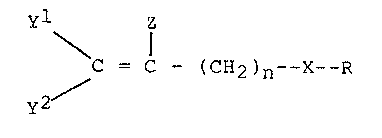Note: Descriptions are shown in the official language in which they were submitted.
7~
--1--
Docket 4605
PESTICIDAL POLYHALOALKENE DERIVATIVES
This invention relates to pesticidal polyhalo-
alkene derivatives and use for combatting infestations
of nel~atodes in soil and in plant systems, particu-
larly agricultural crops, and for combatting plant-
destructive diseases caused wholly or in part by nema-
todes. The invention further concerns anthelmintic
applications of the compounds.
U.S. Patent 3,513,172 - Brokke and divisional
patents thereof disclose nematicidal trifluorobutenyl
derivatives of the formula
F2C=CFCH2CH2-R
where R is selected from various substituents includ-
ing some heterocyclics such as 2-thio-4-alkylthia-
zolyl. These and other patents reflect ongoing
efforts of industry and governmental agencies to find
and commercialize chemicals for combatting nematodes
and nematode-induced plant diseases, to thereby reduce
the substantial economic losses resulting from
nematode infes~ations.
A new class of polyhaloalkene derivatives has now
been found having high nematicidal activity and good
90il mobility, In addition, the compounds exhibit
control of a variety of nematodes, and in some cases
sy~temic activity. The compounds also are efEective
against helminths that are indicators of animal
anthelmintic activity.
The novel nematicidal compounds of the invention
are polyhaloalkene derivatives of the formula (I):
, . . . .
--2--
Y\ Z
y2~ --~CH2 ~ X --R
wherein X is sulfur, oxygen, nitrogen or methylene,
Y and Y independently are fluorine, chlorine or
bromine, Z is hydrogen or the same as Y or Y ,
~nd n is 1-4, preferably 1 or 2; provided that:
(A) when X is sulfur, R is thiazolyl, optionally
substituted thienyl, optionally substituted thia-
naphthyL, optionally substituted thiazolinyl, option-
ally substituted thiadiazolyl, optionally substituted
oxadiazolyl or 3,4,4-trifluoro-3-butenyloxycarbonyl-
methyl;
(B) when X is oxygen, R is C(O)R wherein Ris perfluoroalkyl, optionally substituted phenyl,
optionally substituted thienyl, optionally substituted
furanyl, optionally substituted pyrrolyl or dihydro-
thiazolylthiomethyl;
(C) when X is nitrogen, R taken with the nitro-
gen is an isothiocyanate, succinimide or saccharine
group; and
(D) when X is methylene, R is hydroxy.
Other aspects of the invention include methods of
controlling nematode populations and arresting plant
and animal diseases caused by nematodes and helminths,
and nematicidal and anthelmintic formulations based on
the polyhaloalkene derivatives. Typical nematode
species controlled in accordance with the invention
are the root-knot, stunt, lesion, cyst and C.
nematodes.
In subclasses A and B of the compounds of formula
I above, available carbon atoms of the heterocyclic
rings other than thiazolyl optionally may be substi-
tuted with any group or groups which are non-destruc-
.~ - , . .. . .
7766~3
tive of the nematicidal or anthelmintic activity of
the compounds. Typical substituents include alipha-
tic, aromatic and heterocyclic groups, halo, nitro,
cyano, alkoxy, alkylthio, haloalkyl, haloalkoxy,
halo-, nitro-, cyano- or alkoxy-substituted phenyl,
polyhaloalkenylthio, phenylalkylthio, phenylthioalkyl-
thio, propargylthio, cycloalkylmethylthio, and the
like, further including straight and branched chain
structures, and the various isomers of such substi-
tuents.
Throughout this specification the alkyl, alkenyland alkynyl groups may contain 1-11 or more carbon
atoms and may be straight chain or branched. Cyclo-
alkyl groups may contain 3-8 or more carbon atoms.
Preferably, alkyl, alkenyl, alkynyl and alkoxy are
lower alkyl, l~wer alkenyl, lower alkynyl or lower
alkoxy, meaning that these groups contain 1-8 carbon
atoms, more preferably 1-4 carbon atoms such as
methyl, propenyl ana methoxy. Halo or halogen means
fluorine, chlorine, bromine or iodine, preferably
fluorine, chlorine or bromine. Aromatic substituents
include phenyl, naphthyl, anthracene, diphenyl, and
the like.
Representative compounds of formula I are listed
in Tables 1, la, lb and lc appended.
The preferred compounds,of formula I are those of
subclass A wherein R is defined as follows:
(1) R is a thiadiazolyl group of the structure:
R2S 3 4
1,2,4-thiadiazol-5-yl
'~S ~ ~ Or 1,2,4-thiadiazol-3-yl
wherein R is 3,4,4-trifluoro-3-butenyl, or a
phenylmethyl or phenylthiomethyl group each
.
-4
optionally substituted with halogen or nitro.
The R S group may be in the 3- or 5-position
of the 1,2,4-thiadiazole ring;
(2) R is a thiadiazolyl group as in ~1) above but
with iodo in place of R S;
(3) R is a thiadiazolyl group of the structure:
3N N4
3 S ~ 1,3,4-thiadiazol-5-yl
wherein R is aryl, arylalkyl, aryloxyalkyl,
alkylthio, haloalkylthio, cyanoalkylthio, aryl-
alkylthio, aryloxyalkylthio, arylthioalkylthio,
heterocycloalkylthio, alkenylthio, haloalkenyl-
thio, halocycloalkylalkenylthio, or an amino
group mono- or disubstituted with members
selected independently from alkyl, alkylcarbonyl,
haloalkylcarbonyl, aryl, arylaminocarbonyl, aryl-
alkylcarbonyl, arylalkoxycarbonyl and 3-(2,2-di-
chloroethenyl)-2,2-dimethylcyclopropanecarbonyl;
(4) R is an oxadiazolyl group of the structure:
R\3 4
~ ~ 1,2,4-oxadiazol-5-yl
or 1,2,4-oxadiazol-3-yl
wherein R is aryl. or arylalkyl substituted
with chloro, fluoro, alkyl, haloalkyl, alkoxy, or
nitro. The R4 group may be in the 3- or 5-
position of the 1,2,4-oxadiazole ring; or
(5) R is an oxadiazolyl group of the structure:
3N - N4
R 1 1,3,4-oxadiazol-5-yl
~2~71Ei~
wherein R is alkyl, haloalkyl, aryl, aryl-
alkyl, aryloxyalkyl, arylthioalkyl, heterocyclo-
alkyl, arylalkenyl or alkynyl (C2-Cll).
Aryl and the heterocycles described in (3) and
(4) above are optionally substituted with one or more
qroups selected independently from halogen, alkyl,
haloalkyl, alkoxy, haloalkoxy, cyano, nitr~ and
phenyl. Aryl and the heterocycles described in (5)
above are op.tionally substituted with one or more
groups selected independently from halogen, alkyL,
alkoxy, nitro, amino, hydroxy, acetyloxy and
alkylaminocarbonyloxy.
(6) R is a thiazolyl group.
Synthesls
The compounds of formula I are prepared in a
known manner. For example, a polyhaloalkene such as
4-bromo-1,1,2~trifluoro-1-butene i5 reacted with a
mercaptothiazole (prepared by reaction of thiazole and
elemental sulfur) or mercaptothiazoline in a reaction
solvent medium containing sodium ethoxide to form the
.thia~.olyl or thiothiazoline deriva~ive of ~he poly-
haloalkene. Examples 1, 2, 8-14, 16 and 17 below are
representative of this and other reaction schemes for
synthesis of the subclass A compounds of formula I
(X = sulfur). Compounds of formula I wherein X is
oxygen (subclass B) may be prepared as described in
Examples 3 and 4. Similarly, the subclass C (X =
nitrogen) and subclass D compounas (X = -CH2-) are
prepared as described in Examples 5 and 6 (subclass C)0 and 7 (subclass D).
Ot~er polyhaloalkenes may be used in known ways
to prepare other compounds of formula I. For example,
trifluoroethylene can be chain-extended with methyl
dibromide and the 1,3-dibromo-1,1,2-trifluoropropane
product then reacted with a mercaptan to form a thio
1~:~6Çi~
--6--
intermediate. The intermediate is then dehydrohalo-
genated, as follows, wherein "BP" is benzoyl peroxide
and "DBU" is 1,8-diazabicyclo~5.4~0]undec-7-ene
catalyst, as described by Tarrant and Tandon, J. Org.
Chem. 34(4), 864 (1969):
cH2~r2 ~ F2C = ~HF ~ BrcH2cRFcF2Br
BP
~ N
t S ~
15 ~ S ~ S - -- CR2~ CF2 ~ DBU ~ ~ S C 2 2
Dihalopropene derivatives within the scope of
formula I may be prepared by the following general
reaction, wherein Y~ and Y are as defined above
and one of Y and Y also may be hydrogen:
yl / yl
CH2~r2 + ~C CH2 E`P -~ 8rCH2CH2C
/ C \> ~ SH
~ ~ yl
\ ~ S ~ CH2C~ ~ C /
S ~2
.. . .
... ... .. .. .
~2t7~6~8
Trihalopropene derivatives also may be prepared
in a manner and reaction similar to the Tarrant and
Tandon scheme to form other compounds of formula I,
wherein yl~ y and Z are as defined in formula I:
yl z 2 yl
y rCH~CHf
~5}~
~ \~ S - C~2~ = C\ 2
Methods of preparing propenes, butenes and other
alkenes having mixed halogen substituents and there-
fore useful in the invention for preparing compounds
Of formula I are described in a Ph.D. thesis of M. R.
Lillyguist, University of Florida (1955), pages 9, 39,
59 and 60. It will be apparent, therefore, that the
polyhaloalkenes and heterocyclic or other compounds
used to prepare the compounds of formula I generally
are known materials or can be synthesized by known
procedures.
The following examples further describe methods
of preparing the compounds of the invention. In the
examples all parts and percentages are by weight and
all temperatures are C unless otherwise stated. The
. .
~27~76~i8
--8--
products of Examples 1-9 correspond to compounds 1-9
listed in Tables 1 and lb. Examples 10-17 identify
the tabulated compounds to which they relate.
Example 1
.
2-(3,4,4-Trifluoro-3-butenylthio)~
4,5-dihydrothiazole
Sodium ethoxide was prepared by stirring 0.25
gram (0.011 mole) of sodium metal in 30 ml of absolute
ethanol. To this was added 1.2 grams (0.01 mole) of
2-mercaptothiazoline. The xeaction mixture was
stirred for one hour and the excess ethanol was
removed under reduced pressure. The residue was dis-
solved in 35 ml of rnethyl ethyl ketone and 2.0 grams
(0.01 mole) of 4 bromo-1,1,2-trifluoro-1-butene was
added. The reaction mixture was stirred at ambient
temperature for four hours, then concentrated under
reduced pressure to a residue. The residue was
dissolved in 50 ml of toluene and washed with three 25
ml portions of water. The organic layer was dried
with sodium sulfate and filtered. The filtrate was
concentrated under reduced pressure to give 1.7 grams
of 2-(3,4,4-trifluoro-3-butenylthio~-4,5-dihydrothia-
zole as an oil. The nmr and the ir spectra were
consistent with the proposed structure.
Example 2
5-Methylthio-2-(3,4,4-trifluoro-
3-butenylthio)-1,3,4-thiadiazole
A solution of 2.0 grams (0.012 mole) of 2-mer-
capto-5-methylthio-1,3,4-thiadiazole in 25 ml of
distilled acetone was added to a stirred mixture of
0.84 gram (0.006 mole) of potassium carbonate and 0.2
gram (0.001 mole) of potassium iodide in 25 ml of
distilled acetone. With continued stirring 2.2 grams
(0.012 mole) of 4-bromo-1,1,2-trifluoro-1-butene was
added dropwise. Upon completion of addition the reac-
tion mixture was heated under reflux for four hours.
.
~ 7~6~3
The reaction mixture was cooled, filtered, and the
filtrate concentrated under reduced pressure to a
residue. The residue was dissolved in diethyl ether
and washed with aqueous 5% sodium hydroxide. The
organic layer was dried with magnesium sulfate and
filtered. The filtrate was concentrated under reduced
pressure to give 1.3 grams of 5-methylthio-2-(3,4,4-
trifluoro-3-butenylthio)-1,3,4-thiadiazole as an oil.
The nmr and the ir spectra were consistent with the
proposed structure.
Example 3
~3,4,4-Trifluoro-3-butenyl) heptafluorobutyrate
(A) A stirred solution of 2.6 ml (0.02 mole) of
heptafluorobutyric acid in 50 ml of water was warmed
to 50C and 5.1 grams (0.022 mole) of silver oxide was
added. Upon completion of addition the reaction
mixture temperature was maintained at 50-60C for two
hours. The reaction mixture was allowed to cool to
ambient temperature, then it was filtered. The
filtrate was concentrated under reduced pressure to
give 6.4 grams of the silver salt of heptafluoro-
butyric acia as a solid.
(B) To a stirred mixture of 3.2 grams (0.01
mole) of the silver salt of heptafluorobutyric acid in
40 ml of diethyl ether was added dropwise 1.9 grams
(0.01 mole) of 4-bromo-1,1,2-trifluoro-1-butene in 10
ml of diethyl ether. Upon completion of addition the
reaction mixture was stirred for two hours at ambient
temperature, then was heated under reflux ~or one
hour. The ether solvent was removed by distillation
and the residual oil distilled under reduced pressure
to give 1.0 gram of (3,4,4-trifluoro-3-butenyl)
heptaEluorobutyrate; b.p. 25C/4.0 mm Hg. The nmr
spectrum was consistent with the proposed structure.
.... ..
-- 10 --
Example 4
(3,4,4-Trifluoro-3-butenyl) 4-chlorobenzoate
To a stirred solution of 1.6 grams (0.01 mole) of
4-chlorobenzoic acid in 35 ml of acetonitrile was
added 1.5 ml ~0.01 mole) of 1,8-diazabicyclo[5.4.0]-
undec-7-ene, followed by 1.9 grams (0.01 mole) of
4-bromo-1,1,2-trifluoro-1-butene. The reaction
mixture was heated under reflux for four hours then
allowed to ~ool to ambient temperature. Water, 25 ml,
was added to the reaction mixture, and the reaction
mixture was extracted with three 20 ml portions of
diethyl ether. The combined extracts were washed in
succession with one 25 ml portion of water, two 25 ml
portions of aqueous 5% sodium hydroxide and, finally,
one 25 ml portion of water. The organic layer was
dried with sodium sulfate and filtered. The filtrate
was concentrated under reduced pressure to give 1.2
grams of (3,4,4-trifluoro-3-butenyl) 4-chlorobenzoate
as an oil. The nmr and the ir spectra were consistent
with the proposed structure.
Example 5
~-(3,4,4-trifluoro-3-butenyl)succinimide
This compound was prepared in a manner analogous
to that of Example 1 using 1.1 grams (0.01 mole) of
succinimide, 1.9 grams (0.01 mole) of 4-bromo-1,1,2
trifluoro-l-butene, ~.25 gram (0.01 mole) of sodium
metal, 30 ml of absolute ethanol and 20 ml of ai-
methylformamide. The yield of ~-~3,4,4-triEluoro-3-
butenyl)succinimide was 0.3 gram as an oil. The nmr
and the ir spectra were consistent with the proposed
structure.
Example 6
(3,4,4-Trifluoro-3-butenyl)isothiocyanate
(A) To a stirred solution of 10.0 grams (0.053
mole) of 4-bromo-1,1,2-trifluoro-1-butene in 50 ml of
.
.. .. . . ..
~277G68
dimethylformamide was added 10.4 grams (0.056) of the
commercially available potassium salt of phthalimide.
The reaction mixture was warmed to 50C where it
stirred for four hours. The reaction mixture was
allowed to cool and 50 ml of chloroform was aaded.
The mixture wa~ poured into 200 ml of water. The
aqueous layer was separated and extracted with two 50
ml portions of chloroform. The combined organic
layers were washed with two 50 ml portions of aqueous
5~ sodium hydroxide and one 50 ml portion of water.
The organic layer was dried with sodium sulfate and
filtered. The filtrate was concentrated under reduced
pressure to give 4.8 grams of N-(3,4,4-trifluoro-3-
butenyl)phthalimide as an oil. The nmr spectrum was
consistent with the proposed structure.
(B) A st;rred solution of 4.2 grams (0.016 mole)
of ~-(3,4,4-trifluoro-3-butenyl)phthalimide and 1.0 ml
(0.032 mole) of anhydrous hydrazine in 50 ml of
methanol was heated under reflux or one hour. The
reaction mixture was allowed to cool and the solvent
removed under reduced pressure. The residue was taken
up in 25 ml of water and 30 ml of concentrated hydro-
chloric acid. The reaction mixture was heated under
reflux for two hours and then cooled to 0C. A solid
was removed from the reaction mixture by filtration.
The filtrate was concentrated under reduced pressure
to a residue. The residue was taken up in 50 ml of
water and made basic with aqueous 10% sodium
hydroxide. The mixture was extracted with two 25 ml
3 portions of diethyl ether. The combined extracts were
dried with sodium sulfate and filtered. The filtrate
was concentrated under reduced pressure to give 0.4
gram of 4-amino-1,1,2-trifluoro-1-butene as an oil.
The ir spectrum was consistent with the proposed
structure.
~2776~
- 12 -
(C) A stirred solution of 0.4 gram (0.004 mole)
of 4-amino-1,1,2-trifluoro-l-butene in 25 ml of
diethyl ether was saturated with gaSeGUS hydrochloric
acid. The reaction mixture was concentrated under
reduced pressure to give 0.4 gram of 4-amino-1,1,2-
trifluoro-l-butene hydrochloride as a solid. The nmr
spectrum was consistent with the proposed structure.
(D) To a stirred solution of 0.4 gram (0.0027
mole) of 4-amino-l,1,2-trifluoro-l-butene hydrochlo-
ride in 15 ml of chloroform was added 0.3 gram (0.003mole) of thiophosgene, followed by 0.7 ml (0.009 mole)
of triethylamine. Upon completion of addition the
reaction mixture was stirred at ambient tPmperature
for three hours. The reaction mixture was then washed
in succession with one 25 ml portion of water, two 25
ml portions of aqueous 5~ sodium hydroxide, and,
finally, one 25 ml portion of water. The organic
layer was dried with sodium sulfate and filtered. The
filtrate was concentrated under reduced pressure to
give 0.3 gram of (3,4,4-trifluoro-3-butenyl) isothio-
cyanate as an oil. The ir spectrum was consistent
with the proposed structure.
Example 7
4,5,5-Trifluoro-4-penten-1-ol
(A) To a stirred mixture of 2.4 grams (0.1 mole)
of magnesium turnings in 100 ml of diethyl ether was
added 18.9 grams (0.1 mole) of 4-bromo-1,1,2-tri-
fluoro-l-butene. Upon completion of addition the
reaction mixture was heated under reflux until the
reaction was complete. The reaction mixture was
cooled to 0C and 9.0 grams (0.2 mole) of carbon
dioxide was bubbled in slowly. Upon completion of
addition the reaction mixture was stirred for 0ne
hour, then lO0 ml of aqueous 20~ hydrochloric acid was
added to destroy the excess magnesium. The reaction
.. .. .. .
- 13 -
mixture was extracted with three 40 ml portions of
diethyl ether. The combined extracts were cooled and
40 ml oE aqueous 25% sodium hydroxide was added
slowly. The organic layer was separated and extracted
with one 40 ml portion of aqueous 25% sodium
hydroxide. The combined base layers were cautiously
acidified with aqueous 20~ hydrochloric acid. The
mixture was extracted with two 100 ml portions of
diethyl ether. The comblned extracts were dried witl-
sodium sulEate and filtered. The filtrate was con-
centrated under reduced pressure to give 6.9 grams of
4,5,5-trifluoro-4-pentenoic acid as an oil. The nmr
and ir spectra were consistent with the proposed
structure.
(B) To a stirred suspension of 0.4 gram (0.01
mole) of lithium aluminum hydride in 20 ml of diethyl
ether was added dropwise a solution of 1.5 grams (0.01
mole) of 4,5,5-trifluoro-4-pentenoic acia in 30 ml of
diethyl ether. Upon completion of addition the reac-
tion mixture was stirred at ambient temperature for
one hour, then 20 ml of water was added carefully.
The mixture was filtered and the filtrate concentrated
under reduced pressure to give 0.8 gram of 4,5,5-tri-
fluoro-4-penten-1-ol as an oil. The nmr and the ir
spectra were consistent with the proposed structure.
Example 8
_T. '
3-Chloro-5-(3,4,4-trifluoro-3-
butenylthio)-1,2,4-thiadiazole
(A) A stirred solution of 5.0 grams (0.026 mole)
of dipotassium cyanoimidodithiocarbonate ~prepared by
the method of L.S. Wittenbrook et al, J. Org. Chem.,
38, 3, 465 (1973)] in 19 ml of acetone and 22 ml of
water was cooled to 0C and 4.9 grams (0.026 mole) of
4-bromo-1,1,2-trifluoro-1-butene in 10 ml of acetone
was added dropwise. Upon completion of addition the
,
~ 2~7~6~3
- 14 -
reaction mixture was allowed to warm ~o ambient tem-
perature where it stirred for 16 hours. The reaction
mixture was concentrated under reduced pressure to a
residual solid. The solid was dissolved in ethyl
acetate and filtered. The filtrate was concentrated
under reduced pressure, and the residual solid dried
in a vacuum oven. The dried solid was dissolved in
hot chloroform - ethyl acetate and filtered. The
filtrate was concentrated under reduced pressure, and
the residual solid dried in a vacuum oven to give 4.4
grams of potassium ~3,4,4-trifluoro-3-butenyl) cyano-
imidodithiocarbonate. The nmr spectrum was consistent
with the proposed st.ructure.
(B) A stirred solution of 2.0 grams (0.008 mole)
of potassium (3,4,4-triEluoro-3-butenyl) cyanoimido-
dithiocarbonate in 10 ml of chloroform was cooled to
0C and 1.2 ~rams (0.009 mole) of sulfuryl chloride
was added dropwise. Upon completion of addition the
reaction mixture was maintained at 0C for one hour,
warmed under reflux for 4 hours, then at ambient
temperature for 16 hours. Stirring was continued
throughout the 21 hour period. The reaction mixture
was filtered and the filtrate concentrated under
reduced pressure. The concentrate was passed through
silica gel using diethyl ether as an eluent. The
ether eluate was fi~tered and the filtrate concen
trated under reduced pressure to a residual oil. The
oil was dried in a vacuum oven to give 0.9 gram of
3-chloro-5-(3,4,4-trifluoro-3-butenylthio)-1,2,4-thia-
diazole. The nmr and the ir spectra were consistent
with the proposed structure.
Example 9
3-Bromo-S-(3,4,4-trifluoro-3-butenyl
thio)-1,2,4-thiadiazole
35A stirred solution o~ 3.0 grams (0.011 mole) of
15 -
potassium (3,4,4-tri~luoro-3-butenyl) cyanoimido-
dithiocarbonate (prepared as in Example 8, Step A3 in
25 ml of water was cooled to 0C and 2.2 grams ~0.~14
mole) of bromine was added dropwise under a positive
gaseous nitrogen pressure. Upon completion of addi-
tion the reaction mixture temperature was maintained
at 0C for one hour, then was allowed to warm to
ambient temperature where it stirred for 16 hours.
Sodium thiosulfate was added to the reaction mixture,
which was then partitioned between chloroform and
additional water. The chloroform layer was separated
and dried with magnesium sulfate. The mixture was
filtered and concentrated under reduced pressure to a
residual oil. The oil was passed through silica gel
using 4:1 - hexane:diethyl ether as an eluent. The
eluate was concentrated under reduced pressure to give
1.1 ~rams of 3-bromo-5-(3,4,4-trifluoro-3-butenyl-
thio)-1,2,4-thiadiazole as an oil. The nmr spectrum
was consistent with the proposed structure.
Example 10 (Compound 2S)
3,5-Di(3,4,4-Trifluoro-3-butenylthio)-
1,2,4-thiadiazole
(A) A stirred solution of 16.0 grams (0.08 mole)
of dipotassium cyanoimidodithiocarbonate (prepared as
in Example 8, Step A) and 2.6 grams (0.08 mole) of
sulfur in 425 ml of methanol was heated under reflux
for 15 minutes. The reaction mixture was allowed to
cool then was concentrated under reduced pressure to a
residual solid. The solid was dried under reduced
pressure to yield 18.1 grams of the dipotassium salt
of 3,5-dimercapto-1,2,4-thiadiazole.
(B) A solution of 1.0 gram (0,004 mole) of the
dipotassium salt of 3,5-dimercapto-1,2,4-thiadiazole
in 35 ml of methyl ethyl ketone was stirred and 1.7
grams (0.009 mole) of 4-bromo-1,1,2-trifluoro-1-butene
~%771~i6~
- 16 -
was added. The reaction mixture was heated under
reflux for two hours then allowed to cool to ambient
temperature where it stirred for 18 hours. The
reaction mixture was concentrated under reduced
pressure to a residue. The residue was stirred in 25
ml of water and the mixture was e~tracted with two 25
ml portions of toluene. The organic layer was dried
with sodium sulfate and filtered. Tlle ~iltrate was
concentrated under reduced pressure to yield 1.1 grams
of 3,5-di-(3,4,4-trifluoro-3-butenylthio)-1,2,4-thia-
diazole as a liquid. The nmr spectrum was consistent
with the proposed structure.
Example 11 (Compound 26)
3-(4-~itrophenylmethylthio)-5-(3,4,4-
trifluoro-3-butenylthio)-1,2,4-thiadiazole
(A) A stirred solution of 24.7 grams (0.109
mole) of the dipotassium salt of 3,5-dimercapto-
1,2,4-thiadiazole (prepared as in Example 10, Step A)
in 200 ml Gf water was acidified with concentrated
hydrochloric acid. The resultant solid was collected
by filtration to yield 17.3 grams of wet 5-amino-
1,2,4-dithiazol-3-thione; m.p. 217-220C.
(B) A solution of 2.2 grams (0.055 mole) of
sodium hydroxide in 7 ml of water and 20 ml of ethanol
was stirred and 4.0 grams (0.027 mole) of 5-amino-
1,2,4-dithiazol-3-thione was added portionwise. After
all of the 5-amino intermediate was in solution, 4.7
grams (0,027 mole) of 4-nitrophenylmethyl chloride was
added dropwise, Upon completion of addition the reac-
tion mixture was stirred at ambient temperature for 16hours, The reaction mixture was concentrated under
reduced pressure to a residue, The residue was dis-
solved in 20 ml of water then extracted with two 25 ml
portions of diethyl ether. The aqueous layer was
acidified with concentrated hydrochloric acid to yield
. .
~Z~77~;~8
- 17 -
a gummy solid. The solid was extracted from the
aqueous layer with two 25 ml portions of ethyl
acetate. The combined extracts were dried with sodium
sulfate and filtered. The filtrate was concentrated
under reduced pressure to yield a gummy solid. The
solid was dissolved in methylene chloride and filtered
to remove a small amount of insoluble material. The
filtrate was concentrated under reduced pressure to
yield 2.8 grams of 3-(4-nitrophenylme~hylthio)-5-mer-
capto-1,2,4-thiadiazole as a soli~. The nmr spectrum
was consistent with the proposed structure.
(C) A solution of 0.25 gram (0.011 mole) of
sodium in 35 ml of ethanol was stirred and 2.7 grams
(0~0095 mole) of 3-(4-nitrophenylmethylthio)-5-mer-
capto-1,2,4-thiadiazole was added. Upon completion of
addition the reaction mixture was stirred at am~ient
temperature for one hour. The ethanol solvent ~as
removed under reduced pressure. The residue was dis-
solved in 35 ml of methyl ethyl ketone and 1.6 grams
(0.0085 mole) of 4-bromo-1,1,2-trifluoro-1-butene was
added. Upon completion of addition the reaction
mixture was stirred for 16 hours, then was concen-
trated under reduced pressure to a residue. The
residue was dissolved in 50 ml of toluene and washed
with 25 ml of water, two 25 ml portions of aqueous 5
sodium hydroxide solution, and 25 ml of water. The
organic layer was dried with sodium sulfate and
Eiltered. The filtrate was concentrated under reduced
pressure to yiel~ a residual oil. The oil was dis-
solved in methylene chloride and passed through a
column of silica gel. The eluate was concentrated
under reduced pressure to yield 2.1 grams o~ 3-(4-
nitrophenylmethylthio)-5-(3,4,4-trifluoro-3-butenyl-
thio)-1,2,4-thiadiazole. The nmr spectrum was con-
sistent with the proposed structure.
~77~6~3
- 18 -
Example 12 (Compound 30)
2-(l-Methylethylthio)-5-(3,4,4-trifluoro-3-
butenylthio)-1,3,4-thiadiazole
A solution of 22~5 grams ~0.15 mole) of 2,5-di-
mercapto-1,3,4-thiadiazole in 200 ml of tetrahydro-
furan was stirred and 21 ml (0.15 mole) of triethyl-
amine was added dropwise. Upon completion of addition
the reaction mixture was stirred at ambient tempera-
ture for 15 minutes, then 28.4 grams (0.15 mole) of
4-bromo-1,1,2-trifluoro-l-butene was added dropwise.
Upon completion of addition the reaction mixture was
heated under reflux for two hours. The cooled reac-
tion mixture was concentrated under reduced pressure
to a residue. The residue was stirred in 250 ml of
diethyl ether and extracted with two lO0 ml portions
of aqueous 10% potassium hydroxide. The combined
extracts were acidified with aqueous 10% hydrochloric
acid, then were extrac~ed with two 100 ml portions of
diethyl ether. The combined ether ex~racts were dried
with sodium sulfate and filtered. The filtrate was
concentrated under reduced prassure to yield, after
drying, 35.6 grams of 2-mercapto-5-(3,4,4-tri-
fluoro-3-butenylthio)-1,3,4-thiadiazole as a solid.
The nmr spectrum was consistent with the proposed
structure~
(~) In a manner analogous to Example 11, Step C,
1,3 grams (0.005 mole) of 2-mercapto-5-(3,4,4-tri-
1uoro-3-butenylthio)~1,3,4-thiadiazole, 0.5 ml (0.005
mole) of 2-iodopropane, 0.15 gram (0.007 mole) of
sodium were reacted in 35 ml of ethanol and 35 ml of
methyl ethyl ketone by heating the mixture under
reflux for five hours prior to stirring at ambient
temperature for 16 hours. The yield of 2-(1-methyl-
ethylthio)-5-(3,4,4-trifluoro-3-butenylthio)-1,3,4-
thiadiazole was 1.3 grams as a liquid. The nmrspectrum was consistent with the proposed structure.
~Z776~3
-- 19 --
Example 13 (Compound 37)
2-~4-Chlorophenyl)-5-(3,4,4-trifluoro-
3-butenylthio)-1,3,4-thiadiazole
~A) A stirred solution of 8.1 grams (0.048 moLe)
of 4-chlorobenzoic acid hydrazide in 300 ml of tri-
ethyl orthoformate was heated under reflux for ~6
hours. The excess triethyl orthoformate was removed
by disti]lation and the residual solid was stirred
with petroleum ether to yield 7.7 grams of 2-(4-
chlorophenyl)-1,3,4-oxadiazole; m.p. 129C. The nmr
spectrum was consistent with the proposed structure.
(B) Under a nitrogen atmosphere, a solution of
17 grams (0.084 mole) of phosphorus pentasulfide in
100 ml of dry xylene was stirred and 7.6 grams ~0.042
mole) of 2-(4-chlorophenyl)-1,3,4-oxadiazole was
added. Upon co~pletion of addition the reaction
mixture was heated under reflux for 30 hours. The
reaction mixture was cooled and 10~ ml of water was
added dropwise. The mixture was filtered through
diatomaceous earth to separate the organic and aqueous
phases. The organic phase (the filtrate) was
extracted with an aqueous 10% potassium hydroxide
solution. The extract was acidified with an aqueous
5~ hydrochloric acid solution, and then was extracted
with diethyl ether. ~he ether extract was concen-
trate~ under reduced pressure to yield 0.3 gram of
2-(4-chlorophenyl)-5-mercapto-1,3,4-thiadiazole; m.p.
178C.
(C) In a manner anaLogous to Example 2, 0.3 gram
(0.0015 mole) of 2-(4-chlorophenyl)-S-mercapto-1,3,4-
thiadiazole, 0.4 gram (0.002 mole) of 4-bromo-1,1,2-
trifluoro-l-butene, 0.2 gram (0.0015 mole) of potas-
sium carbonate, and 0.05 gram of potassium iodide were
reacted in 9 ml of methyl ethyl ketone. The yield of
2-(4-chlorophenyl)-5-(3,4,4-trifluoro-3-butenylthio)-
..
~27~
- 20 -
1,3,4-thiadiazole was 0.1 gram: m.p. 68-6~C. The nmr
spectrum was consistent with the proposed structure
Example 14 (Compound 39)
3-(4-Chlorophenyl)-5-(3,4,4-trifluoro-3-
butenylthio)-1,2,4-oxadiazole
(~) A stirred solution of 4.1 grams (0.03 mole)
of 4-chlorobenzonitrile, 2.1 grams (0.03 mole) of
hydroxylamine hydrochloride, and 2.1 grams (0.015
mole) of potassium carbonate in 10 ml of water and 100
ml of ethanol was heated under reflux for 16 hours.
The reaction mixture was cooled and 50 ml of water was
added. The ethanol solvent was removed under reduced
pressure. The concentrate was cooled in an ice bath
and the resultant solid collected by filtration. The
solid was dried to yield 4.4 grams of N-hydroxy-
imido-4-chlorobenzamide: m.p. 122-130C.
(B) A solution of 4.4 grams (0.028 mole) of
N-hydroxyimido-4-chlorobenzamide in 50 ml of diethyl
ether was stirred and 0.55 ml (0.007 mole) of thio-
phosgene was added dropwise. Upon completion of addi-
tion the reaction mixture was stirred at ambient
temperature ~or 15 minutes then was heated under
reflux for one hour. The reaction mixture was cooled
and filtered to collect bis 0,0'-thiocarbonyl(4-
chLoro-~-hydroxybenzenecarboximidamide). A 100~ yield
was assumed.
(C) A solution of 10.0 grams (0.25 mole) of
sodium hydroxide in 90 ml of water was stirred and 5.4
grams ~0.14 mole) of bis 0,0'-thiocarbonyl(4-chloro-
N-hydroxybenzenecarboximidamide) was added. Upon
completion o addition the reaction mixture was heated
under reflux for one hour. ~he reaction mixture was
cooled and extracted with two 50 ml portions of
diethyl ether. The aqueous layer was acidified with
concentrated hydrochloric acid. The resultant preci-
-
~z7~i6a
- 21 -
pitate was collected by filtration, washed with water,
and dried to yield 1.0 gram of 3-~4-chlorophenyl)-5
mercapto-1,2,4-oxadiazole; m.p. 139-156C, dec. The
nmr spectrum was consistent with the proposed struc-
tureO
(D) In a manner analogous to Example 2, 0.7 gram
(0.003 mole) of 3-(4-chlorophenyl)-5-mercapto-1~2,4-
oxadiazole, 0.6 ~ram (0.003 mole) of 4-bromo-1,1,2-
trifluoro-l-butene, 0.2 gram (O.OQ15 mole) of
potassium carbonate, and 0.1 gram of potassium iodide
were reacted in 40 ml of distilled acetone. The yield
of 3-~4-chlorophenyl)-5-(3,4,4-trifluoro-3-butenyl-
thio)-1,2,4-oxadiazole was 0.3 gram, m.p. 49-52C.
The nmr spectrum was consistent with the proposed
structure.
Example 15 (Compound 43)
_ _ .
5-(3-4,4-Trifluoro-3-butenylthio)-1,3,4-oxadiazole
(A) A solution of 25 grams (0.147 mole) of
4-chlorophenylacetic acid in 250 ml of acetonitrile
was stirred and 15.0 grams (0.0147 mole) of bromo-
ethane, followed by 22.0 grams ~0.147 mole) of
1,8-diazabicyclo[5.4.0]undec-7-ene, were added. Upon
completion of addition the reaction mixture was cooled
in a water bath while being stirred for 18 hours. The
reaction mixture was concentrated under reduced pres-
sure to one-half volume and then was added to 50 1.l1 of
water. The mixture was extracted with two portions of
diethyl ether. The combined extracts were dried with
magnesium sulfate and filtered. The filtrate was
concentrated under reduced pressure to yield 18.2
grams of ethyl (4-chlorophenyl)acetate.
(B) A stirred solution of 18.2 grams (0.091
mole) of ethyl (4-chlorophenyl)acetate and 10 ml of
hydrazine hydrate in 10 ml of ethanol was heated under
reflux for one hour during which time a solid preci-
- .
~2~
-22-
pitated. The solid was collected by filtration to
yield, when dried, 14.9 grams of 4-chlorophenylacetic
acid hydrazide; m.p. 159-161C. The nmr spectrum was
consistent with the proposed structure.
(C) A stirred solution of 7.0 grams (0.038 mole)
of 4-chlorophenylacetic acid hydrazide, 3.0 grams
(0.039 mole) of carbon disulfide and 2.8 grams (0.050
mole) of potassium hydroxide in 10 ml of water and 200
ml of ethanol was heated under reflux for four hours.
The ethanol was removed under reduced pressure. The
concentrate was taken up in water and the mixture
washed with diethyl ether. The aqueous layer was
acidified with aqueous 5% hydrochloric acid and then
was extracted with diethyl ether. The extract was
dried with magnesium sulfate and filtered. The
filtrate was concentrated under reduced pressure to
yield 3.9 grams of 2-(4-chlorophenylmethyl)-5-mer-
capto-1,3,4-oxadiazole; m.p. 115C. The nmr spectrum
was consistent with the proposed structure.
(D) In a manner analogous to Example 2, 2.
grams (0.011 mole) of 2-(4-chlorophenylmethyl)-
5-mercapto-1,3,4-oxadiazole, 2.0 grams (0.011 mole) of
4-bromo-1,1,2-trifluoro-1-butene, 1.5 grams (0.011
mole) of potassium carbonate and 0.5 gram of potassium
iodide were reacted in 45 ml of acetone. The yield of
2~(4-chlorophenylmethyl)-5-(3,4,4-trifluoro-3-butenyl-
thio)-1,3,4-oxadiazole was 1.5 grams as a li~uid. The
nmr spectrum was consistent with the proposed struc-
ture.
Example 16 (Compound 230)
Synthesis of 2-(3,4,4-trifluoro-3-
butenylthio)thiazole
Under a nitrogen atmosphere, a stirred solution
of 2.0 grams (0.024 mole) of thiazole in 30 ml of dry
35 tetrahydrofuran is cooled to -65C and 16 ml of 1.55
: . . ~ : . - ;-
.
q6~3
-23-
molar n-buty]lithium is added dropwise. ~pon comple-
tion of addition the reaction mixture is stirred for
45 minutes and 0.8 gram (0.024 mole) of elemental
sulfur is added portionwise. The reaction mixture is
5 stirred for an additional one hour, the temperature
then being adjusted to -60C, and 4.5 grams (0.024
mole) of 4-bromo-1,1,2-trifluoro-1-butene is added
dropwise. ~pon completion of addition the reaction
mixture is stirred for three hours during which time
it is allowed to war~ to ambient temperature. The
solvent is removed under reduced pressure. The resi-
due is dissolved in diethyl ether and washed with two
portions of an aqueous solution saturated with sodium
chloride. The organic layer is dried with magnesium
sulfate and fiLtered. The filtrate is concentrated
und~r reduced pressure to a residual semi-solid. The
semi-solid is subjected to column chromatography on
silica gel. ~lution is accomplished with 1:1 -
hexane:diethyl ether. The appropriate fractions are
combined and concentrated under reduced pressure to
give 0.4 gram of 2-(3,4,4-trifluoro-3-butenylthio)
thiazole as an oil. The nmr and ix speatra are con-
sistent with the proposed structure.
Example 17 (Com~ound 231)
Synthesis of 2-(2,3,3-trifluoro--2-
propenylthio)thiazole
(Al A stainless steel autoclave is charged with
50 grams (0.6 mole) of trifluoroethylene, 300 grams
(1.7 moles) of dihromomethane, and 5 grams (0.02 mole)
of benzoyl peroxide. The reaction mixture i9 stirred
and heated at 100C for six hours, then is cooled to
-70C. The autoclave i5 opened and the reaction
mixture fractionally distilled. The appropriate frac-
tions are combined to give 1,3-dibromo-1,1,2-tri-
fluoropropane.
~7~
-24-
(B) Under a nitrogen atmosphere a stirred solu-
tion of 20 grams (0.24 mole) of thiazole in 300 ml of
dry tetrahydrofuran is cooled to -65C and 160 ml of
n-butyllithium (1.55 molar) is added dropwise. Upon
completion of addition the rea~tion mixture is stirred
for 45 minutes and 8.0 grams (0.24 mole) of elemental
sulfur is added portionwise. Upon completion of addi-
tion the reaction mixture i5 stirred for one hour and,
at -60C. 61.4 grams (0.24 mole) oE 1,3-dibromo-
1,1,2-trifluoropropane is added dropwise. Upon com-
pletion of addition the reaction mixture is allowed to
warm to ambient temperature where it stirs for three
hours. The reaction mixture is concentrated under
reduced pressure to a residue. The residue is dis-
solved in diethyl ether and is washed with two por-
tions of aqueous sodium chloride. The organic layer
is dried with magnesium sulfate and filtered. The
filtrate is concentrated under reduced pressure to a
residue. The residue is purified by column chromato-
graphy to give 2-(3-bromo-2,3,3-trifluoropropylthio)-
thiazole.
(C) A stirred solution of 2.9 grams (0.01 mole)
of 2-(3-bromo-2,3,3 trifluoropropylthio)thiazole and
1.5 grams (0.01 mole) of 1,8-diazabicyclo[5.4.0]-
undec-7-ene in 40 ml of toluene is heated under reflux
for two hours. The solvent is removed by distillation
and the residue is purified by column chromatography
to give 1.1 grams of 2-(2,3,3-trifluoro-2-propenyl-
thio)thiazole.
The appended Tables 1, la, lb and lc list
compounds prepared as in the foregoing Examples. In
Tables la and lc the compounds are those of formula I
wherein yl~ y and Z are fluoro, based upon the
use of 4-bromo-1,1,2-trifluoro-1-butene as the
starting material in the synthesis.
~L27~ i8
-25-
Pesticidal Use
-
The compounds of the invention can be used
against a variety of pes~s that attack plants and
animals. In agriculture, they are useful as nemati-
cides, particularly against plant-parasitic nematodes
and "free-living" nematodes, i.e., nematodes not
dependent on any specific plant or other host. An
example of the latter is the microbivorous nematode
Caenorhabditis elegans. This nematode will feed on
bacteria such as Escherichia coli and is used as a
screen for both agricultural and veterinary nemati-
cides or anthelmintics.
When used as anthelmintics, in veterinary treat-
ments for treatment of infestations of Ascaris lumbri-
coides (roundworm in pigs) for example, the compoundsmay be administered ora~ly, parenterally or topicalLy
either alone but more usually in a pharmaceutically
acceptable carrier, to provide an appropriate dosage.
Such carriers incluae one or more of water, gelatine,
sugars, starches, organic acids such as stearic or
citric acid and salts thereof, talc, vegetable fats or
oils, gums, glycols and other excipients, for admini-
stration as solids ~e.g., tablets or capsules) or
liquids (e.g., soIutions, suspensions or emulsions).
The compositions may also contain preservatives,
stabilizers, wetting or emulsifying agents, buffers,
salts and other therapeutic agents. The compositions
may be formulated by conventional methods to contain
about 5 to 95~ by weight of the anthelmintic compound,
preferably about 25 to 75~ by weight. Further
guidance to anthelmintic activity, formulations and
modes of treatment, utili~ing the compounds of the
invention, is available from publications on the
subject, such as the article "Chemotherapeutics,
Anthelmintic" in Kirk-Othmer, Encyclopedia of Chemical
-26-
Technology, Third ed., 5 ~51-468, and articles cited
therein, and in the patent literature, such as U.S.
Patent 3,576,89~, col. 3, lines 29-56.
In using the compounds of the invention as agri-
cultural nematicides, the compounds, like most agri-
cultural chemicals, are generally not applied full
strength, bu~ are formulated with agriculturally
acceptable carriers and various additives normally
employed for facilitating the dispersion of active
ingredients, optionally with other active ingredients,
recognizing that the formulation and mode of applica-
tion of a toxicant may affect the activity of the
material. The present compounds may be applied, for
example, as powders or liquids, the choice of applica-
tion varying with the nematode species and environ-
mental factors present at the particular locus of
infestation. Thus, the compounds may be formulated as
granules, dusts, wettable powders, e~ulsifiable con-
centrates~ solutions, suspensions, dispersions, con-
trolled release compositions, and the like.
~ typical formulation may vary widely in concen-
tration of the active ingredient depending on the
particular agent used, additives, carriers or other
active ingredients used, the nematode species to be
controlled, and the desired mode of application. With
due consideration to these factors, the active in~e-
dient of a typical formulation may, for example,
suitably be present at a concentration of from about
0.5% up to about 99.5~ by weight of the formulation.
Surface active agents, if employed in the formulation,
may be present at various concentrations, suitably in
the range of 1 to 30~ by weight.
Dusts are admixtures of the active ingredient
with Einely divided solid carriers and/or diluents
such as talc, natural clays, kieselguhr, pyrophyllite,
lZ~6~3
chalk, diatomaceous earths, calcium phosphates,
calcium and magnesium carbonates, sulfur, lime,
flours, and other organic and inorganic solid
carriers. These finely divided formulations generally
have an average particle size of less than about 50
microns (325 mesh, Standard U.S. Sieve Series). In
most cases, the active ingredient will be present in
dust formulations at a concentration in the range o~ 1
to 15%, and occasionally from 1% to about 30~, the
balance of the composition typically comprising one or
more agriculturally acceptable inerts as adjuvant,
carrier, or diluent.
The nematicidal compounds of the invention may
also be formulated as wettable powders. These formu-
lations are in the form of finely divided particleswhich disperse readily in water or other liquid vehi
cles. The wettable powder is ultimately applied as a
dry dust or a dispersion in water or other liquid.
~ypical carriers Eor wettable powders include fuller's
earth, kaolin clays, silicas, and other highly absor~
bent or adsorbent inorganic diluents. The concentra~
tion of active ingredient in wettable powders is
dependent upon physical properties of the active
ingredient and the absorbency of the carriers.
Liquids and low melting solids (mp less than 100C)
are suitably formulated in the concentration range of
5 to 50% by weight; usually 10 to 30~; high melting
solids (mp greater than lOO~C) being formulated in the
range of 5 to 95% by weight, usually 50 to 85%. An
agriculturally acceptable carrier or diluent, fre~
quently including a small amount of a surfactant to
facilitate wetting, dispersion and suspension,
accounts for the balance of the formulation.
Microencapsulated or other controlle~ release
~L27'76G8
-2~-
formulations may also be used for application of
compounds in accordance with this invention.
Emulsifiable concentrates (EC's) are homogeneous
liquid compositions, usually containing the active
ingredient dissolved in a liquid carrier. Commonly
used liquid carriers include xylene, heavy aromatic
naphthas, isophorone, and other nonvolatile or
slightly volatile organic solvents. For application
of the nematicide, these concentrates are dispersed in
lo ~ater, or other liquid vehicle, forming an emulsion,
and are normally applied as a spray to the area to be
treated. The concentration of the essential active
ingredient in ~C's may vary according to the manner in
which the composition is to be applied, but, in
general, is in the range of 0.5 to 95~, frequently 10
to 80%, by weight of active ingredient, with the
remaining 99.5~ to 5~ being surfactant and liquid
carrier.
Flowables are similar to EC's except that the
ingreaient is suspended in a liquid carrier, generally
water. Flowables, like EC's, may include a small
amount of a surfactant, and contain active ingredient
in the range of 0.5 to 95~, frequently from 10 to 50%,
by weight of the composition. For application, flow-
ables may be diluted in water or other liquid vehicle,and are normally applied as a spray to the area to be
treatad.
Typical wetting, dispersing or emulsifying agents
used in these formulations include, but are not
limited to, the alkyl and alkylaryl sulfonates and
sulfates and their sodium or calcium salts; alkylaryl
polyether alcohols; sulfated higher alcohols; poly-
ethylene oxides; sul~onated animal and vegetable oils;
sulfonated petroleum oils: fatty acid esters of poly-
hydric alcohols and the ethylene Gxide addition
.
6~
-29-.
products of such esters; addition products of long-
chain mercaptans and ethylene oxide; and addition
products of alkylphenols such as nonylphenol and
ethylene oxide. Many other types of useful surface-
active agents are available in commerce. The sur-
face-active agent, when used, normally comprises from
1 to 15% by weight of the nematicidal composition.
Other useful formulations include simple solu-
tions of the active ingredient in a relatively
non-volatile solvent such as corn oil, kerosene,
propylene glycol, or other organic solvents. This
type of formulation is particularly useful for
ultra-low volume application.
The concentration of active ingredient in use
dilution is normally in the range of about 2~ to about
0.1%. Many variations of spraying, dusting, and
controlled or 510w release compositions in the art may
be used by substituting or adding a compound of this
invention to compositions known or apparent to the art.
The compositions may be ~ormulated and applied
with other suitable active ingredients, including
other nematicides, insecticides, acaricides, fungi-
cides, plant regulators, herbicides, fertilizers, etc,
In applying the foregoing chemicals, an effective
nematode controlling amount of active ingredient must
be applied, sometimes referred to herein as a "nemati-
cidal amount.`' While the application rate will vary
widely depending on the choice of compound, the formu-
lation and mode of application, the plant species
being protected and the planting density, a suitable
use rate may be in the range of 0.5 to 25 lcg/hectare,
preferably 1 to about 20 kg/hectare.
The compounds of this inv.ention are usually
applied by incorporating a formulation thereof into
the soil in which plants or agricultural crops are or
~77~
-30-
are to be planted, i.e., the locus of infestation.
This may be achieved by incorporating the compounds
into the soil or by broadcasting the formulation over
the planted area or the area to be planted or by
limiting the application to a small area or band in
the root 7one where plants are or are to be planted.
It wi]l be readily apparent where the latter method is
employed ~hat a nematicidal amount, that is, a nemati-
cidal concentration in the soil, must be applied to
the root zone. A suitable concentration for this
purpose is in the range of 0.1 to about 50 parts by
weight of compound of the invention per million parts
of soil.
However, in a significant aspect of the inven-
tion, it has been found that certain of the polyhalo-
alkene deri~atives of the invention have ef f icacy
against nematodes by foliar application, i.e., the
compounds are systemic nematicides. This aspect is
exemplified he~einafter.
The following are specific examples of formula-
tions which may be utilized in accordance with the
present invention. In these formulations the percen-
tages are wt/wt.
1. Typical dust formulation:
25 Test Compound 5%
Base
96~ Attaclay
2~ highly purified sodium
lignosulfonate (100~)
2~ powdered sodium alkylnapthalene
sulfonate ~75%)
.
j6~
-31-
2 Typical emulsifiable concentrates:
(A) Test Compound 5.0
Emulsifier A 4.0~
Emulsifier B 0.4%
Emulsifier C 0.8~
Emulsifier D 1.3%
Refined xylene solvent 88.5%
Emulsifier A is the anionic calcium salt of
dodecylbenzene sulfonate. Emulsifier B is a nonionic
6-molar ethylene oxide condensation product of nonyl-
phenol. Emulsifier C is a nonionic 30-molar ethylene
oxide condensation product of nonylphenol. Emulsifier
D is a nonionic paste of 100% polyalkylene glycol
ether.
(B) Test compound 21.3~
Emulsifier A 4.2%
Emulsifier B 0,5%
Emuisifier C o,g~
Emulsifier D 1.4%
Refined xylene solvent71.7
(C~ Test compound 5.0
Emulsifier E 4,0~
Emulsifier F 3.0%
Emulsifier G 3,0%
Dormant spray oil solvent
(non-volatile) 85.0~
Emulsifier E is an oil-soluble nonionic blend of
polyoxyethylene ethers commercially available under
the trademark and designation '`T Mulz 808A". Emulsi- ;
fier F is a'Eormulated nonionic concentrate commer-
cially available under the trademark and designation
"FloMo 200-4". Emulsifier G is the anionic free acid
of a complex organic phosphate ester commercially
available under the trademark and designation "Gafac
RE-410".
~77~
-32-
3. _ypical granule formulations:
(A) Test compound (technical) 5.0%
Attapulgite carrier/diluent 95.0
The carrier/diluent is a 20/40 or 60/90 mesh
5 hydrated aluminum magnesium silicate of low volatile
matter having 2~ free moisture.
(B) Test compound (technical) 5.0%
Ground corn cobs, 14/40 mesh 95.0
(C) Test cornpound tas emulsifiable
concentrate 2(B) above)23.5%
Attapulgite carrier/diluent
C3(A) above]76.5
4. Typical solution formulation:
Test compound 0.3~
Acetone 55.9%
Water 43.8
Biological Testing
Compounds of this invention were tested as follows
for nematicidal and anthelmintic ac~ivity as dust
formulatlons (initial and residual activity) and as
acetone/water formulations (systemic activity). The
formulations are described above.
1. Initial Root-Knot Nematicidal Activity
The activity against root-knot nematode
(Meloidogyne incognita) was determined by incorporat-
ing the compound of the invention in nematode infested
soil at rates in the range of 10 ppm to 0.078 ppm of
compound. Several tomato or cucumber seedlings were
planted in the nematode in~ested soil. Two weeks
after planting the test pots were evaluated to ascer-
tain the degree of galling (swelling) on the roots of
the plants, indicating the control provided by the
test chemical.
The results expressed as percent control (deter-
mined by knot index) are set forth as averages in
~7~6~8
-33~
Table 2 (appended). Knot inlex is a numerical desig-
nation assigned at evaluation, having the following
meanings:
Knot Index Observations
0 No swellings - complete control
1 7S% less swellings than control plants
2 50~ less swellings than control plants
3 25% less swellings than control plants
4 About same as control plants -
no control.
Percent control is related to knot index as
follows:
Knot Index Percent Control
0 1~0
15 1 75
2 50
3 25
4 0
When the Knot Index is between 0 and 1 it is
further subdivided as follows to indicate how close
the percent control is to 75~ or 100%:
Knot IndexPercent Control
_
0.8 80
0.5 90
250.1-0.4 95_99
The results demonstrate that compounds of this
invention are highly effective against root-knot
nematodes at the application rates tested.
2. Residual Root-Knot Nematicidal Activity
_. _
The ability oE nematicidal compounds of the
invention to control root-knot nematode infestations
in soil over a period of time after treatment was
evaluated. ~ust formulations of test compound (5~)
were incorporated into soil samples at test compound
rates of 5 and 10 ppm. Subsequently, the treated soil
~277~8
34-
samples were inoculated with nematode inoculum at
weekly intervals, and Knot Index and Percent Control
determined on seedlings planted in the soil samples.
Specifically, soil treated with test compound was
5 placed in 7.6 cm diameter fiber pots and stored in a
greenhouse. At one, two and four weeks post-treat-
ment, the appropriate number of pots was infested with
root-knot nematode eggs and larvae. A cucumber or
tomato seedling was planted in each pot and evaluated
approximately two weeks after the soil infestation to
obtain the test results reported in Tables 3 and 3a
appended. The data shows that as compared with
untreated, but nematode-inoculated control soil,
planted with seedlings (which showed no nematode
control), substantial residual activity was exhibited
with most of the test compounds at the application
rates tested.
3. Stunt Nematode Test
The procedure was substantially the same as
in the initial root-knot nematode tests described
above except that rates of application of formulated
compound were 2.5 and 5 ppm in soil containing a corn
seedling, with subsequent inoculation of the soil with
combined larvae and adult stunt nematodes. The
samples were evaluated approximately four weeks after
infestation. The results (Tables 4 and 4a appended)
indicate good control at the test application rates as
compared with untreated samples where no control was
observed. "Percent control" means the difference
between average population counts between untreated
and treated samples, divided by average population
count of untreated sample, multiplied by 100.
4. Lesion Nematode Test
The procedure was substantially the same as
in the stunt nematode test described above except that
7~8
-35-
pea seedlings were used. The results (Table 5
appended) show good control with many of the compounds
at the application rates tested as compared with
untreated samples (no control). "Percent Control" is
defined as follows:
IPopulation Count Population Count
lin Check in Trtmt
t of Roots Wt of Roots
lin Check Plant in Treated Plants
10 1 I x 100
I Population Count in Check
I Wt of Roots in Check Plant _
5. Cyst ~ematode Test
The procedure was substantially the same as
described in the stunt nematode test except soybean
seedlings were used. "Percent Control" (Table 6
appended) is as defined in the stunt nematode test
results. The data indicate good control by most of
the compounds at the application rates tested.
6. Soll Mobility
The ability of nematicidal compounds of the
invention to move through nematode-infested soil and
to control the nematodes was evaluated by incorporat-
ing 5% dust formulations of test compound at 30 ppm
rates into pots of root-knot nematode infested soil,
and subsequently eluting the soil with 15 cm of water
(equivalent to 15 cm of rainfall) into a series of two
or more pots of untreated, but nematode-inEested,
soil. Specifically, the pots were 8 cm diameter
plastic pots containing a lO cm layer of sand over
a coarse grade filter paper disc. Sufficient soil was
placed over the sand to fill the pots, and a second
filter paper disc was placed over the soil. Each test
compound-treated pot was nested over a series of two
or more pots containing untreated, but nematode-
~77668
-36-
infested soil, also containing sand filter paper discs
as described for the treated soil pots. Fifteen cm of
water was slowly dripped into the top pots and the
pots were allowed to drain for 16-18 hours to remove
excess water. The top filter of each pot was then
removed and the pots were planted with a cucumber or
tomato seedling. The seedlings were evaluated
approximately two weeks after planting to give the
test results reported in Tables 7 and 7a appended.
The data indicate good soil mobility and nematicide
control at the application rates tested as compared to
untreated systems which showed no nematode control.
"Knot Index" and "Percent Control" are as defined in
the initial root-knot nematode tests above.
8. Systemic Activity
Compounds of the invention were tested for
basipetal systemic activity against the root-Xnot
nematode. In this test, tomato plants are grown in
10.2 cm diameter fiber pots containing steam-pas-
20 teurized soil mix (50% soil, 50% sand) until 4-6 true
leaves appear. Three of the pots are then placed on a
turntable in a spray hood and the plants sprayed with
50 ml of water/acetone solution containing the test
compound. The soil surface is covered during the
spraying to prevent spraying of the soil. After
treatment, the potted plants are placed in a lighted
drying chamber. The plants are then grown in a growth
chamber at 25C for three days and inoculated with a
standard nematode culture by incorporating the
inoculum into the top cm of soil in the pots. The
plants are returned to the growth chamber for about
two weeks at which time the pots are allowed to dry
until the plants begin to wilt. The roots are shaken
free of soil and the degree of galling (swelling)
noted as compared to galling of untreated control
~;~7~76613
-37-
plants. The results are expressed as Knot Index and
Percent Control as defined in the initial root-knot
nematode activity tests reported above in Table 2.
Table 8 appended reports the test results. The data
5 indicate that many of the compounds exhibited good
systemic nematicidal activity at the application rates
tested as compared with untreated plants wherein no
nematicidal activity was evident. Systemic nema-
ticidal activity of any substantial degree is highly
unusual and desirable and i5 not available from any
commercial nematicides.
9. C~ Elegans Nematode Screening Test and
Evaluation
This in-vitro test using the free-living
nematode Caenorhabditis elegans, is a modification of
the assay developed by Simpkin and Coles, J. Chem.
Tech, Tiotechnol, 31:66-69 (1981). In this ~est,
nematicidal activity is evaluated by placing a suspen-
sion of C. elegans nematodes in a medium containing a
food source ~E. coli) and a candidate nematicide at
test rates of 5.0-0.156 ppm. One milliliter of a test
medium consisting of 5 mg ampicillin, 10,000 units of
mycostatin and 10 ml of a dense suspension of Escheri-
chia coli per l00 ml of a buffer solution, was
pipetted into each well of a 24-well microtiter
plate, The candidate nematicide, suspended at the
appropriate concentration in dimethylsulfoxide, was
added to the wells in 2,5 1 volume~. Each rate of
application was replicated two to three times. After
thorough mi~ing of the contents of each well, 50 to
100 1 of a nematode suspension in a buffer was added
so that each well received 10-15 nematodes. After the
nematodes were added, the microtiter plates were
incubated at 20C for 5-6 days.
~277G68
-38
The effect of the candidate nematicide on the
survival and the reproduction of C. elegans was then
evaluated by comparison of the level of population
developed in the treated wells with that in untreated
5 wells. Specific effects on population development,
such as reduced egg hatch or molting disruption, were
noted if they were evident. Tables 9 and 10 appended
show high activity test results for many compounds of
the invention at the application rates tested.
- 39
Table I
F2C=C-CH2CH2X-R
Cmpd. No. X R Empirical Formula
S ~ C7H8F3Ns2
~1/5
2 S ~ ~ SCH3 C7H7F3N2S3
3 0 C(O)CF2CF2cF3 CgH4F1002
4 0 4-chlorobenzoyl CllHgclF302
N 1 CgHgF3N02
6 N =C=S CsH4F3NS
7 C~2 OH C5H7F30
8 S Cl C6H4ClF3N2S2
1155W30113Wjd-l
-
~2~
~ 40
Table 1 ~Continued)
Cmpd. No. X R _ Empi rical Fc~rmula
9 S N~NI C6H~BrF3N2S2
1-- C8H 7 F 3 S 2
/~
11 -H cls ,1~! cl3HllF3S2
N- -iN
12 S )~S~\ C14EI21F3N2S3
SC (CH3) 2CH2C (CH3) 3
N N
13 S ~ F Cl~H18F6~2S3
~` S ~SCH2C~12C = CF2
14 S ~ CgH7F3~2S3
C 2 - CH
N - N
)~ S ~I\SCH ~I C gH gF 3N2S 3
N -N
16 S ~ C12HgF3N20S
~O~
17 S N N Cl C12El8clP3N2os
,~ .
1 1 5 5W3 01 1 3Wmd- 2
~;~7~6~
- 41 -
Table 1 (Continued)
Cmpd. No. X R Empirical Formula
18 S -CH2co2cH2cH2cl CF2 CloHloF6o2s
19 O -C(0)CF2CF3 (CH3C~2)2 CllHl4F803
20 . O ~ ~ CgH6F3~OS
C (O)
21 O J r ~ CgH6F3NOs
-C (O) N02
22 O ~ ~ CgH7F3NO2
-C(0)-`N~
H
23 o -C(O)CH2S ~S] CgHloF3No2s2
24 \ S~ ~ CllH8F3NO3S
~Y
O O
1155W30113Wmd-3
~77~
Table la
F\ F
C = C(CH2)2SR wherein
R2s
R is ~S
Compound Empirical Formula
No. R2 M.P. (C)
___. _ _ _
25-CH2CH2CF=cF2 CloH8F6N2s3
liquid
264-nitrophenylmethyl C13Hl oF 3~302S3
liqui~
N - N
R is R3 ~ ~
27 -SCH2CH2F CgH~F4N2S3
liquid
28 -SCH2CH2C--N CgH8F3N3S3
li~uid
29 -SC3H7 CgHllF3N2S3
liquid
-SCH~CH3)2 CgHllF3N2s3
liquid
31 -SCH2C~=CH2 CgHgF3N2S3
liquid
32 -SCH2~ C13HllF3N2S3
liauid
33 -SCH2~, 4-bromo Cl3HloBrF3N2s3
S (49-51)
1155W30113Wjd-4
~2~ i8
- 43 -
Table la (Continued)
Compound Empirical Formula
No. R3 M.P. ( C)
34 -SCH2~, 2-fluoro C13HloF4N2s3
liquid
-SCH2~, 4-nitro C13HloF3N3o2s3
liquid
36 2-thienylmethylthio CllHgF3N2S4
liquid
37 -~,4-chloro C12H8ClF3N2S2
S (68-69)
R4
~---N
R is
R4
38 -CH2~, 4-fluoro C13HloF4N2os
liquid
39 -~, 4-chloro C12H8clF3~2Os
S (49-52)
-~, 4-nitro C12H8F3N3O3s
S (6i-64)
R is Rlo~
R5
41 -C3H7 CgHllF3N2Os
liquid
42 -CH2~ C13HllF3N2Os
liquid
43 -CH2~, 4-chloroCl3HloclF3N2os
liquid
1155W30113Wjd-5
~Z77~ 8
~ 44 ~
Table la (Continued)
Co~pound Empirical For~ula
No. R5 M.P. (C)
44 -CH2~, 2-fluoro C13HloF4N2os
liquld
-CH2~, 4-Eluoro Cl3HloF4~20S
liquid
46 -CH2~, 2,4-difluoro Cl3H9F5N2os
liquid
47 CH2CH2~ Cl4Hl3F3N20s
liquid
48 -~, 3-chloro Cl2H8c(F3)2os
49 -~, 4-bromo S (58-61)
-~, 4-fluoro Cl2H8F4N2~S
S (56-59)
1155W30113Wja~6
Table lb
Cmpd. ~o. Name
1 2-(3,4,4-trifluoro-3-butenylthio)-4~5-
dihydrothiazole
2 2-methylthio-5-(3,4,4-trifluoro-3-butenyl-
thio)-1,3,4-thiadiazole
3 (3,4,4-trifluoro-3-butenyl) heptafluoro-
butyrate
4 . (3,4,4-trifluoro-3-butenyl) 4-chlorobenzoate
S N~(3,4,4-trifluoro-3-butenyl)succinimide
6 (3,4,4-trifluoro-3-~utenyl) isothiocyanate
7 4,5,5-trifluoro-4-penten-1-ol
8 3-chloro-5-(3,4,4-trifluoro-3-butenylthio)-
1,2,4-thiadiazole
9 3-bro~o-5-(3,4,4-trifluoro-3-butenylthio)-
1,2,4-thiadiazole
2-(3,4,4-trifluoro-3-butenylthio)thiophene
11 2-(3,4,4-trifluoror3-butenylthiomethyl)-
thianaphthene
12 2-(1,1,3,3-tetra~ethylbutylthio)-5-(3,4,4-
trifluoro-3-butenylthio)-1,3,4-thiadiazole
13 2,5-di(3,4,4-trifluoro-3-butenylthio)-1,3,4-
thiadiazole
14 2-propargylthio-5~(3,4,4-trifluoro-3-
butenylthio)-1,3,4-thiadiazole
i5 2-cyclopropylmethylthio-5-(3,4,4-trifluoro-
3-butenylthio)-1,3,4-thiadiazole
16 2-phenyl-5-(3,4,4-trifluoro-3-butenylthio)~
1,3,4-oxaaiazole
17 2-(4-chlorophenyl)-5-(3,4,4-trifluoro-3-
butenylthio)-1,3,4-oxadiazole
18 (3,4,4-trifluoro-3-butenyl) (3,4,4-tri-
fluoro-3-butenylthio)acetate
1153~30113Wjd-l
- -4 6 -
Table lb (Continued)
Cmpd. No. Name
__
19 (3,4,4-trifluoro-3-butenyl) pentafluoro-
propionate, mono diethyl etherate
(3,4,4-trifluoro-3-butenyl) 2-thiophene-
carboxylate
21 (.3,4j4-trifluoro-3-butenyl) 5-nitro-2-
furancarboxyIate
22 (3,4,4-trifluoro-3-butenyl) 2-pyrrolecar-
boxylate
23 (3,4,4-trifluoro-3-butenyl) [2-(4,5-di-
hydrothiazolyl)thio]acetate
24 N-(3,4,4-trifluoro-3-butenyl)sacch~rine
3,5-di~3,4,4-trifluoro-3-butenylthio)-
1,2,4-thiadiazole
26 3-(4-nitrophenylmethylthio)-5-(3,4,4-tri-
fluoro-3-butenylthio)-1,2,4-thiadiazole
27 2-(2 fluoroethylthio)-5-(3,4,4-trifluoro-3-
butenylthio)-1~3,4-thiadiazole
28 2-(2-cyanoethylthio)-5-(3,4,4-trifluoro-3-
butenylthio)-1,3,4-thiadiazole
29 2-propylthio-5-(3,4,4-trifluoro-3-butenyl-
thio)-1,3,4-thiadiazole
2-(1-methylethylthio)-5-(3,4,4-trifluoro,3-
butenylthio)-1,3,4-thiadiazole
31 2-(2-propenylthio)-5-(3,4,4-trifluoro-3-
butenyl.thio)-1,3,4-thiadiazole
32 2-phenylmethylthio-5-(3,4,4 tri~luoro-3-
butenylthio)-1,3,4-thiadiazole
33 2-(4-bromophenylmethylthio)-5-(3,4,4-tri-
fluoro-3-butenylthio)-1,3,4-thiadiazole
34 2-(2-fluorophenylmethylthio)-5-(3,4,4-tri-
fluoro-3-butenylthio)-1,3,4-thiadiazole
1153W3011~jd-2
~27~76~
Table lb (Cbntinued)
C~pd No. Name
2-(4-nitrophenylmethylthio)-5-(3,4,4-tri-
fluoro-3-butenylthio)-1,3,4-thiadiazole
36 2-(2-thienylmethylthio)-5-(3,4,4-trifluoro-
3-butenylthio)-1,3,4-thiadiazole
37 2-(4-chlorophenyl)-5-(3,4,4-trifluoro-3-
butenylthio)-1,3,4-thiadiazole
38 3-(4-fluorophenylmethyl)-5-(3,4,4-trifluoro-
3-butenylthio)-1,2,4-oxadiazole
39 3-(4-chlorophenyl)-5-(3,4,4-trifluoro-3-
butenylthio)-1,2,4-oxadiazole
3-(4-nitrophenyl)-5-(3,4,4-trifluoro-3-
butenylthio)-1,2,4-oxadiazole
41 2-propyl-5-(3,4,4-trifluoro-3-butenylthio~-
1,3,4-oxadiazole
42 2-phenylmethyl-5-(3,4,4-trifluoro-3-butenyl-
thio)-1,3,4-oxadiazole
43 2-(4-chlorophenylmethyl)-5-(3,4,4-trifluoro-
3-butenylthio)-1,3,4-oxadiazole
44 2-(2-fluorophenylmethyl)-5-(3,4,4-trifluoro-
3-butenylthio)-1,3,4-oxadiazole
2-(4-fluorophenylmethyl~-5-(3,4,4-trifluoro-
3-butenylthio)-1,3,4-oxadia~ole
46 2-(2,4-difluorophenylmethyl)-5-(3,4,4-tri-
fluoro-3-butenylthio)-1,3,4-oxadiazole
47 2-(2-phenylethyl)-5-(3,4,4-trifluoro-3-
butenylthio)-1,3,4-oxadiazole
48 2-(3-chlorophenyl)-5-(3,4,4-trifluoro-3-
butenylthio)-1,3,4-oxadiazole
49 2-(4-bromophenyl)-5-(3,4,4-trifluoro-3-
butenylthio)-1,3,4-oxadiazole
2-(4-fluorophenyl)-5-(3,4,4-trifluoror3-
butenylthio)-1,3,4-oxadiazole
1153W30113Wjd-3
~27~
~ 48 -
Table lb (Continued)
Cmpd. No ~ame
230 2-(3,4,4-trifluoro-3-butenylthio)thiazole
231 2-(2 3,3-trifluoro-2-propenylthio)thiazole
232 2-(4,4-di~luoro-3-butenylthio)thiazole
233 2-(3,3-difluoro-2-propenylthio)thiazole
234 2-(4,4-dichloro-3-butenylthio)thiazole
235 2-(3,3-dichloro-2-propenylthio)thiazole
236 2-(4,4-dibromo-3-butenylthio)thiazole
237 2-t3,3-dibromo-2-propenylthio)thiazole
238 2-(2,3,3-trichloro-2-propenylthio)thiazole
239 2-~3,4,4-trichloro-3-butenylthio)thiazole
240 2-(2,3,3-tribromo-2-propenylthio)thiazole
241 2-~3,4,4-tribromo-3-butenylthio)thiazole
1153W30113Wjd-4
~2~
.~ g .
Table lc
Sub ituted Thiadiazolyl/~xadiazolyl Compounds
F~ I
C = C(CH2)2SR wherein:
F
R S
~r~ N
R is ~S
Compound
No. R2_ M.P._(C)
51 phenylmethyl- liquia
52 4-chlorophenylmethyl- liquid
53 4-chlorophenylthiomethyl- liquid
~~----N
54 R is 11 ~ liquid
~S
R is ~ 1 liquid
~SlS/~
~N02
1153W30113Wjd-25
~2~
- 50 - ~
Table lc (Continued)
__
N3 N
R3
Compour,d S
3 ~ P (C)
~lo. R
56 4-chlorophenylmethyl- liquid
57 -SCH2CH3 liquid
58 -SCE~2CF3 liquid
5 9 -SC4Hg l i qu i d
6 0 - SC~ ( C~13 ) C2H5 l i qu i d
61 -SC~2CH ( CH3 ) 2 liquid
6 2 -S ( CH2 ~ 2CClFCBrF2 1 i qui d
63 S~CH2)7 3 liquid
64 s~CH2)loc 3 liquid
--S ( CH2 ) 2CH=CH2 1 iquid
66 --ScH(cH3)cH=cH2 liquid
6 7 --S CH2 C ( ~H3 ) C1~2 1 i qu i d
68 --SCH2CH=CHCH3 - liyuid
69 S ( CE~ ) 3C 2
-SCH2CH=C ( CH3 ) 2 1 iquid
71 --SCH2C ( Cl ~ =CH2
7 2 -SCH2C ( Br ) =CH2 1 i qu id
73 -SCH2CH=C(Br)2
74 -S(CH2)2C~=C(Cl)- solid ~55 )
-SC~2C=~C=N liquid
76 S(C~2)3 . liquid
77 -S(CH2)4C=~
liqL~id
78 H2 ~ CH3
, H3C
79 2,4-dimethylphenoxymethyl- liquid
3-chlorophenylmethylthio- liquid
81 4-chlorophenylmethylthio- solid (38)
1153W30113Wjd-28
~2~i6~3
- 51
Table lc (Continued
Compound
~ _ 3_ M.P. (C)
82 3~4-dichlorophenylmethylthio- liquid
83 2,6-dichlorophenylmethylthio- liquid
84 2-bromophenylmethylthio- liquid
3-brom~phenylmethylthio- liquid
86 3,5-dibromophenylmethylthio- liquid
87 3-fluorophenylmethylthio- liquid
88 4-fluorophenylmethylthio- liquid
89 2,4-difluorophenylmethylthio- liquid
2,5-difluorophenylmethylthio- liquid
91 3,4-difluorophenyl~ethylthio- liquid
92 2,6-di~luorophenylmethylthio- liquid
93 2,3,4,5,6-pentafluorophenylmethylthio- liquid
94 2-chloro 6-fluorophenylmethylthio liquid
2-iodophenylmethylthio- liquid
96 2-methylphenylmethylthio- liquid
97 3-methylphenylmethylthio- liquid
98 2-trifluoromethylphenylmethylthio- liquid
99 3-trifluoromethylphenylmethylthio- liquid
100 4-trifluoromethylphenylmethylthio- liquid
101 3-methoxyphenylmethylthio- liquid
102 4-methoxyphenylmethylthio- solid (42-44)
103 4-trifluoromethoxyphenylmethylthio- liquid
104 2-cyanophenylmethylthio- liquid
105 3-cyanophenylmethylthio liquid
106 4-cyanophenylmethylthio- solid (51-57)
107 2-nitrophenylmethylthio- liquid
108 3-nitrophenylmethylthio- liquid
109 2-ohloro-4-nitrophenylmethylthio- liquid
110 4-chloro-2-nitrophenylmethyl.thio- liquid
111 2-Eluoro-4-nitrophenylmethylthio- liquid
112 2-methyl-3-nitrophenylmethylthio- liquid
1153W30113Wjd-27
- 52 -
Table lc (Continued
Cbmpound
No. R3 M.P. ( C)
113 2-nitro-5-methylph~nylmethylthio- liquid
114 2-methoxy-5-nitrophenylmethylthio- liquid
,115 3,5-dinitrophenylmethylthio- liquid
116 4-phenylphenyl,methylthio- solid (62)
117 2-methyl-3-phenylphenylmethylthio- solid
118 anthracine-9-ylmethylthio- liquid
119 5-chlorothien-2-ylmethylthio- liquid
120 2-methylthiazol-4-ylmethylthio- liquid
121 2,6-dichloropyridin-4-ylmethylthio, liquid
122 1,3-benzodioxol-5-ylmethylthio- liquid
123 phe~ylthiomethylthio- liquid
124 l-phenylethylthio- liquid
125 2-~4-nitrophenyl)ethylthio- liq~id
126 3-phenoxypropylthio- liquid
127 -NCC(O)CF3]~C2H5] liqu'id
128 -N[C(O)CH3][CH3] liquid
129 -N~C(0)CH3]~4-trifluoromethylphenyl~ solid
130 1,2-bis(4-chlorophenyl)urea- solid
131 -N~C(O)CF3][4-methoxypheny,l] solid
132 4-trifluoromethylphenylamino- solid
133 4-methoxyphenylamino- solid
134 4-~hloro~henylamino- solid
~ H3
135 -N[C(O) \ CH=CC12]~CH3] liquid
136 1-(4-trifluoromethylphenyl)-2-(4- solid
chlorophenyl)urea--
137 1-methyl-2-(4-~hlorophenyl)urea- . solid (141-2)
1153W30113Wjd-28
~771~3
- 53 -
Table lc (Continued)
Compound
No. R3_ M.P. (C)
138 -N[C(O)CH3][4-chlorophenyl] solid (84-85)
139 -N~C(O)CH3][4-fluorophenyl] solid (96-97)
140 -I:~C(0) ~ OE~ 12]C4-methoxy- liquid
phenyl]
141 4-nitrophenylamino- solid
142 1-ethyl-2-(4-chlorophenyl)urea- solid
143 -N[C(O)CH3][4-methoxyphenyl] solid
144 1-(4-fluoroph,enyl)-2-(4-chlorophenyl)- solid
urea
145 -NHC2H5 solid
146 4-fluorophenylamino- solid
147 -N~CH3~[2,4-dichlorophenylmethyl- liquid
carbonyl]
3 ~( 3
148 -~[C(0) CH=~X 12]~C2H5] liquid
149 4-bromophenylamino- solid
3 ~ 3
A
150 -N~C(0)- C:EI=CC12]C4-trifluoro- liquid
methylphenyl3
151 -NCC2H53[phenylmethoxycarbonyl] solid
152 -NCC(O)CE13]~C2H5] liquid
153 bromomethylthio- liquid
154 -N[C(O)C~131[4-bromophenyl] solid
1153W30113~nd-29
~L277~
- 54 -
Table lc (Continued)
Compound
No. _ 3_ M.P. 1C)
3 ~ 3
155 -N[C(O) CH=CC12]~4-nitrophenyl] solid
3 ~! 3
156 -N[C(O) ~ CH=CC12][4-fluorophenyl] liquid
157 -S(CH2)3CH2Cl semi-solid
158 -SCH2Cl liquid
159 -S(CH2)~Cl lqiuid
160 -S(CH2~2C~2Cl liquia
161 S(CH2)3CH2Br solid (67-69)
R is
N~
Co~urr~
~~ _ _ 4 M P. (C)
162 4-chlorophenylmethyl- liquid
163 4-methylphenyl- liquid
164 phenylmethyl- liquid
165 phenyl- liquid
166 2-chlorophenyl- liauid
1153W30113Wjd-30
. .
- 55
Compound
No. _ 4_ M.P. (C)
167 3-chlorophenyl- solid (48-51)
168 3-trifluoromethylphe~yl- solid (41-44)
169 4-methoxyphenyLmethyl- liquid
170 3-nitrophenyl- solid (69-71)
N - ~N
R5
Compound
No. _ 5_ M.P. (C)
171 -CH liquid
172 _~2H5 liquid
173 -CH(CH3)2 liquid
174 CH2CH(CH3)2 liquid
175 -C(CH3)3 liquid
176 ( 2)4 3 liquid
177 (CH2)16CH3 solid (45)
178 -C-C(CH2)4CH3 liquid
179 -CH2CH(CH3)CF3 liquid
180 2-chlorophenylmethyl- liquid
181 2-bromopnenylmethyl- liquid
182 4-bro phenylmethyl- solid (38-40)
183 2-methylphenylmethyl- liquid
184 3-methylphenylmethyl- liquid
185 2-bromo-4,5-dime~hoxyphenylmethyl- solid (54-59)
186 2-nitrophenylmethyl- liquid
187 4-nitrophenylmethyl- liquid
188 thien-2-ylmethyl- liquid
189 1,4-ben~odio~an-6-ylmethyl- liquid
1153W30113Wjd-31
- 56 -
Table lc (Continued)
C~mpound
No~ R5_ _P (C)
190 1,3-benzcdioxol-5-ylmethyl- solid (69-71)
/N _ N
191 \ N - 1N~ liquid
192 l-phenylethyl- liquid
193 2-(4-nitrophenyl)ethyl- liquid
194 2-(4-chlorophenyl)ethenyl- liquid
195 2-(4-bromophenyl)ethenyl- iiquid
196 2-(2-fluorophenyl)ethenyl- liquid
197 3-phenylpropyl- liquid
198 4-phenylbutyl- liquid
199 4-chlorophenoxymethyl- liquid
200 4-methylphenoxymethyl- liquid
201 3-methyl-4-chlorophenoxymethyl- liquid
202 4-nitrophenoxymethyl- liquid
203 1-(4-chlorophenoxy)ethyl- liquid
204 1-(4-methylphenoxy)ethyl- liquid
205 2-(4-chlorophenylthio)ethyl- solid (54-59)
206 1-(4-chlor.ophenoxy)prop~l- liquid
207 2-chlorophenyl- liquid
208 2-bro~phenyl- liquid
209 2,5-dichlorophenyl- liquid
210 4-(1-methylethyl)phenyl- liquid
211 2-methoxyphenyl- liquid
212 3-methoxyphenyl- liquid
213 4-methoxyphenyl- liquid
214 3,4-dimethoxyphenyl- solid (51)
215 4-nitrophenyl- solid (94-96)
216 2-aminophenyl- solid (57-61)
217 4-hydroxyphenyl- solid (96-101)
1153~30113W~d-32
~776~
- 57 -
Table lc (Continued)
Compound
No. R5_ M.P. (C)
218 4-acetyloxyphenyl- solid (63-66D)
219 4-(methylaminocarbonyloxy)phenyl- solid (108-111)
220 4-phenylphenyl- solid (49-52)
221 naphth-2-yl- solid (70-72)
222 naphth-l-yl- solid (68)
223 thien-2-yl~ liquid
224 furan-2-yl- . liquid
225 4-methyl-1,2,3-thiadiazol-5-yl- liquid
226 2-(4-chlorophenyl)ethyl- solid (43-45)
227 2-(2-chlorophenyl)ethyl- liquid
228 2-(4-fluorophenyl)ethyl- liquid
229 phenylmethyl liquid
1153W3011~jd-33
~`~
- 5~ -
Table ld (Cbntinued)
Polyhaloalkenylthio Thiazolyl Cbmpounds
N
F2C=CF (CH2) S--<
Compound
No. n ~I.P. (C)
230 2 liquid
231 1 liquid
1153W30113Wjd-36.
- 59 -
Table 2
Initial Activity Against the Root-knot Nematode
Application Percent
Compound No.Rate (ppm) Control
10. 0 100
5.0 99
2.5 99
5.0 100
2.5 100
1.25 99
0.625 100
0.313 99
0.625 100
0.313 98
0.156 95
0.07~3 56
2 10.0 100
5.0 96
2.5 96
5.0 99
2.5 95
1.25 86
~.625 84
0.313 38
3 10.0 95
5.0 83
2.5 71
4 2.5 99
1.25 56
0.625 44
0.313 38
2.5 81
1.25 70
0.625 6
0.313 0
6 10.0 100
5.0 78
2.5 38
7 10.0 79
5.0 44
2.5 25
1153W30113~jd--4
~2:7~7~;68
- 60 -
Table 2 (Continued)
ApplicationPercent
Compound No. Rate (ppm)Control
8 2.5 100
1.25 100
0.625 100
0.312 100
0.625 100
0.312 98
0.156 95
0.078 58
5.0 99
2.5 95
1.25 81
0.625 63
11 5.0 83
.5 78
1,25 67
0.625 0
12 2.5 100
1.25 79
0.625 69
0.313 8
13 2.5 99
1.25 99
0.625 96
0.313 71
0.625 95
0.313 79
0.156 13
0.078 0
14 2.5 83
1.25 25
0.625 0
0.313
2.5 100
1.25 97
0.625 31
0.313 6
115~30113Wjd-5
- 61 -
Table 2 (Contin~ed)
Application Percent
Compound No.Rate (ppm)Control
16 2.5 95
1.25 44
0.625 25
0.313 0
17 10.0 100
5.0 100
2.5 100
18 10.0 100
5.0 9g
2.5 96
5.0 80
2.5 69
1.25 38
0.625 19
0.313 0
19 10.0 69
5 0 75
2 5 58
2.5 98
1.25 63
0.625 6
0.313
21 2.5 64
1.25 31
0.625 8
0.313
22 2.5 96
1~25 44
0.625 0
0.313 0
23 10.0 96
5.0 84
2.5 76
24 10.0 78
5.0 63
2.5 19
1153W30113Wjd-6
àB
- 62 -
Table 2 (Continued)
AppLication Percent
Compound No.Rate (ppm)Control
2.5 99
1.25 97
.625 97
.312 78
26 2.5 86
1.25 63
.625 0
.312 0
27 2.5 100
1.25 99
.625 79
.312 31
28 2.5 100
1.25 98
.625 86
.312 81
29 2.5 99
1.25 99
.625 99
312 95
2.5 100
1.25 99
.625 99
.312 97
31 2.5 99
1.25 96
.625 7~
.312 69
.625 63
.312 44
.156 13
.078 0
32 2.5 100
1.25 99
.625 97
.312 78
1153W30113~jd-7
_ 63 -
Table 2 (Continued)
Applicat~onPercent
Compound No. Rate ~ppm)Control
32 cont'd .625 98
.312 69
.156 31
.078 8
33 2.5 97
1.25 84
.625 70
.312 38
3~ 2.5 100
1.25 99
.625 99
.312 86
.625 99
.312 86
.156 64
.078 38
2.5 100
1.25 98
.625 70
.312 67
.625 97
.312 51
.156 6
.078
2.5 100
1.25 99
.625 g6
.312 69
.625 99
.312 71
.156 25
.078 0
37 .625 0
.312 0
.156 o
.078
1153W30113Wjd-8
- 64 -
Table 2 (Continued)
Application Percent
Cbn~ound No.Rate (ppm) Control
38 2.5 98
L.25 96
.625 76
.312 63
39 2.5 100
1.25 98
.625 84
.312 19
2.5 100
1.25 99
.625 83
.312 19
2.5 99
1.25 84
.625 56
.312 0
41 2.S 100
1.25 100
,625 98
,312 76
.625 100
.312 97
.156 ~4
,078 31
42 2.5 100
1.25 98
.625 98
.312 86
.625 96
.312 83
.156 50
,078 38
43 .62~ 99
.312 78
.156 38
.078 13
1153W30113Wjd-9
- 65 -
Table 2 (Continued)
Application Percent
Compound No.Rate (FPm) Control
43 cont'd .5 99
2.5 97
1.25 56
.~25 13
44 2.5 98
1.25 86
.625 76
.312 50
2.5 99
1.25 99
.625 99
.312 95
46 2.5 99
1.25 98
.625 98
.312 85
47 2.5 1~0
1.25 96
.625 95
.312 95
48 2.5 9~
1.25 95
.625 78
.312 64
.625 87
.312 56
.156 38
.078 6
49 2.5 100
1.25 100
.625 100
.312 98
2.5 100
1.25 ~9
.625 gg
.312 78
1153W30113Wjd-10
- 6~ -
Table 2 (Continued)
Application Percent
Compound No. Rate (ppm) Control
230 2.5 100
1.25 100
0.625 81
0.313 44
1153W30113Wjd-12
- 67 -
Table 3
Residual Activity Against the Root-knot Uematode
Inoculation,
Cmpd. Application Weeks After Percent
No.Rate (ppm)TreatmentControl
1 10 1 98
2 44
4 0
1 g9
2 75 .
4 42
1 99
2 63
4 25
2 10 1 77
2 56
4 69
1 96
2 38
4 5~
1 98
2 38
~ 42
8 5
4 98
13 10 1 99
2 99
4 95
1 98
2 98
4 75
18 10 1 0
2 0
. O
21 5 1 13
aS o
1153W30113Wjd~11
68
Table 3a
Residual Activity Against The Root-Knot Nematode
5~ Dust - Application Rate: 5 ppm
Inoculation Percent
Co~pound No.Post-TreatmentControl
1 Week 100
2 ~eeks 97
4 Weeks 98
27 l Week 99
2 Weeks 97
4 Weeks 99
28 1 Week lO0
2 ~Jeeks 100
4 Weeks 8
29 1 Week 100
2 Weeks 98
4 Weeks 81
l Week 98
2 ~eeks 97
4 Weeks 83
31 l Week g7
2 Weeks 97
4 Weeks 95
32 1 Week 99
2 l~eeks 99
4 Weeks 82
1 Week 99
2 Weeks 99
4 Wee,ks 98
l '~eek 99
2 Weeks 96
4 Weeks 84
36 l Week 98
2 Weeks 99
4 Weeks 95
1153W30113Wjd-12
- 6 9 -
Table 3a ~Cbntinued)
Inoculation Percent
Compound No.P~st-TreatmentControl
41 1 Week 97
2 Weeks 63
4 Weeks 6
42 1 ~eek 99
2 Weeks 97
4 Weeks 0
43 1 Week 100
2 Weeks 100
4 Weeks 100
44 1 Week 100
2 Weeks 100
4 Weeks 100
1 Week 99
2 Weeks 100
4 Weeks 98
46 1 Week 99
2 Weeks 100
4 Weeks 99
47 1 Week 96
2 Weeks 25
4 Weeks 0
48 1 Week 97
2 Weeks 97
4 ~Jeeks 69
1153W30113Wjd-13
~ 70 -
Table 4
__
Initial Activity A~ainst the Stunt Nematode
Rate of Percent
Cbmpound. No. Application (ppm) Control
1 10 61
2 5 72
6 5 5
1153W30113Wjd-14
~ 71 ~
Table 4a
Initial Activity Against the Stunt Nematode
Application rate: 5 ppm
Percent Percent
Com_ und No. Control Compound No. Control
89 ~1 29
56 42 q6*
27 62 33
28 38 43 81
29 56 76
66 62
31 52 44 75
32 84 ~5 80*
33 52 46 65*
34 88 47 35
56 48 82
77 66
83 49 58
36 64 50 53
91
*Some phytotoxicity
1153W30113Wjd-15
72 ~
Table 5
Initial Activity Against the Lesion Nematode
Rate of Percent
Co~pound No. Application (ppm) Control
1 5 82
52
2.5 0
2 5 0
2.5 33
18 5 0
21 2.5 54
2.5 77
2.5 79
27 2.5 63
28 - 2.5 45
29 2.5 68
2.5 75
31 2.5 11
32 2.5 28
2.5 L5
1.25 o
.625 0
33 2.5 46
3~ 2,5 52
2.5 58
2.5 55
2.5 52
2.5 78
1153W30113Wjd-16
~ 73
Table 5 (Continued)
Rate of Percent
Compound No. plication ~ppm) Control
36 2.5 5g
2.5 19
1.25 2
.625 0
2.5 71
41 2.5 64
42 2.5 73
82
43 2.5 93
2.S 78
44 2.5 88
2.5 ~1
46 2.5 77
47 2.5 69
48 2.5 85
2.5 78
1153W30113Wjd-17
- 74 -
Table 6
Initial Activity Against the Cyst Nemat~de
Rate of Percent
_npound No. Application (ppm) Control
1 5 94
791
2 5 70
- 8 5 92
18 5 8
21 2.5 33
83
27 5 57
31 5 7
32 5 ~6
78
0
36 5 37
41 5 0
42 5 30
44 5 5g
48 5 ~6
~ h~le cysts rather than homogeniæed cysts were used.
1153W30113Wjd-18
:
Z77~
~ 7 5 -
Table 7
Soil Mobility Evaluations Against the Root-knot Nematode
Location
Application of TestPercent
Cmpd. ~o. Rate (ppm) Container Control
1 30 Top 97
Middle 9S
Bottom 85
1 (~rOp) 100
2 100
3 100
4 99
97
6 (Bottom) 42
Top 100
Middle 98
Bottom 99
2.5 Top 100
Middle 100
Bottom 100
1.25 Top 100
Middle 99
Bottom 96
0.625 Top 97
Middle 98
Bottom 77
0.313 Top 77
Middle 75
Bottom 25
2 30 Top 42
~iddle 33
Bottom 25
8 5 Top 100
Middle 100
Bottom 96
18 30 Top 33 ,
Middle 25
Bottom 25
21 30 Top 100
Middle 96
Bottom 99
1153W30113W~d-19
.
3E~8
7 6
Table 7a
Soil Mobility Evaluations Against The Root-Xnot
Nematode - 5% Dust, Application Rate: 5 ppm
Location of Percent
Compound No. Test Container Control
2S TOP 100
MIDDLE 69
BOTTCM 8
27 TOP 99
MIDDLE 100
BOTTOM 100
28 TOP 68
MIDDLE 83
BOTToM 68
29 TOP 98
MIDDLE 81
BCnl~l 50
TOP 99
MIDDLE 100
BOTTQM 17
31 TOP 98
MIDDLE 86
BOTToM 50
32 TOP 99
MIDD~E 75
BOTTOM 25
34 TOP 98
MIDDLE 67
BCn~3M 8
TOP 99
MIDDLE 50
BOTTOM o
36 'roP 97
MI W LE 17
BCTTOM 0
38 TOP 0
MIDDLE 0
BCTrOM 0
115~30113Wjd-20
- 77 -
Table 7a (Continued)
Location of Percent
C~pound ~o. Test Container Control
41 TOP 97*
MIDDLE 95*
BOTTOM
42 TOP 100
MIDDLE 100
BOITOM i00
43 TOP 99
MIDDLE 97
BOTToM 81
44 TOP 100
MIDDLE lC0
~OTT3~ . 96
TOP 98
MIDDLE 97
BOTTOM 83
46 TOP 96
MIDDLE 97
B0~TOM 78
47 TOP 98
MIDDLE 96
BOTrCM 58
48 TOP 50
MIDDLE 0
B~ITOM 0
TOP 96*
MIDDL~, 75
BOTTCM 42
49 TOP 99
MIDDLE 71
BOTTCM 8
TOP 98
MIDDLE 71
BO~OM 33
*Some phytotoxicity
1153W30113Wjd-21
7 8
Table 8
Systemic Activity Against the Root-knot Nematodes
Application Test Percent
Cmpd. No.Rate (ppm) Control
12 2000 73, 99
1000 42, 9S
13 2000 79
2000 83
16 2000 67
17 2000 42
31 2000 71
1250 o
32 2000 25
33 2500 17
2000 0
36 2000 97
1250 17
41 2000 17
48 2000 50
2500 33
1153W30113Wjd-22
i'~C~
79
Table 9
Screen Against C. Elegans - Rate: 5 pp~
Percent
Inhibition of Percent
Compound ~o.ReproductionMortality
100 75
26 100 100
27 38 0
31 38 0
32 100 100
33 100 100
34 100 25
100 100
37 100 100
39 100 100
43 100 25
44 25 0
0
48 100 88
1153W30113Wjd-23
~ ` ~
- 80
Table 10
Evaluations Against C. Elegans
Percent
RateInhibition ofPercent
Compound No. (PPM)Reproduction Mortality
100 100
2.5 58 0
1.25 17 0
26 5 100 100
2.5 100 100
1.25 100 100
32 5 100 8
2.5 42 o
1.25 25 0
33 5 100 100
2.5 100 100
1.25 100 100
34 5 100 100
2.5 100 100
1.25 o 0
100 100
2.5 100 100
1.25 100 100
37 5 100 100
2.5 100 100
1.25 100 100
39 5 100 100
100 100
2.5 100 100
2.5 100 100
1.25 100 67
1.25 100 58
100 100
2,5 100 100
1.25 100 100
43 5 100 83
2.5 100 67
1.25 42 0
48 5 100 100
2.5 25 0
1.25 17 0
1153W30113Wjd-24