Note: Descriptions are shown in the official language in which they were submitted.
~.~'7~73~1
CAPACITOR WITH DIELECTRIC OF PLZT
AND AN INTERGRANULAR BORATE
This invention relates to a ceramic capacitor
having a lead lanthanum zirconate titanate (PLZT) phase
and a small intergranular cadmium- and/or zinc-borate
phase, and more particularly to such a capacitor having
a low temperature coefficient of capacitance and having
gettered lead that escaped from the PLZT phase during
sintering.
Prior art use of a glass sintering aid in PLZT
dielectric ceramics is disclosed in each of my patents
US 4,027,209 issued May 31, 1977, US 4,135,224 issued
January 16, 1979, US 4,219,866 issued August 26, 1980,
and US 4,324,750 issued April 13, 1982.
In US 4,324,750 small amounts of lead are
volatilized and escape from the PLZT as lead oxide (PbO)
during calcining and sintering; precipitation of semi-
conducting PbO at the grain boundaries seriously degrades
capacitor performance.
The addition of glass sintering aids in
US 4,027,209 reduced this source of degradation, but
used alone the addition was far from an adequate solution
to that problem; as seen in US 4,324,750 example 6,~PLZT
with 1.0 wt% of a cadmium zinc borate silicate exhi~ited
an unacceptable life test result. A post-sinter anneal-
ing step in an open atmosphere at about 950C for about5 an hour drove out the unwanted free PbO, thereby
g~
:
'
~ ~7173~
2-
producing good life test results in examples 7 and 8 of
US 4,32~,750.
The annealing step is a solution to the free
PbO problem, but is accompanied by the disadvantage that
optimum anneal time and temperature must be determined for
each formulation, and is further a function of the surround-
ing PbO pressure at sintering and the quantity of ceramic
being sintered in each closed crucible. In short, the
prior art anneal step is difficult to control.
In a drawi.ng which illustrates embodiments of
the invention,
Figure l shows in side sectional view a mono-
lithic ceramic capacitor,
Figure 2 shows in side sectional view a ceramic
lS chip, and
Figures 3 through 6 each show a graph of the
number of capacitors failing under increasing voltage
stress versus the voltage at which failure occurred;
Figure 5 presents the capacitors of this invention.
A feature of this invention is the provision
of a high quality glass-PLZT dielectric ceramic formula-
tion that does not require a post-sintering anneal.
In accordance with this invention a glass
ceramic dielectric body has two spaced apart electrodes
in contact with the body. The body is an antiferro-
electric lead zirconate wherein from 0.07 to 0.16 molar
parts of the lead are replaced by lanthanum and wherein
from 0.10 to 0.40 molar parts of the zirconate are
replaced by titanate, and a glass amounting to from 0.1
to 0.9 wt% of the body. The glass consists of borates
selected from cadmium borate, zinc borate, and combina-
tions thereof.
The lead borate had not been a part of the
start glass used for making the glass-ceramic dielectric
material, but was acquired during sintering. During
sintering a portion of the lead in the ceramic PLZT phase
is volati:Li~ed and, as is we~l-known, tends to precipitate
as semiconducting PbO at the grain boundaries leading to
low breakdown voltages and early failure at life test.
7 ~ 3
--3--
This invention recogniæes that essentially pure
cadmium borate or zinc borate or both, uniquely act as
efficient getters of the unwanted free PbO during sinter-
ing. Zinc and cadmium glass compositions also serve as
effective fluxes in a PLZT composition and they have a
very small effect on the electrical properties o~ the
PLZT.
In a PLZTt a cation from the glass that enters
the grain on a large cation site will displace lead and
exacerbate the free PbO problem, whereas cadmium and zinc
do not appear to do so. The alkali earth metals are such
lead-displacing ions. On the other hand, when the glass
phase includes the glass-former element silicon, lanthanum
is found to preferentially associate with the silicon in
the glass creating a lanthanum silicate phase and thus
changing the stoichiometry of the PLZT matrix. There-
fore, a glass flux containing silicon is to be avoided.
Advantageously the borates of cadmium and zinc
each have a low melting temperature, relative to the
silicates, which glass-melting temperature becomes even
lower as the gettering of lead proceeds during sintering.
Thus sintering the PLZT and borate flux of cadmium and/or
zinc at about the conventional temperature of 1100C
yields a PLZT body having densities near 97% of the
theoretical maximum while essentially all of the free PbO
is incorporated into the glass phase and the need for a
post-sinter annealing step is eliminated.
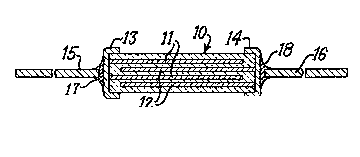
The monolithic ceramic capacitor of Figure 1
has a ceramic body 10 with film electrodes 11 interleaved
with film electrodes 12 within body 10. Conductive ter-
mination coatings 13 and 14 contact electrodes 11 and 12,
respectively. Lead-wires 15 and 16 are attached by
solder bonds 17 and 18 to terminations 13 and 14, respec-
tively. Although the capacitor of Figure 1 has three
active ceramic dielectric layers between adjacent and
oppositely polarized electrodes, experimental ~onolithic
capacitors to be described herein have more active diele-
tric layers.
~.~7~73~78
--4--
The chip capacitor of Figure 2 has a ceramic
body 20 and two film electrodes 21 and 22 on the opposite
major surfaces of body 20, respectively Chip capacitors
may have a rectangular or circular shape, and for high
voltage uses may have a thickness equaling or exceeding
the largest dimension of a major surface.
A brief description of the steps employed for
making experimental capacitors of this invention is as
follows:
A powder blend was prepared consisting by weight
of 55.0 PbO, 5.70 La203, 3.40 ~aO, 0.68 Ag(metal), 1.~7
Bi2O3, 24.7 ZrO2 and 9.0 TiO2. The blend was ball milled
and preclacined at 790C for 5 hours. The resulting cake
was then granulated mechanically and calcined in a closed
high purity aluminum sagger at 1090C for 3 hours. The
calcined cake was crushed and jet pulverized to form a
fine ceramic powder.
The above-noted start materials correspond to
a compound
(pbo~78Lao~llBao~o7Bio~o2Ago.o2)(zrO 6~Tio 36)3
which co~pound is forrned with lead vacancies at calcining
and is structurally that of the grains in the subsequently
sintered dielectric.
This material will sinter well without a low-
melting sintering aid because PbO escapes the PLZT at
sintering; PbO itself is molten above 890C and will
promote liquid-phase sintering. Thus the addition of a
sintering aid or flux to a PLZT ceramic heretofore pro-
vides a source of cations that may be incorporated in
the ceramic grains to effect desired changes in the
temperature coefficient, and/or to moderate the detri-
mental presence of PbO at the grain boundaries after
sintering. However, no additives have been known that
essentially eliminate the residual free lead at the grain
boundaries after sintering.
_5_
At this early point in the process 0.5 wt% of
a glass powder is added to the calcined and pulverized
PLZT start powder. The different glass fluxes used in
the following experimental examples are:
Table I
Glass Flux Compound(s)
GF-l 25(PbO), 24(Bi203), 36(CdO), (Al203),
4(ZnO), 5(B203), 5(SiO3)*
GF-2 5 CdO 2 SiO2
GF-3 BaO B203
GF-~ 3ZnO B203
GF-5 3CdO B O
GF-6 68(3ZnO B203) 32(3CdO-B203)*
GF-7 CdO 2ZnO-B203
GF-8 43ZnO 21CdO 21B203 l5Al2o3
* by wtV/o
In a series of experiments the relative merits
of four glass compositions in a PLZT glass dielectric were
explored. These glasses are identified in Table I as GF-l,
GF-2, GF-7, and GF-3. To make these glasses, the powder
oxides of glass cations and alumina milling balls were
mixed in acetone and milled in a polyethylene bottle or
two hours. After drying, the balls were removed and the
powder mixture was calcined at 550C to form the solid
solution indicated in Table I for each case.
Four groups of experimental monolithic ceramic
capacitors identified respectively as Examples 1, 2, 3 and
4 have PLZT ceramic bodies with 0.5 wt% of the four glass
powders GF-l, GF-2, GF-7, and GF-3, respectively.
The PLZT and glass powder mixture was stirred
in an organic binder medium of essentially turpentine~ 6%
pine oil and 5% lecithin to produce a dispersion or slurry
containing about 70% by weight o solids. This slurry
was balled milled for about 10 hours.
.~ 7
-6
Groups of experimental monolithic capacitors
were produced by applying successive coatings of the
above-noted milled slurry to a substrate, drying each
layer in turn, and screen printing an electroding paste
S of 70% silver and 30V/o palladium particles onto each
(except the last) of the dried layers of the dielectric
material. Each dielectric layer is about 1 mil (0.025
mm) thick.
This assembly of dried layers with seven inter-
leaved films of electroding paste was then diced into amultiplicity of square bodies and baked at 870C to
remove the organic material. The electrodes were so
arranged that, after dicing, each body had alternate
electrodes extending to one cut end of the body, with
the other electrodes extending to the opposite cut end
of the body, as illustrated in Figure 1. The body was
subsequently sintered in a closed alumina crucible at
a peak temperature of 1100C for 2~ hours. Closed con-
tainer sintering is preferred wit'n the container substan-
tially filled with the bodies to be sintered, because
this results in maintaining a positive atmosphere of
` lead oxide vapor leading to the retention during sinter-
ing of liquid lead oxide that acts as a sintering aid so
that densification is achieved at a low sinte~ing tempera-
ture. An open atmosphere sintering may result in a
poorly sintered porous body and uncontrollable loss of
lead from the PLZT body. In these experimental capacitors
the active dielectric layer between adjacent buried elec-
trodes is 1 mil (25 microns) thick.
Free lead at the grain boundaries was then
removed by annealing the sintered bodies for 2~ hours at
950C in air. A silver paste was applied to the opposi~e
ends of each sintered body and the body was heated to
about 760C for 5 minutes to form cured terminals, e.g.
termina~s 13 and 14 in Figure 1.
,
.~n~
--7--
The four groups of capacitors were subjected to
an accelerated life test at 160VDC and 150C. The number
of failures experienced at 250 hours is shown in Table II:
Table II
Example Glass # Failures/# Tested
1 GF-l 1/10
2 GF~2 9/9
3 GF-7 0/10
~ GE-3 3/8
These four procedures were repeated except for
omitting the annealing step in Examples 5, 6, 7 and 8.
A substantially worse failure rate was experienced except
for the capacitors for Example 7 containing the cadmium
zinc borate glass, in which case there were again zero
failures as indicated in Table III.
Other capacitors of Examples l through 8 were
not subjected to the above-mentioned life test, but were
sub~ected to an increasing voltage at room temperature
until each capacitor broke down. In Figures 3 through 6,
breakdown voltage data (circles) for annealed capacitors
in Examplex 1, 2, 3 and ~ and comparative breakdown
voltage data (crosses) for unannealed capacitors in
Examples 5, 6, 7 and 8 are plotted in the fields defined
by voltage breakdown versus number failed. In each graph,
data points are connected by a curve designated by the
corresponding Example number plus 30.
Only in Figure 5 curve 33 for annealed capaci-
tors and curve 37 for unannealed capacitors, all having
the cadmium zinc borate glass flux, are essentially
indistinguishable. This series of tests is even more
indicative of the superiority of the cadmium zinc borate
glass in Examples 3 and 7.
These data strongly suggest that in this system,
the barium glass of Examples 4 and 8 enters the PLZT crys-
tal laktice on the large sites displacing even more leadand exacerbating the already serious free lead problem.
It is also theorized that lanthanum is pre-ferantially
drawn to the silicon in the silica containing glasses of
~ ~7~7~
--8--
Examples 1 and 5 and Examples 2 and 6, robbing the PLZT
grains of lanthanum, and changing the PLZT lattice.
Table III
Glass Post Sinter Life Test Breakdown Voltage
Example FluxTreatment Results Results
1 GF-l a G G
2 GF-2 a ~ B
3 GF-7 a G G
4 GF-3 a B B
GF-l ua ~ B
6 GF-2 ua B B
7 GF-7 ua G G
8 GF-3 ua B B
Note: a = annealed; ua = unannealed; G = good; B = bad.
Neither cadmium nor zinc is believed to leave
the glass to any significant extent at sintering and
enter the PZLT grains, contrary to their behavior in
barium titanate. This theory is consistent with the
fact that there is no change in the lower Curie tempera-
ture as defined in the dielectric constant function plot
versus temperature.
Considering this stability of cadmium and/or
zinc, it is concluded that the glass-forming element boron
is what is needed to getter the free lead. However,
boria or boric acid is not acceptable because both are
hygroscopic. Hygrosocpic materials are eschewed as
causing too drastic a shrinkage as water i5 driven off
in the early phases of heating and sintering bodies.
Also, it has been observed that for additions
of about 1.0 wt% and more of the borate flux, the PLZT
formulation begins to exhibit a greatly reduced dielec-
tric constant. Less than 0.9 wt% of the borate flux is
preferred while at least 0.1 wt% is considered essential
for PbO gettering.
~z~
- 9 -
In another series of experiments the e~fective-
ness in the PLZT system o simple cadmium borate and zinc
borate fluxes were compared with that of the above-noted
cadmium zinc borate. To a sample each of glasses GF-4,
GF-5, GF-6, and GF-7 was added an equal weight of PbO.
The mixture in each case was heated in a platinum dish
to observe the temperatures at which they become sub-
stantially completely liquid. Table IV shows these
results that are considered accurate to within about
10 + 25C:
Table IV
Liquidous Liquidous
Glass Pure with 5~% PbO
GF-4 1090C 725C
GF-5 1060C 675C
GF-6 860C 625C
GF-7 860C 625C
The melting temperature of PbO is 890C. Thus, it is
clear that all of these borates will create similarly
eutectic compounds with fugitive PbO during the sintering
of PLZT ceramics and result in similarly efficient liquid
phase sintering.
Each of these four glasses was employed at the
0.5 wt% flux with the PLZT ceramic. During the ramp-up
of temperature at sintering, shrinkage of the fluxed PLZT
bodies was measured by a dilatometer. The rate and the
ultimate degree of densification were almost identical
for these borates of cadmium and zinc, indicating essen-
tially equal ability to getter free PbO and to aid sinter-
ing. In contrast, a PLZT body without any flux fired inan open air atmosphere does not sinter properly a~ 1100C.
It is partially porous due to the volatility of PbO and
does not fully sinter. This demonstrates the importance
of the free lead oxide as a sintering aid and puts in
perspective the synergism between the cadmium and/or zinc
borate flux leading to efficient sintering at a low 1100C
and at the same t-ime resulting in essentially no free lead
oxide after sintering, and without a post anneal step.
~ ~7~
-10-
In yet another experiment, a comparison was
o~tained between the results wsing the excellent cadmium
zinc borate (G-7), and the same glass with the addition
of alumina (G-8). Again, monolithic PLZT ceramic capaci-
tors were made by the same process used for making thecapacitors of Examples 1, 2, 3 and 4. Again, 0.5 wt%
of the glass G-8 was employed. The capacitors in this
experiment exhibited capacitance, dielectric constant,
temperature coefficient of capacitance, and dielectric
breakdown characteristics that are essentially indistln-
guishable from those characteristic capacitor measures
of Examples 3 and 7 that include the G-7 glass.
During the sintering at 1100C in air of a PLZT
body having no flux, from 1 to 1~ wt% PbO is lost. This
loss is normally anticipated and compensated ~or in
advance by using a start composition that is lead rich.
A fl.ux should be chosen that will not cause a further
loss of lead. Neither should the flux lure away elements
in the PLZT grains nor add impurity atoms to the grains
causing an imbalance in stoichiometry and~or charge
balance in the grains.
Titania and zirconia as additives will getter
free lead but will not reduce the sintering temperature
and do not meet the above criteria for leaving the grains
in balance. Also, even though the amount of flux is less
than 1 wt%, the start 1ux composition itself should con-
tain less than about 2.0 wt% of the oxides of the alkali
metals, Ba, Ca, Sr, and Cu and transition metals includ-
ing Ni, Co, Fe and Mn. Furthermore, the flux should have
less than 2.0 wt% oxides of Sn, Sb, Nb, Ta and Bi, all
of which additionally tend to enter the PLZT grains and
have a substantial modifying effect on the Curie tempera-
ture of Pl,ZT rnaterials. Although Si does not enter the
grains, SiO2 for reasons noted above should also amount
to less than 2.0 wt% of the flux.
The addition of substantial amounts of Al2O3
to the cadmium/zinc fluxes employed in this invention
can actually result in an improved PbO gettering capacity,
although the comparative tests conducted and described
herein were not designed to show such superiority. This
is consistent with the fact that alumina additions to
glass compositions are known to act as an additional
glass-former material~ capable of expanding the quantlties
of cations that the glass composition can accomodate. It
is judged that the flux of this invention may contain up
to 20 wt% alumina.