Note: Descriptions are shown in the official language in which they were submitted.
lZ7~ 66
With the high cost of electrical energy required to
6 operate air conditioners or heat pumps for cooling buildings,
7 and particularly with the heavy demands for commercial building
8 cooling systems at peak use hours, attention has been directed
9 to various types of thermal energy storage systems. Such energy
storage is advantageous since the building cooling and/or
11 heating and process cooling may be generated and stored during
12 off-peak hours at night when most businesses are normally
13 closed, with the ambient outside temperatures being cooler and
14 municipal power requirements reduced.
Most state of the art thermal energy storage systems
16 are based on a solid to liquid phase change using energy storage
17 in a narrow temperature range. Water based systems using ice
18 storage are especially desirable because of low fluid costs and
19 availability. However, the disadvantages of such systems
include low evaporator temperature requirements because thermal
21 gradient forces evaporator temperatures to a level far below
22 32F, also reducing the chiller efficiency, incomplete or low
23 phase change, often in the order of about 50% to 55%, and low
24 overall energy density of 80 BTU/lb. However, due to the excep-
tional environmental acceptability qualities and large
26 availability, water is preferred above more corrosive, volatile,
27 expensive and less readily available energy storage materials.
~Z78~66
The present invention is directed to an energy stor- -
age system utilizing the advantages of a phase change of gaseous
and liquid water. The energy density of such a phase change is
in excess of 1000 ~TU/lb. with no solidification or crystalli-
zation required. Moreover, the present system utilizes relative-
ly low cost and simple apparatus for taking advantage of the
energy storage combined with state of the art heat exchange
e~uipment normally found in modern residential and commercial
buildings. These as well as other advantages will be evident
from the following description of the invention.
According to one aspect of the present invention
there is provided apparatus for transferring heat comprising
a first vessel containing a liquid solution of a compound
selected from the group consisting of alkali and alkaline earth
metal hydroxide, halide and thiocyanate, ammonium halide and
thiocyanate, and mixtures thereof, said solution having an
initial concentration of between about 30% and about 80~, by
weight of said compound, said liquid selected from the group
consisting of water, ammonia an alcohol having between 1 and 8
carbon atoms, glycerol, glycols, polyglycols, glycol ethers,
aliphatic amines and alkanol amines having between 1 and about
6 carbon atoms, and mixtures thereof, and a first space above
the level of said liquid solution and means for pumping said
liquid solution to distribution means for directing said liquid
solution into said first space, a second vessel containing said
liquid without said compound therein, and a second space above
the level of said liquid and means for pumping said liquid to
distribution means for directing said liquid into said second
space, both said first and second vessels being closed to atmos-
phere and capable of holding a vacuum, conduit means
-- 2
1'~78~;fi~;
communi.cating between said first space and said second space forallowing liquid vapor and pressure changes to pass therebetween,
and valve means cooperating with said conduit means for ~ermin-
ating communication between said spaces, heating means for
heating said solution to a temperature of above about 90F, and
cooling means for cooling said liquid to a temperature below
about 55F, first heat exchange means coope.rating with said
first vessel for transferring heat from heated solution therein,
and second heat exchange means cooperating with said second
vessel for transferring heat to liquid therein.
According to a further aspect of the present inven-
tion there is provided a heat exchange process utilizing the
apparatus defined above comprising:
(a) opening said valve means,
(b) heating said solution in said first vessel to a
temperature of above about 85F and evaporating liquid there-
from, directing the liquid vapor from the space in said first
vessel to the space in said second vessel through said conduit
means, concurrently cooling the liquid in said second vessel to
a temperature below about 55F and condensing the water vapor
in the space therein, and continuing said heating and cooling
until the concentration of said material in said first vessel
is between about 6% and about 25% greater than said initial con-
centration,
(c) terminating said heating of said solution and said
cooling of said liquid and closing said valve means thereby
closing communication between said spaces in said first and
second vessels,
~ d) selectively opening said valve whereby liquid therein
is evaporated thereby cooling said liquid in said second vessel
- 2~a -
1278~;66
as liquid vapor pressure differential between said first and
second vessels is eliminated,
(e) exposing said liquid in said second vessel to heat
exchange means and pumping said liquid to said distribution
means and spraying said pumped liquid into said second space; and
(f) concurrently with step (e) exposing said solution in
said first vessel to heat exchange means and pumping said
solution to said distribution means and spraying said pumped
solution into said first space.
The invention will be further described with refer-
ence to the accompanying drawings showing, by way of example,
embodiments of the invention in which:
Figure 1 is a schematic sectional view of a first
embodiment of the system of the invention used in direct heat
transfer;
Figure 2 is a schematic sectional view of the system
of the invention using a direct expansion system; and
Figure 3 is a schematic sectional elevation illus-
trating a system for use in an indirect heat transfer system.
The hasic invention comprises charging the energy
storage system by heating an aqueous salt solution and cooling
water in separate vessels to a liquid temperature differential
of at least 30 F, and preferably 50F or more up to about 180 F
differential. The vessels are separated although the space
above each liquid in the respective vessels are in communication
until the desired vapor mass transfer is completed. Thereafter
- 2b -
~Z'78566
1 the spatial communication is terminated until it is desired to
2 discharge the energy stored in the system as will be described
3 hereinafter.
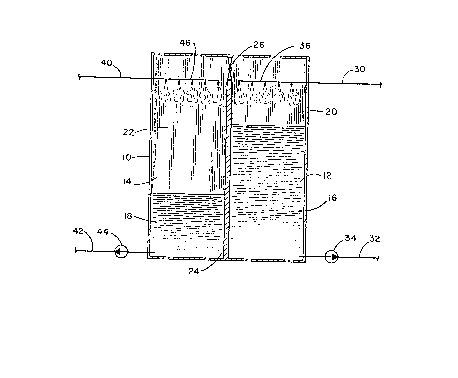
4 In Fig. 1 there is shown an illustration of a first
embodiment of the chemical thermal storage system of the
6 invention. The apparatus illustrated schematically comprises a
7 container lO having two cavities or vessels 12 and 14 separated
8 by a baffle or wall 24. The container must be air-tight so that
9 the two cells or cavities can selectively maintain different
vapor pressures. It will be appreciated that although a single
11 container 10 is shown having the two vessels, different
12 containers may be used, the important consideration being that
13 two vessels or cavities are required for holding two different
14 liquids of the system with means for transferring water vapor
and communicating vapor pressure differentials between the
16 vessels.
17 In the preferred embodiment shown, a valve 26
18 communicates between the spaces of the two vessels above the
19 liquid levels. The valve, conveniently located in wall 24, is
preferably a butterfly valve with an orifice of several inches
21 and associated with convenient means for opening and closing the
22 valve. A first liquid composition 16 is located in first vessel
23 12 and fills the cavity only partially leaving a space 20 above
24 the liquid level. Similarly, in second vessel 14, liquid 18
only partially fills the cavity leaving a second space 22 above
26 the liquid level. It is the spaces 20 and 22 which are in
27 communication via valve 26 which can be selectively opened or
lZ7~3~;66
1 closed to allow for water vapor to pass between the two spaces
2 thereby maintaining vapor pressure differential between the two
3 vessels when the valve is closed.
4 In the first vessel 12 is a liquid solution of an
ammonium halide or thiocyanate, alkali or alkaline earth metal
6 halide, hydroxide or thiocyanate, or mixtures thereof, having
7 initial concentrations of between about 30 and about 80%, and
8 preferably between about 40% and 75%, by weightO In the second
9 vessel 14 is liquid 18. The preferred solvent in vessel 12 and
liquid in vessel 14 is water although ammonia and ammonia/water
11 mixtures may be used for low temperature applications. In
12 addition, lower aliphatic amines, lower alkanol amines,
13 alcohols, glycerol, glycols, polyglycols, alkylene glycol ethers
14 and aqueous solutions or mixtures thereof may also be used in
combination with water or ammonia. Useful alcohol~ are those
16 having between 1 and 12 carbon atoms. Lower alphatic amines and
17 alkanol amines are those of from 1 to about 6 carbon atoms.
18 Examples of the amines are methylamine, ethylamine, etc. while
19 ethanolamine and propanolamine are examples of alkanol amines.
Preferred glycols are ethylene glycol and propylene glycol while
21 suitable glycol ethers include ethylene glycol dimethyl ether,
22 diethylene glycol diethyl ether, etc.
23 Most preferred salts in aqueous systems are the alkali
24 or alkaline earth metal hydroxides especially those of sodium,
potassium, cesium, magnesium, lithium, strontium and calcium.
26 Lithium or calcium chloride or bromide are also preferred.
27 Mixtures of the hydroxides may also be used together with
i~78566
lithium chloride or lithium bromide or calcium chloride as well
as nitrate salts of those metals as corrosion suppressing
additives, in systems where corrosion may be a problem. For
ammonium solvent systems, preferred salts include ammonium
halides such as ammonium bromide and ammonium chloride, ammonium
thiocyanate as well as ammonium metal halide salts, for example
4 4' ( 4)2 n 4 ( 4)3 C15. The above described
organic additives may be used to increase the sorption rates and
to increase the differential pressure, as well as to serve as
freeze point suppressants in aqueous systems in vessels 12 and
14. For this purpose, plastic vessels or containers which are
resistant to the aqueous hydroxide solutions are to be used as
are plastic tubing or conduits. Other materials that are lined
or coated with compositions which are not susceptible to cor-
rosion when exposed to the strong hydroxide compositions may
be used.
The apparatus also includes suitable conduits, pumps
and spray nozzle systems for handling the liquids in the respec-
tive cavities. Thus, conduits 30 and 32, and pump 34 direct
aqueous solution 16 from vessel 12 to a heat exchanger, for ex-
ample, a condenser of a heat pump or chiller or a waste heat
source (not shown) or solar heat means for heating the solution
to a desired temperature of at least 30F and preferably at least
50F higher than the temperature of water in second vessel 14.
Preferred solution temperatures are between about 85 and about
130 F although higher temperatures may be used. The heated solu-
tion is then directed to first space 20. A preferred method of
~278~i6
1 returning the heated aqueous solution to the first vessel
2 incorporates a nozzle or spray nozzle system 36 which simply
3 sprays the heated aqueou~ composition in the form of droplets or
4 a fine mist into first space 20 above the surface of liquid 16
in vessel 12. Any suitable spray no7.zle means may be used for
6 this purpose. Similarly, in second vessel 14, water 18 is
7 pumped to a heat exchanger by pump 44 via line 42 where it is
8 cooled, for example, by an air conditioning or heat pump
9 evaporator, cooling tower or other evaporative cooling means or
an air to air means after which the cooled water is directed via
11 pipe 40 to second space 22 above the water level using a spray
12 nozzle means 46.
13 In operating the above-described apparatus in a
14 storage system of the invention, preferably, during the night,
or o~herwise at relatively low ambient temperature conditions
16 and when area or municipal use loads are at below peak or high
17 requirements, aqueous solution 16 is ~eated with condenser heat
18 from a building heat pump system, or otherwise heated
19 conveniently to a temperature of above about 85F up to about
130F. At the same time, water 18 i8 cooled to a temperature of
21 below about 55F, and preferably below 35F, using an evaporator
22 from a building heat pump or air conditioning system. Because
23 the vapor pressure of the aqueous solution, for example 42%
24 NaOH, by weight, at 120F is higher than the vapor pressure of
water at 34F, the solution will desorb water in the form of
26 water vapor via open valve 26 into second space 22 which
27 condenses into the liquid water 18. This process continues
- 6 -
~78~66
1 until a solution concentration of approximately 52% of NaOH, by
2 weight, is achieved. ~t that point, the system is charged, and
3 valve 26 is closed to separate the first and second spaces in
4 the two vessels and maintain vapor pressure differential which
thereby allows the charged system to remain stored for an indef-
6 inite period of time.
7 When it is desired to utilize the stored energy in the
8 system created by the above-described charging process, the cold
9 water is circulated to a heat exchanger for the building, for
example, passed through a cooling coil in an air handler for
ll cooling the building. During this discharge period, again, the
12 water is pumped via pipe 42 using pump 44 to the building air
13 handler or other heat exchange cooling means, and returned via
1~ pipe 40 to second vessel 14 using the spray nozzle device 46.
The water thus becomes heated as it absorbs or picks up building
16 heat through the heat transfer system during this discharge
17 phase. Concurrently, hot aqueous solution 16 is pumped via pipe
18 32 and pump 34 to outside air heat exchangers or coolers such as
19 evaporative coolers, cooling towers and the like thereby cooling
the aqueous solution which is then returned via pipe 30 and
21 discharged into first space 20 using a spray apparatus 36.
22 During this discharge cycle, valve 26 must be opened
23 and because of the difference in vapor pressure between the
24 first and second spaces, 20 and 22 respectively, water is
evaporated in second space 22 to provide substantial cooling of
26 the liquid in second vessel 14. At the same time, the
27 evaporated water is passed into first space 20 where it is
~Z~3S66
1 absorbed into solution 16, which causes a heat of condensation
2 and solutions in first vessel 12, which heat is again exchanged
3 by the outside cooling means (not shown) previously discussed.
4 Alternatively, the heat of condensation and solutions in first
vessel 12 may be used for heating purposes, for example in a
6 dual temperature storage capability for building heating and
7 cooling.
8 In Fig. 2 there is illustrated another variation or
9 embodiment of a system according to the invention in which a
coolant, such as a refrigerant may be directly cooled or
11 condensed at low temperature during discharge of the energy
12 storage system of the invention. Again, a container 10 having
13 two vessels or cavities as illustrated in Fig. 1 may be used
14 with a divider 24 separating the two cavities. The liquid solu-
tion 16 and water 18 in the respective containers are
16 substantially like that previously described as is the change of
17 vapor pressure and exchange of water vapor between the cavities
18 20 and 22, respectively, through valve 26, in both the charging
19 and discharging phases of the operation of the system. In this
embodiment, the water is cooled during the charging phase by
21 direct exposure to evaporator coils of àn air conditioning
22 system evaporator. For example, the air conditioning system of
23 the building may utilize a cooling conduit 66 which is exposed
24 directly in second cavity 22 through which cold refrigerant is
directed during the charging phase. At that time, water 18 will
26 be pumped via pump 60 and conduit 58 and sprayed through spray
27 nozzle apparatus 46 over the cold pipe or coil 66 to be cooled.
8~;66
1 Concurrently, with valve 26 open during the charginq phase,
2 aqueous solution 16 is heated by pumping the solution via pump
3 54 and conduit 52 through spray nozzle apparatus 36 over heated
4 pipe or coils 56 from a condenser 75 or other heating means
including a heat exchanger of the HVAC (heating ventilation air
6 conditioning) equipment condensing the refrigerant. In that
7 case, hot refrigerant may be directed into pipe or coil 55 via
8 pump 74 from the heat exchanger 75. once the vapor mass
9 transfer between the liquid solution 16 and water 18 is achieved
during this charge phase, further heating and cooling,
11 respectivel~ is terminated and valve 26 is closed thereby again
12 maintaining the energy charged in the respective li~uids stored
13 until its use is desired.
14 During discharge, valve 26 is opened, and the
respective liquids are pumped through their respective nozzle
1~ sprayers over the heat exchange conduits present in the spaces
17 in the respective vessel. Refrigerant directed via conduit or
18 coil 66 is cooled by evaporation of water as water is sprayed
19 over the coil in space 22, the water gradually becoming heated
as it picks up heat as it cools the refrigerant from heat
21 exchanger 72. Similarly, in this discharge phase of operation,
22 heat from solution i6 is removed by heat exchanger 75 which
23 similarly p~lmps a coolant via coil 56 present in space 20.
24 A third apparatus configuration utilizing an indirect
heat transfer system is illustrated in Fig. 3. In this
26 apparatus, substankially like that described in Fig. 2, a heat
27 exchanger 76 is used for directly heating and cooling liquid 16
~Z78566
1 in the charge and discharge phases, respectively of the
2 operation of the system by pumping a heat transfer fluid with
3 pump 74. The heat exchanger illustratad utilizing water with
4 the system is somewhat different in that pump 77 will direct a
heat transfer material such as water, ammonia, methanol,
6 glycol-water mixtures, and the like through remote heat
7 exchanger 71, 73 and 79 at various locations throughout a
8 building in which the system is to operate. Pump 77 simply
g pumps the secondary cooling material via pipe 70 to the
respective heat exchangers, with the pipe 70 being exposed in
11 space 22 over which water is sprayed as previously discussed
12 regarding the apparatus shown in Fig. 2.
13 The specific types of heat exchangers and apparatus
14 used within the purview of the scope of the systems described
herein are not so important, and other types of systems may be
16 used to achieve the same purpose. Thus, the specific design of
17 the systems shown and described herein are for the purpose of
18 illustration only and the invention is not to be necessarily
19 limited thereto. These as well as other modifications,
variations and advantages of the system within the purview of
21 the invention will be evident to those skilled in the art.
-- 10 --