Note: Descriptions are shown in the official language in which they were submitted.
PATENT
Case D 6935/7259
INSECTICIDAL, PEPPER EXTRACT
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to extracts from
pepper having insecticidal activity, to a process for
their preparation, and to a method for their use as
insecticides.
2. Description of Related Art
Although effective, commercial insecticides based
on syntheti~ chemicals are encountering mounting criti-
cism in many countries due to their synthetic origin~Because of this, increasing interest is being shown in
natural vegetable ingredients having insecticidal
activity. The most well known example of this is
pyrethrum from Chrysanthemum cinerariaefolium.
In recent years, it has also been found and
reported that black pepper or rather an extract thereof
is toxic to numerous insects. Originally, it was
assumed that the sharp-tasting principle of the pepper,
piperine or a derivative thereof was responsible for
the insecticidal effect. In later work, however, it
was shown that it is not the piperine, but other alka-
loids of pepper which exhibit the insecticidal activity
5~
(Su and Horvat, Agricult. Food Chem. 29, 115 (1981)).
These authors extracted ground black pepper with ace-
tone and isolated insecticidally active alkaloids con-
taining amide groups from the extract by
chromatographic methods. Black pepper is the dried,
unripe fruit of the tropical plant Piper nigrum L and
contains approximately 5 to 10% of piperine and other
sharp-tasting alkaloids, 1 to 2.5~ of ethereal oils and
small quantities of other alkaloids which are not
included among the sharp components.
In addition, the aromatic constituents of spices,
for example black pepper, have previously been
extracted with a gas supercritical in temperature and
pressure, for example CO2, and the extract separated
by pressure and/or temperature variations (U.S. Patent
Nos. 4,123,559 and 4,198,432). Spice extracts can also
be obtained, for example from black pepper, by removing
the ethereal oils acting as aroma carriers in a first
stage using liquid subcritical CO2 and the components
acting as flavor carriers in a second stage with
supercritical CO2, separating the extracts by tem-
perature and/or pressure variations and optionally
mixing them together (U.S. Patent No. 4,490,398). In
both cases, however, the object of the processes is not
to obtain insecticidally active extracts from black
pepper, but instead to produce flavorings and perfumes
for the food and cosmetic industry.
DESCRIPTION OF THE INVENTION
Other than in the operating examples, or where
otherwise indicated, all numbers expressing quantities
of ingredients or reaction conditions used herein are
to be understood as modified in all instances by the
term "about."
78~
It has now been found that an insecticidally
active, non-sharp-tasting fraction can be extracted
from black pepper using compressed CO2 and can be
obtained in pure form under suitable process con-
ditionsO In this extraction and recovery process, it
is important to ensure that the sharp-tasting consti
tuents, particularly the piperine, are not enriched and
separated during the process because/ in the practical
application of the insecticide, these constituents can
give rise to irritation of the mucous membrane of
people unintentionally or unavoidably coming into con-
tact with the insecticide. In addition, an extraction
and recovery process of the above type must be inex-
pensive and very simple to carry out.
The present invention relates in part to a process
for preparing a pepper extract having insecticidal
activity comprising the steps of:
a) extracting black pepper in ground form with CO2
at a temperature in the range of 30 to 70C and a
press~re in the range of 150 to 500 bar;
b) removing sharp tasting fractions from the resulting
extract in a first expansion step at a temperature
in the range of 25 to 35C and at a pressure in the
range of 70 to 150 bar;
c) separating an o;ly fraction containing the
insecticidally active components as well as most of
the essential oils present in the pepper in a
second expansion step at a temperature in the range
of 15 to 30C and a pressure lower than the
pressure in step b) in the range of 40 to 70 bar;
d) removing the essential oils from the insecticidally
active components in the oily fraction by steam
distillation.
The process can also be carried out by extracting
the black pepper under the conditions of step b) with
C2 at 25 - 35C/70 - 150 bar and separating an oily
fraction therefrom by expansion at 15 - 30C/40 - 70
bar in accordance with step c) and then further
treating the oily fraction obtained in accordance with
d). In this process variant, the sharp-tasting consti-
~2~8S~;~
tuents remain in the extraction residue and can
optionally be recovered therefrom by another extraction
under the conditions of step a).
In another embodiment of the process, the carbon-
carbon double bonds in the insecticidal product of stepd) are hydrogenated in another step e). It is possible
in this way to increase the insecticidal activity.
Hydrogenation is carried out by methods known for
hydrogenating carbon-carbon double bonds, preferably
under pressure in the presence of metal catalysts.
The present invention also relates to the use of
the process products as insecticidal components and to
insecticides containing these components.
The insecticidal components obtained in steps d)
and e) are light-colored, generally yellow, oils which
are not volatile with water vapor and which are highly
effective against hygiene pests and insects, such as
flies, midges, cockroaches, house crickets, silver-
fish, ants, earwigs, wood lice, fleas, lice and bugs,
wasps, plant pests, such as leaf lice, locusts, but-
terfly larvae, beetles and their larvae, stored-food
and material pests, such as meal beetles, grain
weevils, saw-toothed grain beetles, golden spider
beetles, bread beetles, bean weevils, cereal, meal and
dried-fruit moths, clothes moths, carpet beetles,
larder beetles, bacon beetles, and dust lice. In par-
ticular, the insecticidal components have been found to
show a strong synergistic effect in combination with
pyrethrum and with synthetic pyrethroids which enables
considerable savings of these relatively expensive
materials to be made and/or shorter destruction times
to be obtained. The insecticidal components of the
invention are combined with pyrethrum or with synthetic
pyrethroids in a ratio by weight of from 1:0.1 to 1:50
and preferably in a ratio by weight of from 1:0.2 to
1:10.
Even the oily fraction obtained in step c) shows
insecticidal properties and can be used as an active
component in insecticides without any need for further
work up. However, its insecticidal activity is
distinctly lower than that of the components obtained
in step d) or e). In addition, the high percentage of
ethereal oils may be undesirable due to their odor and
the danger of contamination in practical application.
The ethereal oils separated off by distillation
with steam in step d) and the sharp-tasting consti-
tuents of the black pepper obtained in step b) can be
used in known manner for the production of flavoring
concentrates for the food industry and for fragrance
compositions for cosmetic purposes and the like.
The active components can be used in typical
insecticide formulations, for example as solutions,
emulsions, suspensions, powders, aerosols, foams,
pastes or granulates. The formulations are prepared in
known manner, for example by mixing the active com-
ponents with diluents, such as liquid solvents, and/or
solid carriers. Surfactants, i.e. emulsifiers and/or
dispersants and/or foam-generating agents, can addi-
tionally be used in the formulations. The use of auxi-
liary solvents, for example where water i5 used as
diluent, is also possible. The formulations can also
contain sticking agents, dyes and, in the case of aero-
sols, propellent gases. They can also be combined with
other known insecticides and/or synergists, more espe-
cially pyrethrum or synthetic pyrethroids, and mixed
with fungicides, acaricides, repellents, growth promo-
ters and plant nutrients. Formulations such as these
can contain up to 80~ by weight and preferably from
0.02 to 50% by weight of one or more of the insec-
ticidal components from pepper prepared as disclosed
above.
~Z78~2
The invention will be illustrated but not limited
by the following examples.
EXAMPLE 1
Recovery of the insecticidal component
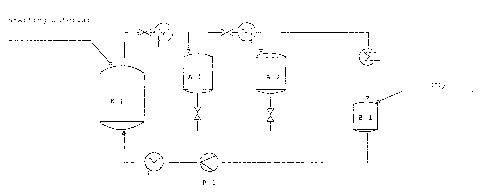
The high-pressure extraction described below was
carried out in the installation shown in Figure 1. To
this end, the extraction vessel E 1 was filled with the
digested starting material, the installation was purged
with CO2 and the extraction conditions established by
compression and tempering of the CO2. Separation from
the supercritical gas was carried out by expansion in
two pressure stages in the two separation vessels A 1
and A 2. The CO2 was reliquefied, collected in the
buffer vessel B 1 and recompressed to extraction con-
ditions in the diaphragm piston pump. The installation
was operated on the batch principle. On completion of
extraction, the extract and extraction residue were
removed from the high-pressure vessels.
Startinq material: 20 kg of ground black pepper from
Lampong: grain fraction 200 to 400 u
Step a): Extraction
Pressure 300 bar, temperature 50C, time 3 h,
C02-circulation
10 kg/kg starting material/hour.
Step b): Separation, first expansion stage (Fig. 1, A 1)
Pressure 80 bar, temperature 35C
1450 g of yellow, solid powder (anhydrous)
Step c): Separation, second expansion stage (Fig. 1 A 2)
Pressure 55 bar, temperature 20C
760 g oE yellow oil (anhydrous)
~7E~5~2
The extract from the second expansion stage (c)
was dehydrated in a continuous centrifuge and was
suitable for use as an insecticide without any further
treatment.
Step d): Distillation with steam
The further purification and recovery of the
insecticidal components were carried out by distilla-
tion with steam as follows:
115 g of oil from the second expansion stage were
initially introduced in 400 ml of water and subjected
to distillation with steam for 4 hours. 60.5 g of
yellow-green pepper oil were separated from the
distillate.
The distillation residue was digested while
stirring ~magnetic stirrer) with 1 liter of ether and
the ether extract separated off. The ether-soluble
insecticidal components (34 g) were obtained as a
golden-yellow oil by evaporation.
EX~MPLE 2
Recovery of a hydrogenated insecticidal component
An oily product was prepared as in Example 1,
steps a) to d).
Step e)
10 g of this oil were dissolved in 25 g of metha-
nol and 1.0 g of palladium carbon (10.15% by weight Pd)
was added, followed by hydrogenation for 20 hours at
room temperature under a hydrogen pressure fo 50 bar.
Filtration and removal of the solvent left 9~3 g of a
pale yellowish oil characterized by a pleasant odor.
~78~
EXAMPLE 3
Testing of insecticidal activity
To test their insecticidal activity, the substan-
ces obtained in accordance with Example 1, process step
c) or d), were diluted with acetone and then introduced
in a quantity of 1.5 mg into lO cm diameter petri
dishes and, after evaporation of the solvent, lO female
flies (Musca domestica) were placed in the covered
dishes. The test was repeated three times; the
following average destruction rates were obtained:
¦ ¦Destruction rate in ~ after minutes ¦
I 1 15_1 20_1_ 30 1 _35 1 --45
¦Substance from
¦ process step c) ¦ 30 ¦ 47 ¦80 ¦ 90 ~_ lO0
¦Substance from
¦ process step d) ¦ 40 ¦ 53 ¦87 ¦ 100
To test the synergistic effect, 1.5 mg of the
substance from process step d) and 0.03 mg of pyrethrin
were tested against resistant flies by the same method,
first individually and then in combination. The
following values were obtained:
-~- ¦ Destruction rate in % after mlnutes¦
I 1 25 1 35_ 1 40 1 50 l 1201150
¦Substance from
¦ process step d) ¦ _~ ¦ ~ ¦ __- ¦ 50 ¦100 ¦ ~
¦Pyrethrin _~ - ¦ 50 ¦ - I _ ¦ - ¦100 ¦
¦Pyrethrin + sub- ¦
¦ stance from
Lprocess step d) ¦ 50 ¦ 90 ~ _100 ¦ -_~
~z~
EXAMPLE 4
Testin~ of insecticidal activity
The substances emanating from process steps d) and
e) of Example 2 were diluted with acetone and intro~
duced in quantities o~ 0.15 mg into 10 cm diameter
petri dishes. After evaporation of the solvent, 10
female flies (Musca domestica) were placed in the
covered dishes. The test was repeated three times and
produced the following average destruction rates:
- '
¦¦Destruction rate in % after minutes¦
I _ 1 25 1 _ 40 1 60 1 90 1 120
¦Substance from
¦ process step d) ¦ - ¦ 3 ¦ 40 ¦ 67_~ 73 _ ¦
¦Substance from
¦ process step e) ¦ 10 ¦ 33 ¦ 73 ¦ 93 ¦ 100
To test the synergistic effect, pyrethrin (0.03
mg) was tested by the same method both on its own and
in combination with 0.15 mg of the substances from pro-
cess steps d) and e) of Example 2. The tests were
carried out three times and produced the following
average destruction rates:
_ _ _ __~
¦ ¦ Destruction rate in % after minutes¦
10 1 20 1 40 1 90 1 150
PYrethrin I _ I _ ¦ 73 ¦ 80 ¦ 87
,
¦Pyrethrin + sub ¦
¦ stance from I I I I t
¦ step d) I _ I ~ ¦ 90 ¦ 100 ¦~
¦Pyrethrin + sub- ¦
¦ stance from
¦ step e) ¦ 50 ¦ 100