Note: Descriptions are shown in the official language in which they were submitted.
~786~
1162
The p:~es~n-t invention rel~-ltec -to a p:rvc~ss .-for free-
ing an aquec,us dispersion of a poly~ner f:rom a monomer
p:reent -the:rei.n, the polym~r, which may ~e a homopolyrner,
graf-t polymcr or copolyrner, having an ex-trerllely mirlor
c~ncerltra-tion of residual rilonomer af-ter purif:icationO
I-t; has long been kno~rn -that aqueous po-ymer disper-
; sions can be freed from volatile cons-tituen-ts 'by ~lol.iing
an iner-t gas or steam at abo-u-t 60-70C through the dis-
persjon, i.e. by subjecting the dispers:ion -to steam di-
stilla--tion. ~'his has 'been described in Ge~rman Published
Specifica-tion ("Auslegeschri~t~ No. 1,2/-~8,943, and in
"Kunststoffe" (1959), volume 49, No. 10, page 499, and
also in "Chemical Engineering", March 1972, page 960
:[n those cases in which aqueous polyvinyl chloride
suspensions are worked up under -the condi-tions just
described, -tne polymer is subsequen-tly dried by means of
air~ whereby it is freed from a fur-ther proportion of
vinyl chloride, e.g. a`bout 2 weight %, based on the
quanti-ty of vinyl chlorlde su'bjected to polymeriza~ti.on7
which is allowed to escape in-to the atmosphere. In other
words, the issuing gas contains vinyl chloride in propor-
tions which are clearly beyond an accepta'ble emiss.ion
limit, namely beyond the limit of 150 mg of vinyl cklo-
- ride per cubic meter of issuing gas. In additLon to this,
excessive proportions of vinyl chloride go into the was-te
water. Despite -this, the final dry polyvinyl chloride
still contains several hundred ppm of monomeric vinyl
chloride, which is absorbed i:n the polyrner and cannot be
- 2 ~
1;~78~8
rernove~rl-there:f:r-(Jm by -the purlfying procedure descri'bed
above.
0~l~ o~ the u~es of polyvin~1 chloride sheets i~ in
packlng Loocl, wh-ch is hazardou~ inasmuch a~ residual
mono~ers con-tail1~d in -the polymer ma-y mi~rat~ i~-to th~?
food, It is -therefore obllga-tory for -th~ dry monomer
ccn-taining polyrners -to be subjected to an additional
special purifying -treatment.
A process wherein dry polyvinyl chloride made in
conven-tion~l manner is freed from resi~ual vinyl chlo-
ridej -~hich is embedded or occluded in -the polymer par-
ticles, has been 'described in German Published Specifi-
cation ("~ffenlegungsschrift") No. 2,331,895~ This pro-
cess, which enables polyvinyl chloride to be freed from
vinyl chloride and further comonomers, if any, comprises:
heating the polymer to a temperature ranging from i-ts
freezing tem'pera-ture to 180C by directly condensing
steam -thereonto; maintaining the polymer at that -tempe-
rature for the period necessary to free it from the bulk
of monomer or monomers therein; and cooling the polymer
down to a temperature lower than i-ts freezing point by
evaporating the s-team condensed on -the polymer. A pre-
ferred embodiment of this process comprises heating -the
polymer to a temperature ranging from 80 -to 1~0C and
allowlng it to remain a-t that temperature for a period
of a'bout 5 minutes up to 2 hours, especially 10 to 60
minutes. Typical of this known process is that the de-
gasifica-tion is effec-ted a-t the dew point of wa-ter, as
described in -the working Examples of that Specifica-tion.
A disadvan-tage encountered with this earlier pro-
786~38
cesx -i~esi(1~s in thC f.lct -t~la-t -the ?o~lymer so plrifled
continu s to pr~serlt rela-5ive^1y high proportions o-~ rno-
norners. As shown in EY~ar~lp1e 1 of ~erri~an P~blished Spe-
cifica-tion ("O1fen]egungsschrift" No. 2~31,89~, the
purified po:Lyrner con-tains 3 g (or 3,000 ppm) of residual
rr.onorners per kg of oolymer. This known process is effect-
ed at ternpera-tures ancl under pressures wh:ich correspond
-to the dew poin-t of water under -the conditions seLecte~,
which naturally means 'nigh a-nd comrnercial~Ly unat-tractive
consump-tion of s-team.
In clear contrast therewi-th, -the present inven-tion
- provides a process permitting an a~ueolls polymer dlsper-
sion -to be freed from`monomeric mat-ter wi-th the resultan-t
formation of a purified product containing a few ppm of
residual monomer(s), the purified produc-t presentinO this
minor concentration of residual Monorner~s) being obtain-
ed much more rapidly than in the prior processes of which
we are aware.
The following proper-ties of a vinyl chloride/water/
P01YVinY1 chloride-sys-tem are of importance to the removal
of monomeric vinyl chloride from an aqueous polyvinyl chlo-
ride dispersion~ and should conveniently be considered in
effecting such operation.
a) Bunsen's solubility coefflcient a of vinyl chloride in
water, which has the following values at temperatures
of from 0.1C up to 100C: -
0.1C 2 unit vol.vinyl chloride/uni-t vol. water
20C 1 unit vol.vinyl chloride/uni-t vol. water
35C 5 unit vol.vinyl chloride/unit vol. wa-ter
60C 0.1 unit vol.vinyl chloride/unit vol. water
100C 0 unit vol.vinyl chloride/uni-t vol. water
12~8~3
b) :Bur~,er~'~ ;o'lllbility coeïEicierl-t ~ of v:iny:l ch'loride
in c)(lueolll PVC~ is?eL,c,ion containing ,5 we:ight ~6 o,
solid ~1'.1 tter, w'n;ch has -the following values:
at; 6C 5 unit vol. -vinyl chloride/uni-t vol. wa-ter
18C 3 uni-t vol. vinyl chloride/unit vol. wa-ter
2~C 2 unit vol~ vinyl chloride/unit vol. wa-ter
5~C 1 unit vol. vinyl chloride/uni-t vol. wa-ter
7~C o.6 uni~t vol. vinyl chloride/uni-t vol. l,Jater
0 C) The solub:ili-ty of vi.nyl chloride in polyvinyl chloride.
The following quanti~t:ies of vinyl chloride have been
~'folmd to be dls~olved a-tl-the'following -temperatures
e.g. in polyvinyl chloride having a mean particle size
of 60 up -to 120 microns and a ~-value of 70.
- ' At 0C 100 g vinyl chloride/kg polyvinyl chloride
2L~oc '50 g vinyl chloridelkg polyvinyl chloride
40C 24 g vinyl chloride/~g polyvinyl chloride
60C 10 g vin-yl chloride/kg polyvinyl ch]oride
100C 4 g vinyl chloride/kg polyvinyl chloride
d) The dis-tribu-tion coefficient of vinyl chloride be-tween
water and PVC, which is equal -to abou~t 1:15.
We have now unexpectedly found that -the phase equili-
bria commence set-ting within the tempera-ture range of 90C
to 100C at intervals of 10 up to 100 seconds under con-
ditions which provide for a very effective exchange of
cons-tituents between -the individual phases of the above
miY,tures a) c). Temperatures around 100C, are, however,
~o known to irnpair the quali-ty of PVC. To avoid -this, i-t is
~7~6.'38
nect~ 1r~y rO~ -the in.~luence of -tempera-tllre o-n YVC to ~be
li1n:L-I;ed -to evera~ n-ilLltcs. In o:rder to e-l~ec-ti-vely re-
rno-~e Monome.ri.e matter ,`rolll an aqueouc PVC-dispeLsion at
-tempera-ture; within -the rarlge 90 and 100C, i-t is f`inally
necessary -to c~stablish condi-t:ions, which p:rovide for an
e~fective exchange of cor.~s-ti-tuen-ts and thereby or a
compLete setting of the phclse equilibria, and also for
-the use o:. a sui-table gas phase enabling the monomeric
matter -to b~ removed
The process of -rhe presen-t in~en.-tion for removi-ng
rnonomeric ma-t-ter from an aqueous dispersion of a polymer
con-taining at least 50 ~eight % of polymeri~ed vinyl chlo-
ride comprises: introducing the dispersion i.nto -the upper
portio.n of a column provided with sie~e plates and con-
tac-ting -the dispersion therein for a period of about 10
seconds up -to 20 mi-nu~tes and under a pressure of abou-t
600 up to 1200 mm Hg wlth ho-t steam a-t abou-t 100 up to
150C flowing countercurrently wi-th respec-t to -the disper-
sion; removing the po]ymer dispersicn so -treated from the
column base portion, and condensing stagewise a vaporous
mat-ter mixture issuing at -the head o:- -the column so as to
recover an aqueous phase and the monomeric ma-tter~
In accordance wi-th a preferred feature of -the pre-
sent Lnven-tion, the aqueous dispersion contains approxi-
mately 10 up to 60 welght %, more preferably 25-up -to
40 wcight %, of polymeri^ solids which in turn should
preferably con-tain a-t leas-t 85 weight % of polymerized
vinyl chloride~ The -term "polymers" as used herein com-
prises polyvinyl chloride homopolymers and vinyl chloride
copolymers, e.g. copolymers of vinyl chloride wi-th. vinyl
-- 6 --
12786;~8
tr-l t~ t i rs cll.o ~dvarlt;ageous for -the polymer ~isper-
s:iorl to con-t.1in betli/ee~l a'bou-t 0.2 up to 5 welghl 5b of
virly~l, ch'lorid~.
A .~llr-th~r preferred fea-ture of the presen-t process
cornp.rises hea-tlllr -the polymer to a -tempera-ture of abou-t
60 up to 90C ancl t;hen introdllcing it into -the colu~n.
~:o n ~c~ cte 6~
The dispersion so prehea-ted is-ee~ R~inslde the column
~ith Ihot si;eam ascending therein~ which prelerably is
at a terrlpexature of 100 up -to 150~, a.nd causes a tem-
0 pe:ratllre 0.~ 90 Up to 100C to be es-tablished ln the
co.Lumn head. The column should preferably be opera-ted
uncle:r a p.ressure of 700 up to 1100 mm Hg. A relatively
mlnor proportion of steam, equal to 1 up -to 5 weight %
' of water, based on the quanti-ty of d1spersion supplied
to the co]umn, escapes at the head of the column. Tha po-
lymer dispersion i-tself is generally allowed to rem.1in
ln -the column over a period of 0.3 up to 10 minu-tes.
The invention also provldes for the aqueous phase
obtained on subjecting the vaporous matter mi~ture issuing
overhead to stagewise condensation to be combined ~lith the
polymer dispersion ahead of the monomer degasi~ica-tion
zone.
The dispersion o~ vinyl chloride homopolymers, graIt
po'lymers or copolymers to be treated in accordance with
the present invention can b'e made by a process,'such as
that'described by H. Kainer in the book enti-tled '~Po]y-
vinylchlorid und Vinylchloride-Mischpolymerisa-t.", pu'--
blished by Springer-Verlag, Berlin/Heidelberg/New York,
1965, pages 12-59.
One exemplary embodirnent of -the process o~ -the present
lZ7~3~.38
i.nventior!-~liL no-~i be ~esc.r:Lbed wi-th reference -to the
IccoTllpanyint~ drcl;~ing. Needles -to say the :inven-tlon is
in r-lo way limL-t;ed -to -the exemp:lary embodi~en-t spec:ifi-
cal~y describecl
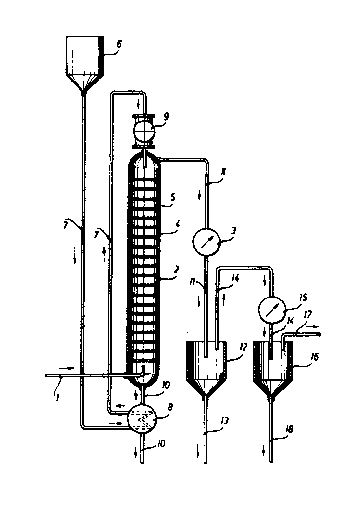
Wi.-th re:~erence -to -the dra~Jing:
S-tearQ is introduced through a li.ne 1 into a s-tri.pp-
- i-ng column 2 for as long as necessary to obtain conden-
sate in a heat exchanger 30 The str:ipping colu~n 2. compri-
ses a vertic~l column having sieve pl.ates 4 at certain
inter~als arranged -therein, perpendicularly with re-
spect -to the column The sieve plates 4 have no par-ti-
cular liquid ma-t-ter inlet or outlet and distinguish in
this feature over the inserts normally used in conven-
tional sieve plate columns, such as those described by
E. Ki.rschbaum in "Destillier- und Rektifizier-technik'~,
. Springer-Verlag, Berlin-Gottingen-Heidelberg (1950)~
page 97. The gas and/or liquid matter apertures 5 pro-
vided in the sieve plates have a diameter between 1
and 10 mm, -the total surface area of those apertures
being equal to 5 up to 50 % of the column's cross-sec-
~tional area. Once the stripping column 2 has been
warmed Up, a polymer dispersion having a mean particl.e
size of about 20 up to 500 microns is in-troduced -there-
i.nto overhead. The dispersion comes from a reservoir 6,
travel.s through a line 7, is prehea-ted in a heat-ex-
changer 8 and supplied in metered ~uanti-ti.es b~ means
of a dosing me-ter 9. Following -this, the strippin~
column is supplied with -the quantity of steam which is
necessary to establish a -temperature of 90 Up -to 100C
in the column nead a.nd to expel the monomeric mat-ter
f`rom the polyrner dispersion. The polyrner di~perslon
-- 8 ~
12786.~8
so ~r~ee~3 Irom monomeric rn~-t-ter is removed at -the base
p~r-t; on ol` st:ripp;ng ~olur~n ~ thro~l~;h ~ llne 10 and -t~he
heat excharlger 8. The hea-t con-tained in the polymer
dispersion is thereby communicated ,,o -the hea t exchan-
ger 8 and used ~or preheat'ing ~resh polymer'disper~cJion.
' The -time during w1~ich -the polymer dispersion re-
rnains ih -the stripping column 2 is critical]y de-te-rmined
~y the number of sieve plates prov:ided, e.g. 5 up to 50
sleve plates, ~nd by the nature of the solid matter par-
ticles in -the dispersion. The steam supplied to the co-
lumn is partially used for effecting warm up of the
dispersion to the necessary temperature, and mainly
used for stripplng off and expelling the monomeric ma-tter
frolrl the dispersion. The vaporous ma-tter mixture of steam
and monomers issuing at the head of the s-tripp:ing column
2 and travelling through a line 11, is cooled down to a
tempera-ture of 5 up to 20C, and only steam is accor-
dingly condensed. The condensate containing some mono-
meric mat-ter, i.e. a minor proportion consistent wi-th the
monomer's solubility in water, can be removed through a
line 13 and combined with the polymer dispersion coming
from the reservoir 6. Gaseous monomeric matter7 w1nich
remains uncondensed~ln -the heat exchanger 3, is dellver-
ed through -the line 11-, a container 12 and a line 14 to
a further heat exchanger 15, in which it is completely
condensed by cooling'down to a temperature of -15~. The
resulting li'quefied monomeric matter is collec-ted in a
container 16 provided with a line 17 for the removal of
gaseous rnonomer, and with a line 18 for the removal of
liquid monomer, ~or further uses.
_ g _
~ ~786.'38
The ~)~ocescs of 1;he ~resent lnvention enab1es poly-
mers -to be fre(-~ more effectively and r~ore reliably from
monomer~ Imder ecologically beneficial conditions, and
-therefo-re coMpares ver-y favorabl~J with -t~e prior ar-t
methods. More par-tlcu1arly, the polyrners so purified
only con-taln -traces of mo~omers, of the approximate
order of 10 ppm. In addi-tion to -this, -the polymers are
very pure, so that they can be used in :Eields no-t acces-
sible to thern here-tofore owing -to -their-inadequa-te puri-
ty and relatively high conten-t of monomers. I-t could no-t
have been foreseen that i-t is possible for the presen-t
process to be successfully carried out in a stripping
column with sieve pla-te inserts therein inasmuch as -the
aper-tures providéd in the sieve plates would have been
expected -to becomel soiled or encrus-ted with material7
which is however not the case. The present process is
generally applicable to the removal of monomeric matter
from an aqueous polymer dispersion containing polymer
particles with a unit weight greater than tha-t of water.
EXAMPLE 1
A polyvinyl chloride dispersion was freed from
vlnyl chloride contained therein. The operation was
effected as shown in the flow scheme of the accompany- -
ing~drawing. The dispe:rsion contained 6 000 ppm of vi- ~
nyl chloride and ~5 weight % of solid matter. The poly- -
vinyl chloride had a K-value of 70, a mean partiGle ~
size of 65 microns and a power for absorbing softener
of 27.7 %. The dispersion was purified in a stripping
column 2 which was provided with 20 sieve plates and
had an in-ternal diame:ter of 100 mm.
- 10 -
~2786~3~
The si(ve pla-tes provide~ in -the column were spaced
apc~rt at intervals o:E 150 mrn arld provide~ ~ith 250
ape-rtures 2.5 mm wide. S-team a-t 10~C was introduced
-thrGugh line 1 into stripping column 2 for as long as
necessary -to obtain condensed water in heat exchanger
3. ~ile the supply of stearn was continued, aqueous
polymer dispersion was in-troduced into -the head of
stripping column 2 at a -throughput ra-te o~ 48 l~h7
corresponding -to a mean sojourn time of 1 mi~ute of
the dispersion in -the column. The column was more par-
tivu~Larly supplied wi-th the quantity of s-team necessary
to obtain, in heat exchanger 3, abou-t 3 weight % of con-
densa-te, based on -the quantity of dispersion supplied
per hour, and to have a tempera-ture of 95 up to 100C in
-the gas phase near the head of the column, and a tempe-
rature of 102 up to 105C in the column base portion.
The differential pressure in the column was 50 up to 80
mm Hg. 1.5 l/h of vinyl chloride-contalning water was
collected in con-tainer 12 and subsequentIy combined with
the polymer dispersion to be purified. The dispersion
-taken from the base portion of stripping column 2 con-
tained less than~1 ppm of vinyl chloride in -the a~ueous
phase, and less than 10 ppm of vinyl chloride in -the po-
lyvinyl ch1orlde phase.~he residual content of vinyl
chloride in the polymer dispersion was iden-tified by gas
chromatography. 125 g/h of vinyl chloride was condensed
in hea-t exchanger 5 at -30C.
EY~MPLE 2
The procedure was the same as that described in
Exarnple 1, save tha-t the polymer dispersion which was
- 11 -
127136:~8
pur i f'ied cor:L-t~i ned :rigid polyviny:L chloride wi th a K-
value of 6~. The polyme:r pal--tlc:Les had a Mean par-ticl.e
si~e o:E 120 m:icrons and ~ power for absorbing soften.er
- of 1,7~ ~ 5 %. The dispe:rsion con-tained 5200 ppm of -vinyl
chlorlde. The dispe:rsion was pu-t through -the colunm
at a ra-te of 48 l/h, corresponding to a mean 2 minute
sojourn time of -the dispersion ln the colu.mn. The column
was supplied wi-th the quantity of steam necessary to ob-
tain, in hea-t exchanger 3, abou-t 5 weight % OI conden-
sa-te, based on the quan-tity of disperslon supplied per
hour. ~he purified dispersion was found to contai.n 10
ppm of viny:L chloride in -the solid ma-tter, and less than
1 ~pm of vinyl chloride in the aqueous phase.
- 12 -