Note: Descriptions are shown in the official language in which they were submitted.
-- 1 --
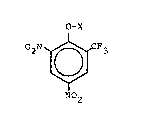
The present invention relates to herbicidal, acaric-
idal and crop regulating compositions containing as active
ingredient 2-trifluoromethyl-4,6-dinitro-phenol and derivatives
thereof t of the general formula I
O -X
02N \ ~ CF3
~ (I)
N02
wherein
stands for hydrogen, alkali metal, alkali earth me-tal,
Cl 10 alkyl - or alkenylcarbonyl, phenylcarbonyl
halogen-substituted phenylcarbonyl, or a group of the general
formula(a)
H N Rl R2 R3 (a)
wherein
Rl, R2 and R3 are the same or di.f~erent and stand for
hydrogen, alkyl, alkoxyalkyl, alkenyl, hydroxyalkyl,
cycloalkyl, phenyl or phenyl substituted by halogen and/or
trifluoromethyl in admixture with a suitable carrier or diluent.
The compounds of the general formula I show herbicidal
and acaricidal and crop regulating activity.
-?-
The compounds can be formulated to herbicidal, acar-
icidal or crop regulating compositions, which preferably contain
0.1 to 80% by weight of active ingredient and the usual carriers
and excipients.
The compositions may be prepared in the form of
emulsifiable concentrates, aqueous suspensions, spray in the form
of dust, oily paste, granulates, etc.
In another aspect the invention relates to a
2-trifluoromethyl-4, 6-dinitro-phenol of the general formula I
O-X
O2N ~ F3 (I)
N02
wherein ~.
X stands for hydrogen, alkali metal, alkali earth metal,
Cl 10 alkylcarbonyl, C2 10 alkenylcarbonyl, phenyl-
carbonyl, or halogen-substituted phenylcarbonyl
or a group of the general formula (.a~.
+
H N Rl R2 R3
wherein Rl, R2 and R3 are the same or different and can stand for
hydrogen, alkyl, alkoxyalkyl, alkenyl, hydroxyalkyl, cycloalkyl,
phenyl or phenyl substituted by halogen and/or trifluoromethyl.
~o~
-2a-
The present invention further provides a process for
the preparatIon of the compounds of the general formula I by
alkaline hydrolysis of 2~chloro~3,5-dinitro-benzotrifluoride in
aqueous medium without catalyst, and if desired followed by the
conversion of the obtained phenolate to the end product by method
known per se.
The structurally similar 2-methyl-4,6-dinitro-phenol,
i.e. the 50 called dinitro-ortho-cresol (DNOC) and alkali metal
salts thereof are known to show herbicidal and acaricidal activlty.
~. ~
-~sr 1~
`` 1~8~
-- 3 --
Known compositions containing such active ingredients
are Novenda and Krezonit Similarly 2-sec.-butyl-4,6-dinitro-
-phenol /DN~P/ of similar chemical structure is a known
herbicide, used at smaller ~4~ as biostimulator in corn
cultures /Ohlrogge, A.J D The Development of DNBP /Dinoseb/
as a Biostimulant for Corn in Plant Crowth Re~ulators,
editor: Stutte, 5.A. 79-87 pp~ Advances in chemistry series,
159 American Chemical Society, Washington, 1977./
No technical reference could be found concerning the
preparation or biological activity of the compounds of the
general formula I accordin~ to the invention only for
similar compoùnds, In C~ PS 1 378 994 the preparation of
2,4-bis-/trifluoromethyl/-6-nitro-phenol and derivatives
thereof is disclosed, In US PS 3 813 446 the preparation
of 2,6-dinitro-4-trifluoromethyl-phenol and derivatives
thereof is described. The known processes are performed
~ oh ~sæ
in an or6anic solvent, optionally in the presence of ~k~is_
transfer catalysts, In said processes undesired side-re-
actions take place, such as ether derivative formation
in methanol or hydrolysi~ of the trifluoromethyl group
into carboxylic group. According to the present ïnvention
no ~uch side reactions occur~
The process of the invention is suitable for the
preparation of the compounds of the general formula I
; 25 at industrial scale economically and in pure state.
We have also found that the new compounds show
a surprisin~ly good biological activity. After subjecting
the various compositions of the invention containing
4~
_ 4
the compounds of formula I as active ingredient we have found
that new herbicidal, acaricidal and crop regulating compositions
were obtained.
When preparing the compounds of the general formula I
- wherein X stands for hydrogen, alkali metal, alkali earth
metal, Cl 10 alkyl - or alkenylcarbonyl, phenylcarbonyl,
halogen-substituted phenylcarbonyl, or a group of the general
formula (a), wherein Rl, R2 and R3 are the same or different
and stand for hydrogen, alkyl, alkoxyalkyl, alkenyl, hydroxy-
alkyl, cycloalkyl, phenyl or phenyl substituted by halogenand/or trifluoromethyl - one may proceed by first preparing an
alkali metal or alkali earth metal phenolate by reacting
2-chloro-3,5-dinitro-benzotrifluoride with alkali metal or
alkali earth metal hydroxide. The reaction is conducted in an
aqueous medium without catalyst at 20-100 C, preferably a-t
60-80 C at an alkali concentration of 5 to 20 % by weight
preferably 10 to 15 % by weight. The phenolate formed during
the alkaline hydrolysis is, if desired, converted to phenol
with an acid by method known per se. The phenol is, if desired,
converted to a phenolate derivative by using a base corresponding
to the substitutents of the general formula I latter being used
as pesticidal active ingredient after isolation from the mixture.
The pesticidal composition is prepared by using the
conventionally used carriers and excipients. The active
ingredient content can vary within a range of 0.1 to 80% by
weight depending on the formulation type and
i:
~L2
-- 5
method of application. The composition is used as
herbicide, acaricide or crop regulating agent.
The process of the invention is simple, economical
and reproduceable and suitable for the preparation of the
new compounds of the invention which broaden the selection
of pesticide agents due to their bioactivity.
The details of` the invention are illustrated by
the following Examples which serve merely for illustration
and not for limitationO
Preparation of the compounds of the general formula I.
~.
To a 1000 cm3 flask equipped with a stirrer, dropp-
ing funnel, reflux and thermometer 300 g /0.75 mole/ of a
10 % by weight NaOH solution are added, 81.3 g /0,3 mole/
of 2-chloro 3,5-dinitro-benzotrifluoride of a purity Or
95 % are added under stirring, Ihe temperature is raised
to 60 C and the reaction mixture is stirred at 60-65 C
for 4 hours, while the aqueous suspension forms an emulsion.
The reaction mixture is then cooled to room ternperature
/20-25 C/ under stirring and within 1 hour a 37 % hydro-
chlorlc acid solution corresponding to 0.82 mole HCl is
added in equal portions under vigorous stirring. The
precipitated product is filtered, washed with water and
dried to constant weight at room temperature. 2-Trifluoro-
methyl-4,6-dinitro-phenol is obtained /68,lg " purity: 94 %/
Identification was carried out by a mass spectrometer
connected to a gas chromatograph.
Yield: 90 %
6 --
Example 2
To 250 1 enamelled reactor equipped with a mixer,
and reflux 60 kg. of water are added and under stirring 32.5 kg.
of 2-chloro-3,5-dinitro-benzo-trifluoride are added through a
powder funnel. The reaction mixture is heated to 75C and at
equal rate 65 kg. of a 20% NaOH solution are pumped in while the
temperature is maintained at 75-80C. The reaction mixture is
stirred at 75-80C for 3.5 hours. The reaction mixture is cool-
ed to room temperature and within 1 hour 9.5 cc. HCl are added
at equal rate. The precipitated product is centrifuged, washed
wi-th water and dried at room temperature to constant weight.
The obtained product is 2-trifluoromethyl-4,6-dinitro-phenol,
(28 kg.).
Purity: 94.3%, yield: 92.46.
Preparation of the composition
'~A ~
:J .
~8014~0
- 6a - 23305-939
Example 3
60% by weight emulsifiable concentrate (EC)
433 g. of 97% 2-trifluoromethyl-4,6-dinitro-phenol are
dissolved at 40C in a mixture of 200 g. of isophoron and 25 g. of
xylene. The solution is cooled to room temperature and filtered
through 0.1 micrometer GAF filter. To the filtrate 36 g of
Tensiofix *B 7425 (a mixture of calcium dodecyl benzene sulphonate
and ethoxylated and propoxylated nonylphenol) and 6 g. of
Tensiofix ~S (a mixture of calcium dodecyl benzene sulphonate and
ethoxylated nonylphenol) emulsifier are added under stirring.
Example 4
60% by weight of emulsifiable concentrate (EC).
One proceeds as disclosed in Example 3 but isophoron is
replaced by cyclohexanone and xylene is replaced by aromatol
solvent and the following emulsifying agents are used:
*Trade-Mark
-- 7
. . ~ . . .
A s C
.. . .. . _
Tensiofix AS g. 18 - 10
(a mixture of calcium dodecyl benzene
sulphonate and ethoxylated nonylphenol)
Tensiofix LS g. 24
Emulsogen *EL g. (e-thoxylated castor oil - 12 25
(EO number = 36-40/mole))
Sapoegenat *T 180 g. - 10
(ethoxylated tributyl phenol (EO number
= 18/mole))
Sapoegenat T 500 g. - 20 7
(e-thoxylated tributyl phenol (EO number
= 50/mole))
. . _ _ . _ . _ _ _ . . . _ . . . _ _
Example ~
20% by weight of water soluble concen-tra-te (WSC)
To 300 g. of a 30% by weight of 2-trifluoromethyl-
4,6-dinitro-phenol-Na aqueous solution 50 g. e-thylene glycol
and 4 g. of Tensiofix CG-21(a mixture of ethoxylated fatty
alcohol, etho~ylated fatty alcohol phosphate ester, e-thoxylated
nonylphenol and ethoxylated nonylphenol phosphate ester) emulsif-
iers are added. The solution is supplemented to 450 g. with
ionexchanged water under stirring.
Example ~
20~ by weight of oily paste
620 g. of 97% 2-trifluoromethyl ~-4, 6-dinitro-phenol
~ . .
are dissolved in 500 g. of cyclohexanone and to this solution
120 g. of cosmetic Vaseline *oil are poured. The
* Trade Mark
-- 8 --
~L28~
obtained solution is emulsified with a 10% solution of
Tensiofix PO-132(ethoxylated lauryl alcohol)emulsifier by
means of Ultra-turrax mixer while the temperature is maintained
at 30C. After cooling to 15C 50 g. of 0.2% by weight of
Kelsan* (xantham gum type thickening agent~-ethylene glycol
solution is dispersed in the mixture within 1 hour under slow
stirring.
^~ Example ~
Herbicide activity test in green house
In culture dishes white mustard, pig weed, fat hen,
autumn wheat, Italian grass and corn seeds were sown. When
the seeds were sprouted 10% EC and 10% WSC were prepared from
the compounds to be tested, and after dilution with water 0.2%
spray was prepared. 2-~ leaves plants were sprayed with this
liquid so that for each compound a dose corresponding to 2.5-5-
10 kg./ha active ingredient was used. On the l~th day after the
treatment the extent of the injury of -the plants was evaluated
and expressed in %. The results are summarized in Table 1.
* Trade ~ark
- 3 ~ ~2~0~
Table 1
Compound Dose white pig fat Italian autumn corn
Kg/ha mustard weed hen grass wheat
-
2-trifluoromethyl- 1.25 45 50 75 0 0 0
-4,6-dinitro-phenol 2.55 75 85 90 10 5 5
5.0 95 100 100 30 10 15
2-trifluoromethyl- 1.25 40 50 70 0 0 0
4,6-dinitro-phenol- 2.5 60 60 75
diethanol-amine 5.0 80 85 80 10 10 10
2-trifluoromethyl 1.25 45 50 70 0 0 0
4,6-dinitro-phenol- 2.5 55 65 75 0 0 0
dimethyl-amine 5.0 75 80 85 20 10 10
2-trifluoromethyl- 1.25 50 60 70 0 0 0
4,6-dinitro-phenol- 2.5 80 85 90 10 0 10
-Na 5.0 100 100 100 15 15 15
2-trifl.uoromethyl- 1.25 65 45 70 0 0 0
4,6-dinitro-phenol- 2.5 75 85 90 10 0 10
~ 5.0 90 100 100 15 15 15
., ~,
1280~40
Example ~
Determination of Hill-reaction inhibition
By means of the method of Arnon et. al. spinach
chloroplastises were isolated. To 1 g. of leaves freed from
the ribs 6 ml. of a 0.35 molar solution of NaCl and 0.6 ml. of
.
~80~
0~2 molar TRIS~buffer were added, the mixture was ground,
and the homogenizate was filtered through a gauze of
several layers and centrifuged for 5 minutes at a rate of
2000 rotations. The chloroplastis precipitate was suspended
ln a 0 035 molar NaCl, homogenized in a Potter tube and
the thus obtained chloroplastis suspension was held at O C
until use. A reaction mixture of the chloroplastis suspension
K3Fe/CN/6, K~HP04 9 TRIS~HCl buffer, MgC12 of suitable
concentration and ratio and a solution of the compounds of
the ~en~r~1 formula /I/ a~ inhibitor~ and N~/3,4-dichloro-
-phenyl/-N',N'-dimethyl urea /DCMU/ and dinitro-ortho-
-cresol /DNOC/ as standards of a molar concentration ofl x 10
1 x 10 3 were subjected to an exposure of 5000 lux light
intensity for 20 minutes whereafter the reaction was quench-
ed with 25 % trichloroacetic acid solution /TCA/. Theprecipitate was removed by centrifuging and the extinction
of the solution was measured at 420 nm, with a photometer
of typc ~pe-~e~e~-~44 The measuring series was conducted
also with reaction mixtures held in the dark. Hill-reaction
inhibition was deter~nined on the basts of the extinction
differences measured in dark and light reactions and
converted to /ug~ml potassium ferricyanide concentration
on the basis of calibration curve The % values of khe
inhibition are plotted against concentration and
concentrations belonging to 50 % inhibition were read from
this curve /I50/.
Results are shown in Table 2.
- ~ \
4as~3
Table 2
. . _ . ., . ~
Compound I50 micromole/dm3
2-trifluoromethyl- 0.21
4,6-dinitro-phenol
2-trifluoromethyl~ 0.57
4~6 dinitro-phenol-
diethanol-amine
2-trifluoromethyl- 0.52
4,6-dinitro-phenol-
dimethyl-amine
2-trifluoromethyl- 0 25
4,6-dinitro-phenol~NH4
2-trifluoromethyl- 0.20
4,6-dinitro-phenol-Na
2-trifluoromethyl- 0.31
4,6-dinitro-phenol-K
dinitro-ortho-cresol-Na standard 0 86
N~t3,4-dichloro-phenyl/-N',N'- 0.071
dimethyl urea
_ .. . . , _ _ _ ..... __ . _
~ \
- 12 ~2~
One can see that 2-trifluoromethyl-4,6-dinitro-phenol
and salts thereof are similarly to N~/3,4-dichloro-phenyl/-
-N',N'-dimethyl-urea inhibitors of the 2. photochemical system
and their effectivity is in between the effectivity of N-/3,4-
-dichloro-phenyl/-N',N'-dimethyl-urea and dinitro-ortho-
-cresol Among the 2-trifluoro-methyl-4,6-dinitro-phenol
compounds the amine salts are less effective than the alkali
and ammonium salts.
i ~ 10 Exam~le ~
Herbicide test in corn field
The test was carried out with 2-trifluoromethyl-4,6-
~dinitro~phenol-Na 20 WSC formulated according to Example 5
and with 2-trifluoromethyl~4,6-dinitro-phenol 60 EC formulat-
ed according to Example 3, The test was performed on abrown forest soil containing 1,8 % of organic substance.
The soil was seeded on April 25th and 26th with corn of
type Pioneer 3950 with a ~erm number of 73000Jha to a depth
of 8 to 10 cm. Spraying was conducted on May 30th at 1.5 atm,
by means of a parcel sprayine machine equipped with a
.~
sprinkler of type Tee-Jet 11002 with water at a rate of
300 liter/ha. At this time the corn had 3-4 leaves, the main
part of the weeds had 2-4 leaves~ The tests were repeated 4
times. At spraying the field was covered with weeds in 87.5 %,
in 58 % with pig weed, in 27 % with fat hen and the residue
was covered with barnyard grass~ The activity was evaluat-
ed on June 15th. The weed killing activity and the phytotoxic
symptoms observed in corn were evaluated by EWRC scale.
* ~ k
_ 13 ~ ~L2~
The EWRC scale needed for evaluation is contained
in Table 3 and the results are summarized in Table 4.
Data in Table 4 are average values obtained from 4
repetitions .
Table 3
EWBC va lue scale
Value Weed killing effect Killirg
10scale: ~6 injury killing %
1. exce llent 100 no 0
2. excellent 99 very ~light
3, gocd 98 slight 2
4. satisfactory 95 sli~;htly 5
mcderate
5. sufficient 90 m~derate 10
6, insufficient 75 average 25
7, bad 50 strong 50
8 very bad 25 very strong 75
20 9~ tmsuitable ccmplete 100
killing
Table 4
EWRC va lue.s
Composition Dose pig fat Barnyard grass corn
l/ha weed hen
2-trifluoromethyl- 5 3 2 5 1.5
-4,6-dinitro-phenol- 10 1.5 1 2 1.5
Na 20 WSC
2-trifluoromethyl- 1,6 4 3.5 7
4 ,6-dinitro-phenol3 .2 2 1 3 .5
60 EC
J~ - 14 _
... , ~ '~
Crop regulation of corn
Test compound: 2-Trifl~oromethyl-4~6-dinitro-phenol-
-Na 20 % WSC
Corn of the type MVSC 3780 with a germ number
of 55000/ha wa~ s~e~ed on April 20th. Spraying from
helicopter was carried out on July 22nd at the beginning
of the bloomin~ of the corn at a beard length of 4-5 cm.
Application rate 0.25 liter/ha -~0~05 kg./ha active
10 in~redlent~, The land3 of parcels were 4 ha and the test was
repeated twice~ For each ha 50 liters of water was used.
Corn was harvested on November 3rd.
Results are summarized in Table 5.
~
length of length of corn-ear weight of
corn-ear covered by grains corn car
cm cm dkg
1. repetition ~.07~ 2.19 14,52~2.80 22.96+6
62 % cf the
ears is 90.35 % of
defective the corn-ear
len~th i5
covered by grain
2, repetition 17.06+2,71 15.35~3.93 20.84~5
66 /0 of the
ears is 93.34 % of the
defective ears is cwered
by grain
0 control 17.33~1.87 15.33+2,48 19.16~4
84 /0 of the
corn-ears 89.97 % of the
is defec~ive ears is covered
by grain
~28
- 15 --
Crop averages:
1. repetition: 4,25 to/ha = 119.7 %
2, repetition: 4,08 to~ha = 11409 %
0 control: 3,55 to/ha - 100 %,
According to the test 2-trifluoromethyl-4,6-dinitro-
-phenol-Na 20WSC could induce a significant crop increase due
to its effect on the increase of the grain-size and on the
better grain-density.
il
~-, 10 ~
Acaricideactivity test
The test was adjusted on apples of Golden delicious
type planted in a 7,5 m x 4.5 m ~ield in parcels of 5 trees
arranged in rows, Spraying was carried out on March 16th
before budding at a pressure of 20 bar by a manual high
pressure spraying gun of Haflinger Qpraying car. Test-
-compounds; 2-trifluoromethyl-4,6-dinitro-phenol-Na 20 % WSC
and 2-trifluoromethyl-4,6-dinitro-phenol 60 % EC. Protective
activity against eggs of spider mite living through the
winter was evaluated by collecting 5 x 10 pieces of fruit-
-buds from the treated and untreated parcels 48 hours after
the treatment. Observatiors were continued in the laboratory
until the mite hatching terminated ~12 days/. Mortality %
was determined on the b~sis of the number of the hatched
mites and expressed in the % of the control, Results are
shown in Table 6,
- 16
Table 6
Canp~3ition Concentration of number of egg infection
the c~nposition in hatched mortality in % of
the spray liguid mites in % the
% 5xlO fruit- control
-buds pieces
2-trifluarcmethyl-2,5 19 96.35 3.65
4 ,6~dinitro-phenol
-Na 20 WSC
2-trifluaranethyl-0,,8 23 95,59 4.41
4,6 dinitro-phenol
60 ~C
Dinitro~ ho cresol. 2.0 27 94~82 5.18
/DNOC-25~paste
Untreated control - 521 0 100
Compounds of` the general f`ormula I achieved the
activity of Novenda-25 paste containing dinitro-ortho-
-cres o:L