N02
~C~
N-H
~ CN
N02
(2) ~ CH2-C - N
No2
in which N02 may be in ortho, meta or para position with respect to CH2CN.
For example:
_ or~honitrophenyl acetonitrile : ~ ,No2
~ H2-CN
- para nitrophenyl acetonitrile :
O N ~ CH2CN
~;~76a~.4
--5--
Furthermore, the paper will preferably
comprise at least 0.0001 g/m2 of the compound,
and, advantageously, between 0.01 and 0.15 g/m2.
The paper may also comprise sensibilizing
reagents, simi]ar to those already used at present
in safety papers, for example products ensuring
a modification of the appearance of the paper by
contact thereof with acids, oxydizing reagents.
Such products are introduced in known manner either
in aqueous solution, in which case care must be
taken that they are retained on the fibres by direct
bond or via fixing agents, or in the micro-dispersed
or pigmentary precipitate state.
For example, the iron(lII) chloride/
manganese ferrocyanide couple brings a reaction
to attempts at falsifying by acids, oxydizing agents
and erazer products with acid reaction (of the
"corector" type).
There is no problem of compatibility
between the sensibilizing agents and the products
of structure (I), provided that these sensibilizing
agents are neither basic nor reducing. In fact,
as the principal property, subject matter of the
Patent, of products (I) is that of developing a
colouring in the presence of bases or reducing
agents (principal components of ink erazer felt
pens), an association between product (I) and basic
or reducing product would lead to a coloured paper
inert with respect to attempted fa]sification by
a base, a reducing agent or an erazer felt pen.
These papers may also contain, dispersed
in their mass, in the pigmentary state, one or
more water-insoluble but organo-soluble dyes, so
as to preserve writing or inscriptions borne thereon
14
--6--
from attempts at falsification with the aid of
organic solvents. Moreover, such papers may be
water-marked or may contain various contrivances
for ensuring recognition thereof, such as fibres,
chips, coloured and/or fluorescent particles.
A first process for rendering a paper
unfalsifiable consists in incorporating the compound
of formula (I) during manufacture of the paper.
A second process consists in depositing
on one or both faces of a sheet of paper an aqueous
composition comprising a compound of formula (1),
as defined hereinabove, and a coating binder.
Among the coating binders, mention may
be made, by way of indication, of synthetic or
natural polymers with compatible hydroxy termina-
tions, such as starch, polyvinyl alcohol and cellulo-
sic derivatives.
It has been founa that it was particularly
advantageous to include a synthetic surface-active
agent in the composition, in order to improve solubi-
lity and reactivity.
Numerous surface-active agents are known
and it is not the purpose of the specification
to make an exhaustive list thereof.
Briefly, surface-active agents may be
classified in three categories:
- A : anionic detergents (excluding real soaps).
Mention may be made of alcoylsulfates, such as
laurylsulfate, alcoylbenzenesulfonates, sulfonated
olefins;
- B : neutral or non-ionic detergents among which
may be mentioned the detergents known on the market
under trademarks "PLURONIC" or "DISPONIL". These
compounds may be obtained by condensation of ethylene
~ ~76414
oxide with an alcohol having a hydrophobic radical,
for example the condensates of polyethylene oxide
and of alcoylphenols;
- C : cationic detergents. This group of detergents
may be defined as comprising derivatives of aliphatic
quarternary compounds of ammonium, phosphonium
and sulfonium in which the aliphatic radicals may
be with straight or branched chain and where one
of the aliphatic substituents contains about 8
10 to 18 carbon atoms.
A more complete description of such agents
will be found in numerous documents, for example
French Patents Nos. 2 014 675 and 2 062 828.
The category of the surface-active agent
selected will depend on the structure of the compound
corresponding to formula (I) used. Thus, for the
compounds of sub-structure (1), a surface-active
agent of category (B) will advantageously be selected
and, for the compounds of sub-structure (2), a
surface-active agent of group (C) and, from the
latter, the quaternary ammonium compounds.
Activators based on chlorine such as
Javel water may optionally be added to this composi-
tion.
The composition preferably comprises,
in grams for 1 litre of water:
- 0.5 to 50 g of compound of formula (I) alone
or in mixture,
- 10 to 150 g of coating binder (depending on the
binder used),
- 5 to 50 g of surface-active agent,
- 1 to 100 g of the other additives (activators,
etc...).
A third process consists in that a composi-
~Z71~4~
--8--
tion comprising a compound of formula (I), an organicsolvent and a compatible coating binder, is deposited
on one or on both faces of a sheet of paper.
Esters, ketones, alcohols, essences or
aromatics may be employed as solvent.
However, this process leads to a paper
which does not react to the action of the solvent
which has been precisely used in this process.
It should be specified that the term
10 composition designates solutions, i.e. compositions
in which the constituents are in the state of so-
lutes, but also partially or non-solubilized disper-
sions.
These compositions may be deposited with
15 the aid of a coating technique used in paper-making
(size-press, roller, blade systems, etc...).
The invention also relates to the composi-
tions as such.
Example 1
On a paper support containing in mass
reagents (iron(III) salt and precipitate of manganese
ferrocyanide) and one or more dispersed organosoluble
dyes, these products being intended to give the
paper sensitivity to the acids and solvents likely
25 to be used for falsifying the paper, there is deposi-
ted on the surface, by a conventional paper-making
technique (size-press, roller systems), the following
coating solution comprising, per litre of water:
30 - 1 g of paranitrophenylacetonitrile
O2N - ~ CH - CN
- 10 g of dodecylpyridinium chloride,
- 100 g of starch,
~76414
- 0.5 g of activator (Javel water).
The colouring obtained with the ink erazer
pencils and bases is violet--magenta.
With ageing, the colourings produced
with the bases tend towards green.
The paper thus treated further reacts
with Javel water, giving a beige-brown colouring,
with acids and acid-reaction erazer products (of
the "corector" type), producing blue; the solvents
10 dye the paper differently depending on their nature.
Example 2
On a paper support containing in mass
reagents (iron(III) salt and precipitate of manganese
ferrocyanide) and one or more dispersed organosoluble
15 dyes, these products being intended to give the
paper the sensitivity to the acids and solvents
likely to be used for falsifying the paper, there
is deposited on the surface, by a conventional
paper-making technique (size-press, roller systems),
the following coating solution comprising, per
litre of water:
- 10 g of orthonitrophenyl acetonitrile NO
~ 2
- 10 g of dodecyl pyridinium chloride,
- 20 g of polyvinylic alcohol Rhodoviol 30-5 (R)
(by RHONE-POULENC)
The instantaneous colouring obtained
with the ink erazer pencils, reducers and bases
is pink. The acids and acid-reaction erazer products
(of the "corector" type) lead to a blue colouring,
Javel water to a brown colouring and solvents to
variable colourings depending on the solvent and
the dye introduced into the mass.
~ 7~;4~4
-10-
Example 3:
On a paper containing simply a dye disposed
in the mass, it is pos~ible to obtain rose-magenta
colourings obtained with ink erazer felts, bases
and reducers, and variable colourings with solvents
~depending on the solvent and the dye used) with
a surfacing solution containing per litre:
- 4 g of 2-amino 5-nitro benzonitrile (SANDOZ product)
N2~--N
~ CN
- 24 g of Disponil SML 120 (product marketed by NH2
HENKEL)
- 20 g of polyvinyl alcohol Rhodoviol 30-5 (by
RHONE-POULENC).
The reaction to acids, to Javel water
and to the acid-reaction erazer products (of the
"corector" type) may be obtained by adding conventio-
nally known products in the coating solution o~
in the mass.
Example 4:
On a paper support, identical to that
of Example 1, there is deposited on the surface
the following coating solution containing:
- 3,5-dinitro benzonitrile at a concentration of
at least 0.5 g per litre, 2 ~
N ~ CN
2
- hexadecyl pyridinium chloride, for example at
12 g per litre,
- a coating binder which may be starch or polyvinyl
alcohols.
Reactions to attempted falsification provoke
a pink-red colouring with the reducers, bases and ink
erazers, blue with acids and the acid-reaction
erazer products (of the "corector" type), brown with
~76~
Javel water and variable as a function of the solvents
used.
Example 5:
On a paper suppo:rt presenting the products
already mentioned (iron(III) sal~, precipitate
of manganese ferrocyanide, organo-soluble dye)
adapted to be incorporated in the mass, there is
deposited on the surface the following coating
solution containing: NO2
1~ - 5 g of 2-chloro 5-nitro benzonitrile
~c~
- 24 g of disponil SML 120 (R) (HEN~EL product)
- 20 g of polyvinyl alcohol Rhodoviol 30-5 (R)
of RHONE-POULENC.
The paper thus treated colours violet-
magenta in the presence of bases, reducers or erazer
felts and, as before, blue with acids and the acid-
reaction erazer products (of the "corector" type),
brown with Javel water and a variable colouring
depending on the solvents (as a function of the
dye used).
Example 6:
When manufacturing a paper support, there
is introduced into the mass, in addition to the
reagents described hereinabove such as an iron(III)
salt, a precipitate of manganese ferrocyanide and
one or more dispersed organosoluble dyes, paranitro-
phenyl acetonitrile:
r--~
O2N - ~ 2
finely divided, in association with hexadecyl pyri-
dinium chloride. After formation of the sheet,
-12-
a reactivity is obtained which is identical to
that described in Example 1 (magenta colouring
with the erazer pencils, bases and reducers, blue
with acids and acid-reaction erazer products (of
the "corector" type), brown with javel water, va-
riable with the solvents).
Example 7:
On the surface oE a paper support contai-
ning in the mass an iron(III) salt and manganese
ferrocyanide, but no organo-soluble dye, there
is deposited, by a conventional coatin~ techni~ue
(engraved roller, roller systems, flexographic
printing, etc...) the following solution:
- 1 g of p-nitrophenyl acetonitrile
O2N ~ CH2CN
-10 g of dodecyl pyridinium chloride,
- 1000 g of ethyl acetate,
- 50 g of Ixan SGA (R) (polyvinylidene chloride
marketed by SOLVAY).
Reactions to attempted falsifications
provoke a pink colouring with the reducers, blue
with acids and acid-reaction erazer products (of
the "corector" type), violet with the bases, brown
with Javel water. Erazer pencils form violet-magenta
traces, stable in time.
Example 8:
On a paper support containing in the
mass reagents (iron(III) salt and precipitate of
manganese ferrocyanide) and one or more dispersed
organosoluble dyes, there is deposited on the sur-
face, by a conventional paper-making technique
(size-press, roller system, etc...), the following
preparation comprising per litre of water:
~7~ 4
- 0.05 g of N-N di (2-cyano ~-nitro phenyl~ amine
N02
CN
~ CN
N02
- 24 g of Disponil SML 102 (R) (HENKEL product)
- 20 g of polyvinyl alcohol Rhodoviol 30-5 (R)
(RHONE-POULENC product).
Reactions to attempted falsification
provoke a pink-magenta colouring with the reducers,
bases, erazer pencils, blue with acids and acid-
reaction erazer products (of the "corector" type),
brown with Javel water and variable as a function
of the solvents used (and of the dyes in the mass).
In all the foregoing Examples, the paper
is white and non-fluorescent after surfacing.
All these Examples have been given only
by way of illustration and the man skilled in the
art may modify them or complete them by known techni-
cal solutions or products without departing from
the scope of the invention.