Note: Descriptions are shown in the official language in which they were submitted.
;2
- 1 - 61936-1682
HERBICIDAL 5-AMINO-3-OX0-4-(SUBSTITUTED-PHENYL)-
2,3-DIHYDROTHIOPHENE AND DERIVATIVES THEREOF
BACKGROUND OF THE INVENTION
This invention relates -to 5-amino-3-oxo-4-(sub-
stituted-phenyl)-2,3-dihydrothiophene derivatives and to the use
of such compounds as herbicides and plant growth regulators.
Japanese Patent No. ]9090 (Chemical Abstracts 69P10352e)
generical]y discloses certa~n 5-amino-3-oxo-4-(phenyl or
10 halophenyl)-2,3-dihydrothiophenes, including 5-amino-3-oxo-4-
(phenyl and 4-chlorophenyl)-2,3-dihydro-thiophenes, as pharmaceu-
ticals. Based on Chem. Abstracts 95: 24799e, Russian Patent SU
767105, discloses 5-amino-3-oxo-4-(4-methoxyphenyl)-2,3-dihydro-
thiophene.
Chemiker-Zeitung 104 (1980) No. 10, Pages 302-303, is an
academic paper disclosing the ring closure of l-(dimethyl-
amino`)-2~4-diphenyl-l-buten-3~4-dione to yield 5-dimethyl-
amino-2,4-diphenyl-2,3-dihydrofuran. British Patent
No. 1,521,092, disc]oses certain 3-phenyl-5-substituted-4(1H)-
pyrid-ones or -thiones as herbicides. Japanese Patent Application
13,710/69 (Chemical Abs-tracts 71:61195e) discloses 5-amino-3-oxo-
4-(phenyl or 4-chlorophenyl)-2,3-dihydroEurans and Japanese Patent
No. 68/21423 discloses p-(2-amino-4,5-dihydro-4-oxo-3-thienyl)-
benzene sulfon;c acid. ~Ielvetica Chemlca Acta, Volume 66, Pages
362-378 (1983) discloses 5-N-cyc]opropyl-4-phenyl-2-methoxy-
carbonylmethylene-3-furanone as part of an academic chemical
~ " ~j `1
t~
" 1,2"~t7~X
- 2 - 61936-16~2
synthesis discusslon. U.S. Patent No. 4,441,910 discloses
herbicidal ureldosulfonylfu~ans and ureidosulfonylthiophenes.
In my co-pending Canadian application Serial No.
4S6,115, filed June 7, 1984, I disclose certain herbicidal
5-amino-3-oxo-4-(subs-ti-tuted phenyl)-2,3-dihydrofurans and
derivatives thereof.
SUMMARY OF THE INVENTION
The present invention provides compounds having both
pre-emergence and post~emeryence herbicidal activity and having
especially good pre-emergence activity against a broad spectrum of
both broad-leaf weeds and grassy weeds. At lower application
ra-tes the compounds can be applied as plant growth regulators.
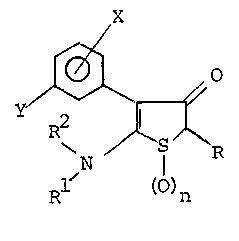
The compounds of the present invention can be
represented by the following formula:
1~1
Y
R2 ~ ~ (I)
R ()n
wherein n is 0, 1, ar 2; R is lower alkyl having L
through 4 carbon atoms; cycloalkyl having 3 -through 7 carbon
atoms, (cycloalkyl)alkylene havlng 3 through 7 carbon atoms in the
cyc]oalkyl moiety and 1 through 3 carbons in the alkylene moiety;
lower alkenyl; haloalkyl having 1 through ~ carbon atoms and 1
through 3 halo a-toms independently selected from the group of
fluoro, chloro, bromo or iodo; haloalkenyl having 2 through 4
5~
- 2a - 61936-1682
carbon atoms and l through 3 halo atoms at the terminal carbon
atom, which halo atoms are independently selected froM the group
of fluoro, chloro, bromo, or iodo; lower alkoxy; lower alkylthio;
lower alkoxyalkyl wherein the alkoxy and alkyl moiety thereof
independently have 1 through 3 carbon atoms; lower alklylthioalkyl
wherein the alkyl moieties independently have 1 through 3 carbon
atoms; phenyl, naphth-l-yl, .inden-l-yl; 4-fluorophenyl;
arylalkylene having 1 through 3 carbon atoms in the alkylene
moiety and wherein said aryl moiety is phenyl, naphth-l-yl or
inden-l-yl; or substituted
~.~
8~t~
01 -3-
aryl or arylalkylene selected from the group having
the formulas:
05
R4 R5 R4 R5
'V ~'
-(R3) ~ o~; (R3) ~
R7~ R6R9 ~ R6 ; or
R4 R5
-(R3) ~ 1 0 ~
~ 6
~ R
R8 R7
wherein one, two or three of R4, R5, R6'
R7, R8, and R9 are independently selected from
the group of lower alkyl, lower alkoxy, halo,
nitro, or haloalkyl having 1 through 3 carbon
atoms and 1 through 3 of the same or different
halo atoms, and the remainder of R4, R5, R6, R7,
R8 and R9 are hydrogen; and R3 is a single bond
or an alkylene having 1 through 3 carbon atoms;
R1 is hydrogen or alkyl having 1 through 4
carbon atoms;
R2 is hydrogen, alkyl having 1 through 4 carbon
atoms, alkenyl having 3 or 4 carbon atoms, alkoxy-
carbonylalkyl having from 1 through 4 carbon atoms in
the alkoxy moiety and from 1 through 4 carbon atoms
in the alkyl moiety; alkoxyalkyl wherein the alkoxy
and alkyl moieties independently have 1 through 3
7~2
- 4 - 61936-1682
carbon atoms, or alkylthioalkyl wherein the alkyl moieties
independently have 1 through 3 carbon atoms; or
Rl and ~2 toge-ther with the nitrogen to which they are
joined form a saturated or unsaturated nitrogen heterocycle
having from 3 through 6 ring atoms one of which is nitrogen and
the remainder of which are carbon atoms;
X is hydrogen, lower alkyl, lower alkoxy, halo, or
trifluoromethyl and can be at any avai.lable position on the
phenyl ring; and
0 ~ i5 lower alkyl; lower alkoxy; halo; lower haloallcyl
having 1 through 4 carbon a-toms, and 1 through 3 of the same or
different halo atoms; lower haloalkoxy having 1 through 4 carbon
atoms and 1 through 3 of the same or different halo atoms, or
lower haloalkylthio having 1 through 4 carbon atoms and l through
3 of the same or different halo atoms; with the proviso that when
Y is other than tri.fluoromethyl and Rl is hydrogen and R2 is
hydrogen then R is methyl, ethyl, propyl, 2-halophenyl, 2-lower
alkylphenyl or 4-fluorophenyl.
The invent;.on al.so comprises compatible salts oE the
compound of Formula (I), for example sal-ts obtained via
replacement of the amino hydrogen (i.e., Rl and R2 is hydrogen)
with a compatible cation or enolation of -the 3-oxo group
following replacement oE the ~mino hydrogen.
The compounds of Formul.a I can exist as oxo-enol
tautomers. The compounds of Formula (I) also have an asymmetric
carbon atom and when n=l have an asymmetric sulfur and can exist
c~t~
5~
- 4a - 61936-1682
as optical isomers and/or diastereomers. The above formula is
intended to encompass the respective -tautomers and optical and
geometric isomers where they exist as well as mixtures -thereoE and
the respective isomers as well as mixtures thereof are encompassed
within the invent;.on.
~.~
S~
Ol ~5-
It has also been discovered that generally thepresence of a 3-trifluorometh~l substituent on the
05 4-phenyl group of the compounds of the present invention
very substantially enhances herbicidal activity.
In a further aspect the invention provides a
herbicidal composition comprising a compatible carrier and
a herbicidally effective amount of the compounds of
10 Formula (I), or compatible salts thereof, or mixtures
thereof.
The present invention also provides a method for
preventing or controlling the growth of unwanted vegeta-
tion, which comprises treating the growth medium and/or
15 the foliage of such vegetation with a herbicidally effec-
tive amount of the compound(s) of Formula (I) and/or com-
patible salts thereof.
In another aspect, the present invention pro-
vides a plant growth regulating composition comprising a
~0 compatible carrier and a plant growth regulating amount of
the compound of Formula (I), compatible salts of
Formula (I), or mixtures thereof, effective to alter ~he
normal growth pattern of said plants.
-~ The present invention also provides a method for
25 regulating plant growth which comprises treating the
growth medium and/or the foliage of such vegetation with a
plant growth regulating effective amount of the com-
pound(s) of Formula (I) and/or compatible salts thereof,
effective to alter the normal growth pattern of said
30 plants.
The present invention also provides chemical
intermediates and processes for preparing the compounds of
Formula (I).
The invention will be further described herein-
35 below.
Ol -6~ 5~
FURTHER DESCRIPTION OF THE
INVENTION AND THE PREFERRED EMBODIMENTS
05 Illustrations of typical compounds of
Formula (I) of the present invention can be had by refer-
ence to Examples 1, 3-8 set forth hereinbelow on
Pages 29-31, 33-54. In terms of substituents, the pre-
ferred compounds are those wherein R is hydrogen, methyl,
lO ethyl, propyl, phenyl or monosubstituted phenyl, more
preferably, methyl, ethyl, n-propyl, phenyl or monohalo-
or monomethyl-substituted phenyl, and especially ethyl,
n-propyl; phenyl, 2-chlorophenyl, 2-methylphenyl and 2-
fluorophenyl, n is preferably 0, Rl and R2 are preferably
15 independently hydrogen, methyl, ethyl or n-propyl, and
more preferably one of Rl or R2 is hydrogen and the other
is methyl, ethyl or n-propyl, preferably methyl or ethyl;
Y is preferably lower haloalkyl and especially trifluoro-
methyl. X is generally preferably hydrogen. The prefer-
20 red compounds have at least one preferred substituent(preferably the Y substituent) and more preferabl~ have a
combination of preferred substituents.
Synthesis
The compounds of Formula (I) wherein Rl and R2
25 are each hydrogen can be prepared by the following sche-
matically represented process:
X X
I
30 ~ O R ~ ~ O
fHCC-SCH3 ~ cyclizing agent > ~ ~
CN H2N S~R
(A) (Ia)
wherein X, Y, and R are as defined hereinabove.
This process can be conveniently effected by
contacting Compound (A) with a cyclizing agent, under
reactive conditions, preferably in the presence of an
40 inert organic solvent.
01 ~7~
Typically, this process is conducted at tempera-
tures in the range oE about from 0 to 200C, pre~erably
05 about from 115 to 120C, for about from 10 to 120 minutes,
pre~erably about from 10 to 30 minutes, using about from 1
to 10, preferably 1 to 2 moles of cyclizing agent per mole
of Compound (A). Suitable cyclizing agents which can be
used include, for example, strong anhydrous acid, for
10 example, sulfuric acid, hydrogen ehloride, hydrogen
bromide, trifluoroaeetic acid, methane sulfonic aeid, and
the like. Suitable organie solvents whieh can be used
inelude, for example, aeetie aeid, propionie aeid, butyrie
aeid, toluene, xylene, and the like, and compatible
15 mixtures thereof.
Best results are obtained using anhydrous
sul~uric acid as the eyelizing agent.
The starting materials of Formula A wherein R is
hydrogen, lower alkyl, eyeloalkyl, alkoxy, alkoxyalkyl,
20 alkylthioalkyl, lower haloalkyl, lo~er haloalkenyl,
arylalkylene, substituted arylalkylene or alkenylalkyl
(e.g., -CH2CH=CH) ean be prepared by the following
schematieally represented overall reaetion equ~tion:
X
O H
CH-C-CH-SCH3
CN (1) /
~ (A')
X
~' O H
r ~ e ~ I 2M~
~ ~ C - C-C-SCH3 R'X; ~ (A wherein R=R')
35CN (A'')
wherein X' is chloro, bromo, or iodo (preferably
iodo); M is sodium or lithium, R' is hydrogen, lower
~0
~`~ -8- ~2~ 75~
alkyl, cycloalkyl, alkoxy, alkoxyalkyl, alkylthio-
alkyl, lower haloalkyl, lower haloalkenyl aryl-
05 alkylene, substituted arylalkylene or alkenylalkyl;
and ~ and Y are as defined hereinabove.
This process is conveniently conducted in two
steps by first contacting compound (A') with an alkali
metal amide (preferably [(CH3)3Si]2NeLi~) under reactive
10 conditions to form an intermediate dianion salt. Step 1
is preferably conducted in an inert organic solvent. In
the seconcl step, preferably conducted in situ, the reac-
tion product of the first step (i.e., A'') is contacted
with the appropriate R'X', under reactive conditions to
15 yield the desired R substitution. This reaction is also
preferably conducted in an inert organic solvent. Also,
both steps of this process are preferably conducted under
anhydrous conditions under an inert atmosphere such as,
for example, nitrogen.
Typically, step 1 of this process is conducted
at temperatures in the range of about from -100 to 25Ct
preferably about from -78 to 25C, for about from 1/2to 5
hours, preferably l/2to 11/2hours, using about ~rom 1 to 5
moles, preferably 2 to 2.5 moles, of alkali metal amide
salt per mole of compound A. Suitable alkali metal amides
which can be used include, ~or example, lithium bis(tri-
methylsilyl)amide (i.e. [(CH3)3Si]2NeLi~); sodium
bis(trimethylsilyl)amide; potassium bis(trimethyl-
silyl)amide; lithium diethylamide; lithium diisopropyl-
amide; sodium dimethylamide, and the like. The alkalimetal amides are generally known compounds and can be
prepared by known procedures, or obvious modiEications
thereof, Eor example, by the reaction of a secondary amine
with n-butyl alkali metal. Lithium bis(trimethylsilyl)-
3S amide is preferred as it gives very good results and canbe conveniently obtained Erom commercial sources. Suit-
able inert solvents, which can be used, include, for exam-
ple, tetrahydroEuran, dioxane, dimethoxyethane, diethyl
ether, diisopropyl ether, and the like and compatible
mixtures thereof.
01 _9_ ~2~ 7~
The second step of this process is typically
conducted at temperatures in the range of about from -30
05 to 30C, preEerably, 22 to 25C for about from 1 to 18
hours, preferably 1 to 5 hours using about from 1 to 10
moles, preferably 1 to 1.5 moles of RX' per mole of ~'.
The R'X' halides are generally known compounds and can be
prepared by known procedures or obvious modifications
thereof (e.g., substitution of appropriate reactants and
solvents).
The starting materials of Formula (A') can be
prepared by the following schematically represented
process:
X
~ H2CN + CH3S-CH2-C-oR5 -> (A')
(B) (C)
wherein R5 is lower alkyl (e.g., methyl or
ethyl) , aryl (e.gO phenyl) or arylalkylene (e.g.
benzyl); and, Y and X are as defined hereinabove.
This process can be conveniently effected by
contacting Compound (B) with Compound (C), and a strong
base under reactive conditions, preferably in an inert
organic solvent.
Typically, this process is conducted at tempera-
tures in the range of about from 0 to 100C, preferably
75 to 85C, for about from 5 to 36 hours, preferably 18 to
24 hours, using about from 1.0 to 10.0, preerably 1.0 to
1.2 moles of Compound (C) per mole of Compound (B).
Typically, about from 1.0 to 10.0 moles of base are used
per mole of Compound (C).
Suitable strong bases which can be used include,
for example, alkali metal alkanolates, for example, sodium
methoxide, sodium ethoxide, potassium ethoxide, sodium
hydride, potassium hydride, and the like. The strong base
ol --lo-- ~ 75~
should preferably be one which does not yield water as a
by-product in this reaction system.
05 Suitable inert solvents which can be used
include, for example, lower alkanols (for example,
methanol, ethanol, and propanol) tetrahydrofuran,
dimethoxyethane, dioxane, and the like, and compatible
mixtures thereo~. Conveniently, the alkali metal
10 alkanolate is prepared in situ by reacting an alkali metal
with excess alkanol which in turn serves as solvent ~or
the above reaction.
The starting materials of Formulas (B) and (C)
are generally known materials and can be prepared by known
15 procedures, or obvious modifications thereof (i.e., sub-
stitution of appropriate starting materials). The pre-
paration of Compound (B) is for example described in
Org. Syn. Coll., Volume 1, 107 (1941), and the preparation
of Compound (C) is described in Methoden Der Organischen
20 Chemie (Houben-Weyl) vol. IX page 107 (1955).
A general procedure for preparing the starting
materials of Formula A can be made by the following
schematically represented by the overall reaction
equation:
X
R O
y ~CH2CN +CH3S-CH--C-oR5 ~ (A)
(B) (C' )
wherein R, X, and Y are as defined hereinabove
and R5 is lower alkyl, pre-ferably methyl.
This process can be conveniently effected by
contacting Compound (B) with Compound (C'), and a strong
base, under reactive conditions, preferably in an inert
organic solvent.
Typically, this process is conducted at tempera-
tures in the range of about from 0 to 100C, preferably
0~ 2~ 75~2
75 to 85C for about from 5 to 36 hours, preferably 18 to
24 hours, using about from 1.0 to 10.0, preferably 1.0 to
os 1.2 moles of Compound (C') per mole of Compound (B). This
process can also be conveniently conducted at room temper~
ature. Typically, about from 1.0 to 10.0 moles of base
are used per mole of Compound (C').
Suitable strong bases which can be used include,
10 Eor example, alkali metal alkanolates, for example, sodium
methoxide, sodium ethoxide, potassium ethoxide, sodium
hydride, potassium hydride, and the like. The strong base
should preferably be one which does not yield water as a
by-product in this reaction system.
Suitable inert solvents which can be used
include, for example, lower alkanols (for example,
methanol, ethanol, and propanol) tetrahydrofuran,
dimethoxyethane, dioxane, and the like, and compa-tible
mixtures thereof. Conveniently, the alkali metal
20 alkanolate is prepared in situ by reacting an alkali metal
with excess alkanol which in turn serves as solvent for
the above reaction.
As pointed out above, the starting materials of
Formula B are known compounds or can be prepared by
25 obvious modifications of known procedures. The starting
materials of formula C' can be prepared by the following
schematically represented overall reaction equation:
R O
1"
~-CHCoR5 ~ CH3SH > (C')
(D) (E)
wherein ~ is chloro or bromo and R and R5 are as
defined hereinabove.
This process can be conveniently effected by
contacting Compound (D) with methyl mercaptan (E) under
reactive conditions preferably in an inert organic solvent
and in the presence of a scavenger base to react with the
40 hydrogen halide by-product of the reaction.
` 01 -12- ~2~0~7~
Typically, this process is conducted at temper-
atures in the range of about from 0-40C, preferably
05 0-25C, using about from 0 8 to 2 moles, preferably 1.1
to 1.5 moles of methyl mercaptan per mole of Compound (D).
Suitable solvents which can be used include for example
methylene chloride, tetrahydrofuran, 1,2~dichloroethane,
chloroform, carbon tetrachloride and the like and
10 compatible mixtures thereof. Suitable scavenger bases
which can be used include, for example, triethylamine;
pyridine; methylpyridine; 1,5-diazabicyclo [4.3.0] nonene;
1,8-diazabicyclo [5.4.0] undec-7-ene; sodium bicarbonate,
potassium bicarbonate, sodium hydroxide, potassium
15 hydroxide, alkali metal alkoxides (e.g., sodium methoxide,
potassium ethoxide) and the like. Typically about 0.9 to
1.5 mole equivalents of scavenger base are used per mole
of Compound (D).
The starting materials of Formula (D) can be
20 prepared by applying the procedure described in Org. Syn.
Coll. Vol. III, 381 (1955) using the appropriate starting
materials. The starting materials of Formula (D) wherein
R - phenyl or substituted phenyl can also be prepared via
the following schematically representPd overall reaction
25 equation:
RCH2CoR5 ~ ~ > (D)
O I o
(F) (G)
wherein R, R5 and Z are as defined hereinabove.
This process can be conveniently conducted by
contactin~ Compound F with N-bromo or chloro-succinimide
(G) under reactive conditions preferably in an inert
organic solvent.
Typicall~, this process is conducted at temper-
40 atures in the range of about from 40 to 100C, preferably
.. .
~ 13~ 75~
60 to 80C, using about from 0.9 to 1.5 moles of the
N-halosuccinimide (G) per mole of Compound (F). Suitable
05 solvents which can be used include, for example, carbon
tetrachloride; 1,2~dichloroethane, methylene chloride,
chlorobenzene, chloroform, and the like and compatible
mixtures thereof.
The starting materials of Formula F are
generally known materials and can be prepared by known
procedures, or obvious modifications thereoE (i.e., sub-
stitution of appropriate starting materials). A
preparation for Compound (F) is described in Org. Syn.
Coll., Volume 1, 270 (1941). N-bromo and N-chloro-
succinimide are, of course, well known commercialcompounds.
The compounds of Formula (I) wherein Rl and R2
are each hydrogen and R is aryl or substituted aryl are
preferably prepared via the following schematically
represented process:
X X
O R6 O
25 ~ ~ IHC CH2 > ~ ~ C - C - CHR6 2M~
Y Y
(A")
/ X
Y H2N S/ R6
(Ib~)
wherein R6 is aryl or substituted aryl, M is an
alkali metal anion, and X and Y are as defined here-
inabove.
5~2
01 -14-
This process is conveniently conducted in twosteps by first contacting compound A" with an alkali metal
S amide under reactive conditions, preferably in an inert
organic solvent or organic carrier liquid. The reaction
product of the first step can then be contacted with
elemental sulfur, conveniently in situ, under reactive
conditions.
The first step is typically conducted at temper-
atures in the range of about from -78 to 25C, preferably
-30 to 22C, for about from 1/2 to 5 hours, preferably about
rom 1/2to 2 hours, using abouk from 2 to 10 moles, pref-
erably 2 to 2.5 moles, of alkali metal amide per mole of
compound A". Suitable alkali amides and organic solvents
or carrier liquids which can be used include those
described with respect to alkali metal reaction previously
described hereinabove.
The second step of this process can then be
effected by contacting the reaction product of the first
step with elemental sulfur, preferably in an organic
solvent or organic carrier liquid and most conveniently is
conducted in situ. This step is typically conducted at
temperatures in the range of about from 20 to 30C,
preferably 22 to 25C, for about from 1 to 2~ hours,
preferably 18 to 24 hours, using about from 1 to 5,
preferably 1 to 1.1 moles of elemental sulfur per mole of
compound A". Suitable inert organic solvents or organic
carrier liquids include those described with respect to
the first step of this process.
The starting materials of formula A" can be
prepared via the following schematically represented over-
all reaction equation:
X
R6 o
CH2CN + H2C - C-oR5 ~ (A")
/ (B) (C')
y
01 -15- ~2 ~7~2
wherein R5 and R6 are as defined hereinabove.
This process can be effected in the same manner
~5 as described hereinabove with respect to the reaction of
compounds B and C but replacing compound C with
compound C'. This process can also be used to prepare the
correponding analogs of A" wherein R6 is lower alkoxy or
haloalkoxy by using the corresponding R6 alkoxy or
lO haloalkoxy analog of C'.
The compound of Formula (I) wherein one or both
of Rl and R2 are lower alkyl or lower alkenyl and n=0 can
be prepared by alkylation (or alkenylation) of the amino
group:
X X
\N ~ R
(Ib) (Ic)
wherein R, Rl, and X are as defined hereinabove;
R is as defined for R2 but is not hydrogen3 and R3Z'
is an alkylation agent having the desired R group or
Rl group if dialkylation is desired.
This process can be effected by contacting
Compound (Ib) under reactive conditions with a suitable
alkylation agent capable of alkylating primary or
secondary amino groups.
For example, this can be effected by contacting
Compound ~Ib) with a R3 halide, preferably R3I or R3Br,
preferably in an inert organic solvent and preferably in
the presence of a scavenger base and a phase transfer
agent. Typically, this process is conducted at tempera-
tures in the range o about from 0 to 100C, preferably
20 to 45C for about Erom 1.0 to 72.0, preferably 2.0 to
4Q
01 -16- 1~ 8~ ~2
18.0 hours~ Where it is desired to monoalkylate, typi-
cally about from 1.0 to 1.1 molas of R3Z' reactant is used
05 per mole of Compound (Ib). Where it is desired to
dialkylate, typically about from 1.9 to 4.0 moles o~ R3Z'
are used per mole of Compound (Ib). In the case where it
is desired to prepare the compound wherein R3 is
alkoxyalkyl or alkylthioalkyl, it is preferred to use a
10 large excess of R3 halide even where monoalkylation is
desired; for example 3 to 6 moles oE R3Z' per mole of
Ib. Further alkylation can be effected in a second step
if desired~ Variation in Rl and R2 can be effected by
first alkylation of only one of the two amino hydrogens
15 and then alkylating the second amino hydrogen with an
alkylating agent having a different R3 alkyl or alkenyl
group. The compounds wherein Rl and R2 together with the
amino nitrogen atoms form a saturated heterocycle can be
prepared by using the appropriate Z''-(CH2)2_5-Z', wherein
20 Zll and Z' are I or Br alkylating agent. The RlR2N
unsaturated heterocycle can be prepared by using the
appropriate cis-alkenyl dihalide, wherein one of the halo
atoms is on each o~ the terminal alkenyl carbons.
Suitable inert organic solvents which can be
25 used, include, for example, liquid halogenated alkanes,
for example, methylene chloride, carbon tetrachloride,
dichloroethane; tetrahydrofuran and the like. Suitable
scavenger bases include, for example, alkali hydroxides or
the bases described hereinabove with respect to the
30 reaction of Compound (B) with Compound (C). Suitable
phase transfer agents are agents which transfer
hydrophilic ions into a lipophilic organic medium and
include, for example, benzyl triethylammonium chloride,
tetra-n-butylammonium chloride, methyltrioctylammonium
35 chloride, and the like.
The compounds of Formula (Ic) wherein R3 is
lower alkyl (e.g. methyl) and Rl is hydroge~ or lower
alkyl, are advantageously prepared using dialkyl sulEate
as the alkylating agent. This can be conveniently
40 effected by contacting the compound of Formula (Ib) with
01 -17- ~28V~5~
the desired lower alkyl sulfates in the presence of a
strong base and preferably in an inert organic solvent in
05 the presence o~ a phase transfer agent. Typically, this
process is conducted at temperatures in the range of abou~
from 0 to 100C, preerably 20 to 45C, using about from
1.0 to 4.0 moles of dialkyl sulfate per mole of
Compound (I'). An excess, typically about 2.5 mole of
10 base is used. Preferably, this process is also conducted
in an inert organic solvent such as, for example,
tetrahydrofuran, carbon tetrachloride, dichloroethane, and
the like.
Suitable strong bases which can be used include,
15 for example, sodium hydroxide, potassium hydroxide, sodium
ethoxide, sodium carbonate, potassium carbonate, and the
like. Suitable phase transfer agents are agents which
transfer hydrophilic ions into a lipophilic organic medium
and include, for example, benzyl triethylammonium
20 chloride, tetra-n-butylammonium chloride, methyltrioctyl-
ammonium chloride, and the like.
My colleagues M. Haire, et al., have discovered
that conducting the alkyl iodide and alkyl sulfate
alkylation processes using an inert organic solvent in
25 which the base is insoluble (for example, using potassium
carbonate or sodium hydroxide in methylene chloride) in
the absence of a phase transfer agent improves the
selestivity of ~he process for monoalkylation. The speed
of the reaction is increased by using a phase transfer
30 agent (e.g., benzyl triethylammonium chloride) but
selectivity is lost. The process is further improved by
using a phase transfer agent which functions as both the
phase transfer agent and the base, such as benzyltrimethyl
ammonium hydroxide.
The alkylation etc., can also be felicitously
effected via the procedure of my colleague P. Pomidor, by
contacting co~npound Ib with the desired aqueous R3 primary
amine, in methanol or ethanol at elevated temperatures
(e.g., 90-120C) and pressures (e.g., 4-8 atmospheres).
~0
.. . .
52
~1 -18-
The sulfoxide and sulfones of the invention can
be conveniently prepared:
05 X X
y~/ O y~/ O
~ I oxidation ~ Rl ~ R
lOl,N S R 2,N
()n'
(Ia)
wherein R, Rl, R2, X and Y are as defined herein
above and n' is 1 or 2.
Any suitable oxidation procedùre can be used to
effect the oxidation. Basically, the same procedure is
used to prepare the sulfoxides and sulfones with the
exception of the severity of the reaction conditions
20 and/or the amount of the oxidizing agent.
In the case of the sulfoxides (n'=l) the
oxidation can be effected by contacting the corresponding
compound of formula Ia with about from 1.0 to 1.5 moles o~
oxidizing agent under reactive conditions preferably in an
inert organic solvent. Typically, the oxidation is
conducted at temperatures in the range of about from 0 to
45C, preferably 20 to 25C for about from 1.0 to 48.0
hours, preferably 12.0 to 24.0 hours, uslng about from l.0
to 2.0 moles, preferably 1.0 to 1.5 moles of oxidizing
30 agent per mole of compound Ia.
In the case of the sulfones (n'=2) the reaction
is typically conducted at temperatures in the range oE
about from 0 to 45C, preferably 20 to 25C, for about
from 24 to 72 hours, preEerably 24 to 48 hours using about
from 1.0 to 6.0 moles, preferably 2.0 to 4.0 moles of
starting materials per mole of compound Ia.
Suitable oxidizing agents which can be used
include, for example, m-chloroperbenzoic acid, hydrogen
peroxide, sodium periodate, potassium permanganate,
peracetic acid, and the like. Suitable solvents which can
01 -19- ~ 2 ~7~
be used include, for example, methylene chloride, chloro-
form, carbon tetrachloride, acetic acid, water, and the
~5 like, and compatible mixtures thereof.
The compatible salts of Formula (I) can be pre
pared by conventional procedures by tr0ating the compound
of Formula (I) with a suitable strong base such as, for
example, n-butyllithium, sodium hydride, potassium
10 hydride, and the like, having the desired cation, by con-
ventional procedures. The enolate salts can be prepared
by treating the Rl and/or R2 cation salts with base in
accordance with conventional procedures. Additional
variations in the salt cation can also be effected via ion
15 exchange with an ion exchange resin having the desired
cation.
G0neral Process Conditions
In the above-described processes, it is gener-
ally preferable to separate the respective products before
20 proceeding with the next step in the reaction sequence,
except where described as an in situ step or unless other-
wise expressly stated. These products can be recovered
from their respective reaction product mixtures by any
suitable separation and purification procedure, such as,
25 for example, recrystallization and chromatography. Suit-
able separation and purification procedures are, for exam-
ple, illustrated in the Examples set forth hereinbelow.
Generally, the reactions described above are
conducted as liquid phase reactions and hence pressure is
30 generally not significant except as it affects temperature
(boiling point) where reactions are conducted at reflux.
Therefore, these reactions are generally conducted at
pressures of about from 300 to 3,000 mm of mercury and
conveniently are conducted at about atmospheric or ambient
35 pressure.
It should also be appreciated that where typical
or preferred process conditions (e.g., reaction tempera-
tures, times, mole ratios of reactants, solvents, etc.)
have been given, that other process conditions could also
40 be used. Optimum reaction conditions (e.g., temperature,
-20- ~ 7 S~
reaction time, mol ratios, solvents, etc.) may vary with
the particular reagents or organic solvents used but can
05 be determined by routine optimization procedures.
Where optical isomer mixtures are obtained, the
respective optical isomers can be obtained by conventional
resolution procedures. Geometric isomers can be separated
by conventional separation procedures which depend upon
10 differences in physical properties between the g00metric
isomers.
Definitions
As used herein the following terms have the
following meanings unless expressly stated to the
15 contrary
The term "lower alkyl" refers to both straight-
and branched-chain alkyl groups having a total of from 1
through 4 carbon atoms and includes primary, secondary and
tertiary alkyl groups. Typical lower alkyls include, for
20 example, methyl, ethyl, n-propyl, isopropyl, n-butyl,
t-butyl.
The term "alkylene" refers to both straight
chained and branched chained alkylene groups. The term
"lower alkylene" refers to alkylenes having 1 through 4
25 carbon atoms and includes, for example,
ICH3
-CH2-; -CH2-CH2-; -CH2CH2- and the like.
The term "lower alkenyl" refers to alkenyl
30 groups having 2 through 6, preferably 2 through 4, carbon
atoms and includes, for example, vinyl, l-propenyl,
2-propenyl, l-methylvinyl, l-butenyl, 2-methylprop-1-enyl
and the like.
The term "lower alkoxy" refers to the group -OR'
35 wherein R' is lower alkyl.
The term "lower alkylthio" refers to the group
-SR' wherein R' is lower alkyl.
The term "lower alkoxalkyl" refers to the group
R'OR''- wherein R' and R'' are independently straight
~0
~' -21~ 75~
chain or branched chain alkyl groups having l through 3
carbon atoms.
05 The term "lower alkylthioalkyl" refers to the
group R'SR" - wherein R' and R'' are independently
straight chain or branched chain alkyl groups having 1
through 3 carbon atoms.
The term "lower alkoxycarbonylalkyl" refers to
10 the group
o
R'OCR''-
15 wherein R' is lower alkyl and R " is alkylene having 1
through 4 carbon atoms and can be straight or branched
chained. Typical alkoxycarbonylalkyl groups include for
example, -CH2C(O)OCH3; -CH(CH3)C(O)OC2H5, and the like.
The term "halo" refers to the group of fluoro,
20 chloro, bromo and iodo.
The term "lower haloalkyl" refers to haloalkyl
compounds having 1 through 4 carbon atoms and 1 through 3
halo a~oms independently selected from the group of
fluoro, chloro, bromo and iodo. Preferably the lo~er
25 haloalkyl group has 1 or 2 carbon atoms.
The term "lower haloalkoxy" refers to "lower
alkoxy" groups having 1 through 3 halo atoms independently
selècted from the group of fluoro, chloro, bromo or iodo.
The term "aryl" refers to aryl groups having 6
30 through 10 carbon atoms and includes, ~or example, phenyl,
naphthyl, indenyl. Typically the aryl group will be
phenyl or naphthyl as compounds having such groups are
more readily available commercially than other aryl
compounds.
The term "substituted aryl" refers to aryl
groups having 1 through 3 substituen~s independently
selected from the group of lower alkyl, lower alkoxy,
halonitro, or haloalkyl having 1 through 3 carbon atoms
and 1 through 3 halo atoms. Typical substituted aryl
-22- ~2~7S~
groups include, for example, 2-fluorophenyl, 2-
chlorophenyl, 2,6-dimethylphenyl, ~-fluorophenyl,
2-methylphenyl, 2-chloro,3-chloromethylphenyl, 2-nitro,5-
methylphenyl, 2,6-dichlorophenyl, 3-trifluoromethylphenyl,
2-methoxyphenyl, 2-bromonaphth-1-yl, 3-methoxyinden-1-yl,
and the like.
The term "arylalkylene" refers to the group
10 ArR3 - wherein Ar is aryl and R3 is alkylene having 1
through 3 carbon atoms~ R3 includes both straight-
chained and branched-chained alkylenes, for example,
methylene, ethyl, l-methylethyl, and propyl.
The term "(substituted aryl)alkylene" or
15 "ring-substituted arylalkylene" refers to the group
Ar'R3 - wherein Ar' is substituted aryl and R3 is
alkylene as defined with respect to arylalkylene.
The term "cycloalkyl" refers to cycloalkyl
groups having 3 through 7 carbon atoms, for example
20 cyclopropyl, cyclopentyl, cyclohexyl or the like.
The term "(cycloalkyl)alkylene" refers to the
group Y'R3 wherein Y' is cycloalkyl and R3 is alkylene
as defined hereinabove with respect to arylalkylene.
The term "saturated nitrogen heterocycle" as
25 used herein with respect to Rl and R2 of formula I refers
to the groups having the formula:
3~ / N \
& J
(CH2)n
whereirl n is 1, 2, or 3.
The term "unsaturated nitrogen heterocycle" as
used herein with respect to Rl and R2 oE formula I refer
to the groups having the formulas:
01 -23- ~ 8~7 32
N N N N~
05
I l l I
~ ~ ~ and
The term "compatible salts" refers to salts
which do not significantly adversely alter the herbicidal
15 properties of the parent compound. Suitable salts include
cation salts such asl for example, the cation salts of
lithium, sodium, potassium, alkali earth metals, ammonia,
quaternary ammonium salts, and the like.
The term "room temperature" or "ambient
20 temperature" refers to about 20-25C.
Utilit~
The compounds of Formula (I) exhibit both
pre-emergence and post-emergence herbicidal activity and
exhibit especially good pre-emergence herbicidal
25 activity. Also by varying the dosage rate certain of the
compounds exhibit acceptable safety with respect to
certain broadleaf crops, notably soybean crops, while
retaining a broad spectrum of pr0-emergence herbicidal
activity against both broadleaf weeds and grasses.
Generally, for post-emergent applications, the
herbicidal compounds are applied directly to the foliage
or other plant parts. For pre-emergence applications, the
herbicidal compounds are applied to the growth medium, or
prospective growth medium, for the plant. The optimum
35 amount of the herbicidal compound or composition will vary
with the particular plant species, and the extent of plant
growth, if any, and the particular part of the plant which
i5 contacted and the extent of contact. The optimum
dosage can also vary with the general location, or
~0 environment (e.g., sheltered areas such as greenhouses
-24- ~ 752
compared to exposed areas such as fields), and type and
degree of contr~l desired. Generally, for both pre- and
05 post-emergent control, the present compounds are applied
at rates of about from 0002 to 60 kg/ha, preferably about
from 0.02 to 10 kg/ha.
Also, although in theory the compounds can be
applied undiluted, in actual practice they are generally
10 applied as a composition or formulation comprising an
effective amount of the compound(s) and an acceptable car-
rier. An acceptable or compatible carrier (agriculturally
acceptable carrier) i5 one which does not significantly
adversely af~ect the desired biological effect achieved by
15 the active compounds, save to dilute it. Typically, the
composition contains about from 0.05 to 95% by weight of
the compound of Formula (I) or mixtures thereof. Concen-
trates can also be made having high concentrations
designed for dilution prior to application. The carrier
20 can be a solid, liquid, or aerosol. The actual composi-
tions can take the form of granules, powders, dusts, solu-
tions, emulsions, slurries, aerosols, and the like.
Suitable solid carriers which can be used
include, for example, natural clays (such as kaolin, atta-
25 pulgite, montmorillonite, etc.), talcs, pyrophyllite,diatomaceous silica, synthetic fine silica, calcium alu-
minosilicate, tricalcium phosphate, and the like. Also,
organic materials, such as, for example, walnut shell
flour, cotton-seed hulls, wheat flour, wood flour, wood
30 bark flour, and the like can also be used as carriers.
Suitable liquid diluents which can be used include, Eor
example, water, organic solvents (e.g., hydrocarbons such
as benzene, toluene, dimethylsulfoxide, kerosene, diesel
fuel, fuel oil, petroleum naphtha, etc.), and the like.
35 Suitable aerosol carriers which can be used include con~
ventional aerosol carriers such as halogenated alkanes,
etc.
The composition can also contain various promo-
ters and surface-active agents which enhance the rate of
transport of the active compound into the plant tissue
~ -25- ~8~7~
such as, for example, organic solvents, wetting agents and
oils, and in the case of compositions designed for pre-
05 emergence application agents which reduce the leachabilityof the compound or otherwise enhance soil stability.
The composition can also contain various
compatible adjuvants, stabilizers, conditioners, insecti-
cides, fungicides, and if desired, other herbicidally
10 active compounds.
At reduced dosages the compounds o~ the present
invention also exhibit plant growth regulating activity
and can be used to alter the normal growth pattern of
green plants.
The compounds of Formula (I) can be applied as
plant growth regulators in pure form, but more pragmatic-
ally, as in the case of herbicidal application, are
applied in combination with a carrier. The same types of
carriers as set forth hereinabove with respect to the
20 herbicidal compositions can also be used. Depending on
the desired application, the plant growth regulating com-
position can also contain, or be applied in combination
with other compatible ingredients such as desiccants,
defoliants, surface-active agents, adjuvants, fungicides,
25 and insecticides. Typically, the plant growth regulating
composition will contain a total of about from 0.005 to
90 wt. % of the compound(s) of Formula (I) depending on
whether the composition is intended to be applied directly
or diluted first.
A further understanding of the invention can be
had in the following non-limiting Preparation and
Examples. Wherein, unless expressly stated to the con-
trary, all temperatures and temperature ranges refer to
the Centigrade or Celcius system and the term "ambient" or
"room temperature" refers to about 20-25~C. The term
"percent" or "%" refers to weight percent and the term
"mole" or "moles" refers to gram moles. The term
"equivalent" refers to a quantity oE reagent equal in
moles, to the moles of the preceding or succeeding reac-
tan~ recited in that example in terms of finite moles or
01 -26- ~2~5~
finite weight or volume. ~here given, proton-magnetic
resonance spectrum (p.m.r. or n.m.r.) were determined at
05 60 mHz, signals are assigned as singlets (s), broad
singlets (bs), doublets (d), double doublets (dd), trip-
lets (t), double triplets (dt), quartets (q), and multi-
plets (m); and cps refers to cycles per second. Also
where necessary preparations and examples are repeated to
10 provide additional starting material for subsequent
examples.
PREPARATIONS AND EXAMPLES
PREPARATION 1
(3-Trifluoromethylphenyl)-benzylcarbonyl-acetonitrile
In this example, 4.91 g of metallic sodium was
added to lL0 ml of anhydrous ethanol at room temperature
and stirred until all of the sodium dissolved. A mixture
containing 18.76 g of (3-trifluoromethylphenyl) aceto-
nitrile and 21.73 g of ethyl phenylacetate was then added
dropwise and the resulting mixture was stirred at reflux
for about 18 hours. The mixture was then poured into
300 ml water and then extracted three times with ethyl
ether. The pH of the extracted aqueous layer was then
adjusted to a pH of about 1 with aqueous 10 wt. % hydro-
chloric acid and then again extracted three times with
ethyl ether. The organic layer was then washed twice with
sa~urated aqueous sodium bicarbonate, dried over magnesium
sulfate and evaporated to dryness under vacuum affording
22.6 g of the title compound.
Similarly, by applying the above procedure using
the appropriately substituted-phenyl acetonitrile and
ethyl-substituted acetate starting materials, the
following compounds can be prepared:
(5-chloro-3-trifluoromethylphenyl)-benzylcar-
3 bonyl-acetonitrile;
(4-chloro-3-trifluoromethylphenyl)-benzylcar-
bonyl-acetonitrile;
(2-bromo-3-trifluoromethylphenyl)-benzylcar-
bonyl-acetonitrile;
01 -27- ~X8~7~
(6-fluoro-3-trifluoromethylphenyl)-benzylcar-
bonyl-acetonitrile;
05 (4-methyl-3-trifluoromethylphenyl) benzylcar-
bonyl-acetonitrile;
(5-methoxy-3-trifluoromethylphenyl)-benzylcar-
bonyl-acetonitrile;
(6-iodo-3-tri~luoromethylphenyl)-benzylcarbonyl-
10 acetonitril~;
(3,5-di-tri~luoromethylphenyl)-benzylcarbonyl-
acetonitrile;
(3-trifluoromethylphenyl)-(2,6-difluorobenzyl-
carbonyl)-acetonitrile;
(3-trifluoromethylphenyl)-(3-iodobenzyl)-aceto-
nitrile;
(3-trifluoromethylphenyl~-naphth-1-ylmethylene-
acetonitrile;
(3-trifluoromethylphenyl)-(2-methylnaphth-1-
20 ylmethylene)-acetonitrile;
(3-trifluoromethylphenyl)-(3-ethoxynaphth-1-
ylmethylene)-acetonitrile;
(3-n-butylphenyl)-benzylcarbonyl-acetonitrile;
(3-n-butoxyphenyl)-benzylcarbonyl-acetonitrile;
(3-trifluoromethylthiophenyl)-benzylcarbonyl-
acetonitrile;
(3-difluoromethoxyphenyl)-benzylcarbonyl-aceto-
nitrile;
(3-chloromethylthiophenyl)-benzylcarbonyl-aceto-
nitrile;
(3-bromophenyl)-(2-ni-trobenzylcarbonyl)-aceto-
nitrile;
(2-chloro-3-propylphenyl)-(6-nitronaphth-1-
ylmethylene)-acetonitrile;
(3-bromo-2-ethylphenyl)-naphth-1-yl~ethylene-
acetonitrile;
. (3,6-difluorophenyl)-beta-naphth-1-ylethyl-
acetonitrile;
(3-iodo-4-methylphenyl)-(2,7-difluoronaphth-1-
ylmethylene)-acetonitrile;
01 -28~ 75~
(3-chlorophenyl)-benzylcarbonyl-acetonitrile;
[3-(2-fluoropropylthio)phenyl]-benzylcarbonyl-
05 acetonitrile;
(3-t-butoxyphenyl)-benzylcarbonyl-acetonitrile;
[3-(2,3-dichloropropylthiophenyl)-ben~yl-
carbonyl acetonitrile;
(3-bromophenyl)-benzylcarbonyl-acetonitrile;
(3-iodophenyl)-(2,3-dinitrobenzylcarbonyl)-
acetonitrile;
(3-1ùorophenyl)-(8-trifluoromethylnaphth-1-
ylmethylene)-acetonitrile;
(3-isopropoxyphenyl)-2-naphthylmethylene-aceto-
15 nitrile;
(3-fluorophenyl)-(6-butyl-8-chloronaphth-1-
ylmethylene-acetonitrile;
(3-trifluoromethylphenyl)-(3-nitronaphth-1-
ylmethylene)-acetonitrile.
(3-iodophenyl)-(3-nitrobenzylcarbonyl)-
acetonitrile;
(3-trifluoromethylphenyl)-(2,3-dichlorobenzyl-
carbonyl)-acetonitrile;
(3-methoxyphenyl)-1-naphthylmethylenecarbonyl-
25 acetonitrile;
(3-trifluoromethyl)-(3-chloro-8-fluoronaphth-1-
ylmethylenecarbonyl)-acetonitrile;
: (3-trifluoromethyl)-[(2-trifluoromethyl-3-
methyl-8-methoxy-naphth-1-yl)methylenecarbonyl]-aceto-
nitrile;
(3-trifluoromethyl)-(inden-1-ylmethylene-
carbonyl)-acetonitrile; and
~3-trifluoromethyl)-(2-fluoroinden-1-yl-
methylenecarbonyl)-acetonitrile.
Similarly, by applying the same procedure to the
appropriate ethyl alkoxy substituted acetate the
corresponding alkoxy acetonitrile analogs of the above
compounds can be prepared, for example:
(3-trifluoromethylphenyl)-dimethoxyacetyl-
acetonitrile;
01 -29- ~ ~ 8~ 5
(3-triEluoromethylphenyl)-(butoxy-
methoxyacetyl)-acetonitrile, etc.
05 The alkoxy compounds can be converted to the
corresponding 2-alkoxy compounds of the invention via the
procedure described in Example 3A hereinbelow.
Example 1
2-Phenyl-3-oxo-4-(3-trifluoromethylphenyl)-
~ 5-amino-2,3-dihYdrothiophene
u
In this example a solution containing 2.0 g of
(3-trifluoromethylphenyl)-benzylcarbonyl-acetonitrile in
30 ml of tetrahydrofuran was added dropwise to 13.2 ml of
a 1 molar mixture o lithium bis(trimethylsilyl)amide
15 under anhydrous conditions at -70C. The resulting
mixture was stirred for ten minutes and then allowed to
rise to room temperature and stirred for another 20
minutes. 0.21 g of powdered elemental sulfur was then
admixed therewith and the resulting mixture stirred for
20 about 18 hours. The mixture was then added to 200 ml of
aqueous saturated ~,lmonium chloride solution and then
extracted three times with ethyl ether. The combined
ether extracts were dried over magnesium sulfate and then
concentrated by evaporation under vacuum affording 1.8 g
; 25 Of a crude solid of the title compound. The crude solid
was chromatographed on silica gel eluting with 30% vol.
ethyl acetate:70% petroleum ether to afford 0.4 g of the
title compound.
Similarly, by applying the above procedure to
30 the compounds listed in Preparation 1, the following
compounds can be prepared:
2-phenyl-3-oxo-4-(5-chloro-3-triEluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(4-chloro-3-trifluoromethyl-
35 phenyl)-5-amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(2-bromo-3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(6-fluoro--3-tri1uoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
" 01~30- ~'~8~75~
2-phenyl-3-oxo-4-(4-methyl-3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
052-phenyl-3-oxo-4-(5-methoxy-3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(6-iodo-3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3,5-di-trifluoromethylphenyl)-
10 S-amino-2,3-dihydrothiophene;
2-(2-nitrophenyl)-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-(3-iodophenyl)-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
152-(naphth-1-yl)-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-(2-methylnaphth-1-yl)-3-oxo-4-(3-trifluoro-
methylphenyl)-5-amino-2,3-dihydrothiophene;
2-(3-ethoxynaphth-1-yl)-3-oxo-4-(3-trifluoro-
20 methylphenyl)-5-amino-2,3-dihydrothiophene; and
2-phenyl-3-oxo-4-(3-n-butylphenyl)-5-amino-2,3-
dihydrothiophene;
2-phenyl-3-oxo-4-(3-n-butoxyphenyl)-5-amino-2,3-
dihydrothiophene;
252-phenyl-3-oxo-4-(3-trifluoromethylthiophenyl)-
5-amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-difluoromethoxyphenyl)-5-
amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-chloromethylphenyl)-5-amino-
2,3-dihydrothiophene;
2-(2-nitrophenyl)-3-oxo-4-(3-bromophenyl)-5-
amino-2,3-dihydrothiophene;
2-(6-nitronaphth-1-yl)-3-oxo-4-(2-chloro-3-
propylphenyl)-5-amino-2,3-dihydrothiophene;
352-(naphth-1-yl) 3-oxo-4-(3-bromo-2-ethylphenyl)-
5-amino-2,3-dihydrothiophene;
2-(naphth-1-yl)-3-oxo-4-(3,6-difluorophenyl)-5-
amino-2,3-dihydrothiophene;
2-(2,7-difluoronaphth-1-yl)-3-oxo-4-(3-iodo-4-
methylphenyl)-5-amino-2,3-dihydrothiophene;
~,ao7~'~
01 -31-
2-phenyl-3-oxo-4-(3-chlorophenyl)-5-amino-2,3-
dihydrothiophene;
052-phenyl-3-oxo-4-[3(2-fluoropropylthio)phenyl]-
5-amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-t-butoxyphenyl)-5-amino-2,3-
dihydrothiophene;
2-phenyl-3-oxo-4-[3-(2,3-dichloropropylthio-
10 phenyl)]-5-amino-2,3-dihydrothiophene;
2-phenyl-3-oxo 4-(3-bromophenyl)-5-amina-2,3-
dihydrothiophene;
2-(2,3-dinitrophenyl)-3-oxo-4-(3-iodophenyl)-5-
amino-2,3-dihydrothiophene;
152-(8-trifluoromethylnaphth-1-yl)-3-oxo-4-(3-
fluorophenyl)-5-amino-2,3-dihydrothiophene;
2-(naphth-1-yl)-3-oxo-4-(3-isopropoxyphenyl)-5-
amino-2,3-dihydrothiophene;
2-(6-butyl-8-chloronaphth-1-yl)-3-oxo-4-(3-
20 fluorophenyl)-5-amino-2,3-dihydrothiophene;
2-(3-nitronaphth-1-yl)-3-oxo-4-(3-triEluoro-
methylphenyl)-5-amino-2,3-dihydrothiophene;
2-(3-nitrophenyl)-3-oxo-4-(3-iodophenyl)-5-
amino-2,3-dihydrothiophene;
252-(2,3-dichlorophenyl~-3-oxo-4-(3-trifluoro-
methylphenyl)-5-amino-2,3-dihydrothiophene;
2-(1-naphthyl)-3-oxo-4-(3-methoxyph~nyl)-5-
amino-2,3-dihydrothiophene;
2-(3-chloro-8-fluoronaphth-1-yl-3-oxo-4-(3-tri-
30 fluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-(2-trifluoromethyl-3-methyl-8-methoxy-naphth-
l-yl)-3-oxo-4-(3-triEluoromethylphenyl)-5-amino-2,3-
dihydrothiophene;
2-inden-1-yl-3-oxo-4-(3-triEluoromethylphenyl)-
5-amino-2,3-dihydrothiophene; and
2-(2-fluorcinden-1-yl)-3~oxo-4-(3-trifluoro-
methylphenyl)-5-amino-2,3-dihydrothiophene.
~O
Ol -32- ~ 7~2
Example 2
(3-Trifluoromethylphenyl)-(2-methyl-
05 thiopropionyl)-acetonitrile
In this example 6~0 g of (3-trifluoromethyl-
phenyl)-(methylthioacetyl)-acetonitrile in 15 ml of tetra-
hydrofuran was added dropwise to 43.96 ml of a 1 molar
solution of lithium bis(trimethylsilyl)amide, i.e.
[(CH3)3Si]2NeLi~, under anhydrous conditions at -70C.
The temperature of the resulting mixture was allowed to
rise to room temperature and it was then stirred at room
temperature for 45 minutes. 3.12 g of methyl iodide was
then slowly added and the resulting mixture stirred
15 overnight (about 12-16 hours) under a nitrogen
atmosphera. The mixture was added to 200 ml of saturated
ammonium chloride solution and then extracted three times
with ethyl ether. The ether extracts were combined, dried
over magnesium sulfate and concentrated in vacuo yielding
a crude oily residue. The residue was chromatographed on
silica gel eluting with 70:30 vol. hexane:ethyl acetate
yielding 2.9 g of the title compound as an oil.
Similarly, by following the same procedure using
the appropriately substituted phenyl-(methylthioacetyl)-
acetonitrile and the appropriate R iodide, the correspond-
ing star~ing materials for Example 3, hereinbelow, can be
prepared.
Example 2A
(3-Trifluoromethylphenyl)-(2-phenyl-
~ 2-methylthiopropionyl)-acetonitrile
i In this example a mixture containing 12 g of t3-
trifluoromethylphenyl)-acetonitrile and 14 g of methyl
alpha-thiomethylphenylacetate was added to a stirred
slurry at room temperature containing 3.4 g of sodium
hydride in 150 ml of tetrahydrofuran. The mixture was
stirred for 11/2hours at room temperature under a nitrogen
atmosphere. The mixture was then added to 250 ml of water
extracted twice with ether. The organic (THF ~ ether)
layer was then washed twice with water. The aqueous
layers were combined and then acidified to pH 1 with 10%
hydrochloric acid, extracted three times with ether,
, . , .: ;. ,
~X8~1~S2
01 -33-
washed with saturated aqueous sodium bicarbonate solution,
dried over magnesium sulfate and evaporated under vacuum
05 affordiny 6~5 g of the title compound as a brown oil.
Similarly, by following the same procedure using
the correspondingly substituted starting materials the
starting materials for the product listed in Example 3A
hereinbelow can be made.
Example 3
2-Methyl-3-oxo-4-(3-trifluoromethylphenyl)-
5-amino-2,3-dihydrothioph_ne
In this example, about 2.0 ml of conGentrated
(98 wt%) suluric acid was added ~o a mixture containing
2.9 g of (3-trifluoromethylphenyl)-(2-methylthiopro-
pionyl)-acetonitrile in 20 ml of acetic acid at room
temperature. The mixture was then warmed to reflux and
re~luxed for thirty minutes. The mixture was then
concentrated by evaporation under vacuum. The concentrate
was then chromatographed over silica gel eluting with a
mixture of 2%, vol., acetone in methylene chloride
affording 0.85 g of the title compound.
Similarly, by following the same procedure using
the corresponding appropriately substituted starting mate-
rials the following compounds can be prepared:
2-ethyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
amino-2,3-dihydrothiophene;
2-vinyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
amino-2,3~dihydrothiophene;
2-(n-propyl)-3-oxo-4-(3-trifluoromethylphenyl)-
5-amino-2,3-dihydrothiophene;
2-(n-butyl)-3-oxo-~-(3-trifluoromethylphenyl)-5-
amino-2,3-dihydrothiophene;
2-allyl-3-oxo-4-(2-methoxy-3-triEluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-trifluoromethyl-3-oxo-4-(3-trifluoromethyl-
~henyl)-5-amino~2,3-dihydrothiophene;
2-(2-chlorovinyl)-3-oxo-4-(5-nitro-3-trifluoro-
methylphenyl)-3-oxo-5-amino-2,3-dihydrothiophene;
01-34- ~Z8~75~
2-methyl-3-oxo-4-(2-methoxy-3-chlorophenyl)-5-
amino-2,3-dihydrothiophene;
- 052-(n-propyl)-3-oxo-4-(3-difluoromethoxyphenyl)-
5-amino-2,3-dihydrothiophene;
2-methyl-3-oxo-4-(3-difluoromethoxyphenyl)-5-
amino-2,3-dihydrothiophene;
2-ethyl-3-oxo-4-(2-chloro-3-fluorophenyl)-5-
10 amino-2,3-dihydrothiophene;
2-vinyl-3-oxo-4-(3-propoxyphenyl)-5-amino-2,3-
dihydrothiophene;
2-allyl-3-oxo-4-(3-butylthiophenyl)-5-amino-2,3-
dihydrothiophene;
lS2-trifluoromethyl-3-oxo-4-(3-trifluoromethyl-4-
bromophenyl)-5-amino-2,3-dihydrothiophene;
2-(2-chlorovinyl)-3-oxo-4-(3-chloromethylthio-4-
methylphenyl)-3-oxo-5-amino-2,3-dihydrothioph~ne;
2-methyl-3-oxo-4-[3-(4-fluorobutyl)phenyl]-5-
amino-2,3-dihydrothiophene;
2-ethyl-3-oxo-4-(3-chlorophenyl)-5-amino-2,3-
dihydrothiophene;
2-vinyl-3-oxo-4-(3-butylphenyl)-5-amino-2,3-
dihydrothiophene;
252-allyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
amino-2,3-dihydrothiophene;
2-triEluoromethyl-3-oxo-4-(3,4-di1uorophenyl)-
5-amino-2,3-dihydrothiophene;
2-ethyl-3-oxo-4-(2-chloro-3-fluorophenyl)-5-
amino-2,3-dihydrothiophene;
2-vinyl-3-oxo-4-(2-nitro-3-butoxyphenyl)-5-
amino-2,3-dihydrothiophene;
2-allyl-3-oxo-4-~2-methyl-3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
352-(trifluoromethyl)-3-oxo-4-(3-tri1uoromethyl-
4-bromophenyl)-5-amino-2,3-dihydrothiophene;
. 2-(2-chlorovinyl)-3-oxo-4-(3-nitro-3-propyl-
phenyl)-3-oxo-5-amino-2,3-dihydrothiophene;
2-hexyl-3-oxo-4-(3-fluoromethylthiophenyl)-5-
amino-2,3-dihydrothiophene;
01 -35- 1'~8~
2-propyl-3-oxo-4-(3-iodophenyl)-3-oxo-5-amino-
2,3-dihydrothiophene;
~5 2-isopropyl-3-oxo-4-(2-chloro-3-fluorophenyl) 5-
amino-2,3-dihydrothiophene;
2-ethyl-3 oxo-4-(3-difluoromethoxyphenyl)-5-
amino-2,3-dihydrothiophene;
2-cyclohexyl-3-oxo-4-(3-trifluoromethylphenyl)-
~0 5-amino-2,3-dihydrothiophene;
2-(2-trifluoromethylbenzyl)-3-oxo-4-(3-tri-
Eluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-(beta-naphth-1-ylethyl)-3-oxo-4-(3-trifluoro-
methylphenyl)-5-amino-2,3-dihydrothiophene;
2-(2-fluoro-3-2',2'-dichloroethylbenzyl)-3-oxo-
4-(3-trifluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-(2,3-dichloro-6-methylbenzyl)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-(beta-phenethyl)-3-oxo-4-(3-trifluoromethyl-
20 phenyl)-5-amino-2,3-dihydrothiophene;
2-[3-(2-bromophenyl)propyl]-3-oxo-4-(3-tri-
luoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-[1-methyl-2-(phenyl)ethyl]-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-naphth-1-ylmethylene-3-oxo-4-(3-trifluoro-
methylphenyl)-5-amino-2,3-dihydrothiophene,
2-(2-fluoronaphth-1-ylmethylene)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-cyclopropylmethylene-3-oxo-4-(3-trifluoro-
30 methylphenyl)-5-amino-2,3-dihydrothiophene;
2-(3-butylnaphth-1-ylmethylene)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-(5-methoxynaphth-1-ylmethylene)-3-oxo-4-(3-
trifluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-(6-nitronaphth-1-ylmethylene)-3-oxo-4-(3-tri
fluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-(7-trifluoromethylnaphth-1-ylomethylene)-3-
oxo-4-(3-trifluoromethylphenyl)-5-amino-2,3-dihydro-
thiophene;
~0
01 -36- ~Z B~ 5Z
2-(2-chloro-8-methylnaphth-1-ylmethylene)-3-o~o-
4-(3-trifluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
05 2-[beta-(8-fluoronaphth-1-yl)ethyl]-3-oxo-4-(3-
trifluoromethylphenyl)-5-amino-2,3-dihydrothiophene:
2-[1-(7-methoxynaphth-1-yl)ethyl]-3-oxo-4-(3-
trifluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2-inden-1-ylmethylene-3-oxo-4-(3-trifluoro-
10 methylphenyl)-5-amino-2,3-dihydrothiophene;
2-(2-fluoroinden-1-ylmethylene)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-amino-2,3-dihydrothiophene;
2 methoxymethylene-3-oxo-4-(3-trifluorornethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-cyclopentylethylene-3-oxo-4-(3-trifluoro-
methylphenyl)-5~amino-2,3-dihydrothiophene;
2-propoxymethylene-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-ethoxymethylene-3-oxo-4-(3-trifluoromethyl-
20 phenyl)-5-amino-2,3-dihydrothiophene;
2-(2-methoxypropyl)-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-amino-2,3-dihydrothiophene;
2-methylthiomethylene-3-oxo-4-(3-trifluoro-
methylphenyl)-5-amino-2,3-dihydrothiophene; and
2-(1-propyl~hioethyl)-3-oxo-4-(3-trifluoro-
methylphenyl)-5-amino-2,3-dihydrothiophene.
Example 3A
2-Phenyl-3-oxo-4-(3-
trifluoromethylphenyl)-5-amino-2,3-dihydrothiophene
In this example a mixture containing 10.8 g of
(3-trifluoromethylphenyl)-(2-phenyl-2-methylthiopro-
pionyl)-acetonitrile and 10 ml of concentrated sulfuric
acid in 50 ml of acetic acid i9 warmed to reflux and
refluxed for 20 minutes. The mixture was then concen-
35 trated under vacuum thereby removing most of the acetic
acid. The concentrate was added to ethyl acetate, washed
twice with lN aqueous sodium hydroxide, then twice with
saturated a~ueous sodium bicarbonate, and dried over
magnesium sulfate. The dried mixture was concentrated by
40 vacuum evaporation affording 7 g of a crude dark oil of
01 ~37~
the title compound. The ~rude oil was chromatographed
eluting within 1:1 by vol. of hexane:ethyl acetate,
05 affording 1.6 g of the title compound.
Similarly, by following the same procedure using
the corresponding starting materials the following
compounds can be prepared:
2-me-thoxy-3-oxo-4-(3-trifluoromethylphenyl)-5-
amino-2,3-dihydrothiophene;
2-t3,4-dichlorophenyl)-3-oxo-4-(3-trifluoro-
methylphenyl)-5-amino-2,3-dihydrothiophene;
2-naphthyl-3-oxo-4-(3-trifluoromethyl-4-
bromophenyl)-5-amino-2,3-dihydrothiophene;
2-(3-methylbenzylphenyl)-3-oxo-4-(3-methoxy-4-
methylphenyl)-3-oxo-5-amino-2,3-dihydrothiophene;
2-(3-fluorophenyl)-3-oxo-4-(3-chlorophenyl)-5-
amino-2,3-dihydrothiophene;
2-(2-fluorobenzyl)-3-oxo-4-(3-methylphenyl)-5-
~ amino-2,3-dihydrothiophene;
2-(3-chlorobenzyl)-3-oxo-4-(3-butylthiophenyl)-
5-amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-beta-chloroethylphenyl)-5-
amino-2,3-dihydrothiophene;
2-methoxy-3-oxo-4-(3-trifluoromethylphenyl)-5-
amino-2,3-dihydrothiophene;
2-n-butoxy 3-oxo-4-(3-tri1uoromethylphenyl)-5-
amino-2,3-dihydrothiophene;
2-(1-naphthyl)-3-4-(3-methoxyphenyl)-5-amino-
2,3-dihydrothiophene;
2-(3-chloro-8-fluoronaphth-1-yl)-3-oxo-4-(3-
trifluoromethylphenyl-5-amino-2,3-dihydrothiophene;
2-(2-trifluoromethyl-3-methyl-8-methoxynaphth-1-
yl)-3-oxo-4-(3-trifluoromethylphenyl)-5-amino-2,3-
dihydrothiophene;
2-inden-1-yl-3-oxo-~-(3-trifluoromethylphenyl)-
5-amino-2,3-dihydrothiophene;
2-(2-fluoroinden-1-yl)-3-oxo-4-(3-tri1uoro-
methylphenyl)-5-amino-2,3-dihydrothiophene;
-38- ~i28~7SiZ
2-(2-chloro-3-propylphenyl)-3-o~o-4-(3-tri-
fluoromethylphenyl-5-amino-2,3-dihydrothiophene;
05 2-(2-nitro-3-methoxyphenyl)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-amino-2,3-dihydrothiophene; and
2-(3-methoxy-5-nitro-7-fluoromethylnaphth-1-yl)-
3-oxo-4-(3-trifluoromethylphenyl)-5-amino-2,3-dihydro-
thiophene;
Example 4
2-Phenyl-3-oxo-4-(-3-trifluoromethylphenyl)-
5-methylamlno-2,3-dihYdrothiophene
This example illustrates a procedure which can
be used to prepare the substituted amine derivatives of
15 the present invention~
In this example about 1 g of solid sodium
hydroxide in 4.0 ml of water is added to a mixture
containing 4.6 9 of 2-phenyl-3-oxo-4-(3-trifluoromethyl-
phenyl)-5 amino-1,2-dihydrothiophene in 80 ml of methylene
20 chloride at room temperature followed by the addition of
1.73 g of dimethyl sulfate and 0.21 g of benzyltriethyl
ammonium chloride. The resulting two-phase mixture was
stirred at room temperature for about 18 hours and was
then washed three times with water, dried over magnesium
25 sulfate and then concentrated by evaporation under
vacuum. The residue was purified by chromatography over
silica gel eluting with 1%, vol., tetrahydrofuran in
chloroform affording 1.8 g of the title compound.
Similarly, by following the same procedure using
30 the products listed in Examples 1 and 3 as starting mate-
rials, the corresponding 5-methylamino homologs thereof
can be prepared, for example:
2-phenyl-3-oxo-4-(5-chloro-3-triflueromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(4-chloro-3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-~2-bromo-3-triEluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(6-fluoro--3-trifluoromethyl-
40 phenyl)-5-methylamino-2,3-dihydrothiophene;
-~ 32~
01 -39-
2-phenyl-3-oxo-4-(4-methyl-3-tri~luoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
05 2-phenyl-3-oxo-4-(5-methoxy-3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(6-iodo-3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3,5-di-tri1uoromethylphenyl)-
10 5-methylamino-2,3-dihydrothiophene;
2-(2-nitrophenyl)-3 oxo-4-(3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-(3-iodophenyl)-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-(naphth-1-yl)-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-(2-methylnaphth-1-yl)-3-oxo-4-(3-trifluoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-~3-ethoxynaphth-1-yl)-3-oxo-4-(3-trifluoro-
~0 methylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-n-butylphenyl)-5-methyl-
amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-n-butoxyphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-trifluoromethylthiophenyl)-
5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-difluoromethoxyphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-chloromethylphenyl)-5-
30 methylamino-2,3-dihydrothiophene;
2-(2-nitrophenyl)-3-oxo-4-(3-bromophenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(6-nitronaphth-1-yl)-3-oxo-4-(2-chloro-3-
propylphenyl)-S-methylamino-2,3-dihydrothiophene;
2-(naphth-1-yl)-3-oxo-4-(3-bromo-2-ethylphenyl)-
5-methylamino-2,3-dihydrothiophene;
. 2-(naphth-1-yl)-3-oxo-4-(2,3-difluorophenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(2,7-difluoronaphth-1-yl)-3-oxo-4-(3-iodo-4-
40 methylphenyl)-5-methylamino-2,3-dihydrothiophene;
01 ~40- ~Z ~7 ~
2-phenyl-3-oxo-4-(3-chlorophenyl)-5-methylamino-
2,3-dihydrothiophene;
05 2-phenyl-3-oxo-4-[3-(2-fluoropropylthio~phenyl~-
5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-t-butoxyphenyl)-5-methyl~
amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-[3-(2,3-dichloropropylthio-
10 phenyl)]-5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-bromophenyl)-5-methylamino-
2,3-dihydrothiophene;
2-(2,3-dinitrophenyl)-3-oxo-4-(3-iodophenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(8-trifluoromethylnaphth-1-yl)-3-oxo-4-(3-
fluorophenyl)-5-methylamino-2,3-dihydrothiophene;
2-(naphth-1-yl)-3-oxo-4-(3-isopropoxyphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(6-butyl-8-chloronaphth-1-yl)-3-oxo-4-(3
20 fluorophenyl)-5-methylamino-2,3-dihydrothiophene;
2-(3-nitronaphth-1-yl)-3-oxo-4-(3-fluorophenyl)-
5-methylamino-2,3-dihydrothiophene;
2 (3-nitrophenyl)-3-oxo-4(3-iodophenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(2,3-dichlorophenyl)-3-oxo-4-(3-trifluoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(1-naphthyl)-3-oxo-4-(3-methoxyphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(3-chloro-8-fluoronaphth-1-yl)-3 oxo-4-(3-tri-
30 fluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(2-trifluoromethyl-3-methyl-8-methoxy-naphth-
l-yl)-3-oxo-4-(3-trifluoromethylphenyl)-5-methylamino-2,3-
dihydrothhiophene;
2-inden-1-yl-3-oxo-4-(3-trifluoromethylphenyl)-
35 5-methylamino-2,3-dihydrothiophene;
2-(2-Eluoroinden-1-yl)-3-oxo-4-(3-trifluoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-methyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
methylamino-2,3-dihydrothiophene;
-41~ 807~
2-ethyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
methylamino-2,3-dihydrothiophene;
052-ethyl-3-oxo-4-,3-difluoromethoxyphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(n-propyl-3-oxo-4-(3-difluoromethoxyphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-vinyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
10 methylamino-2,3-dihydrothiophene;
2-(n-propyl)-3-oxo-4-(3-trifluoromethylphenyl)-
S-methylamino-2,3-dihydrothiophene;
2-(n-butyl)-3-oxo-4-(3-trifluoromethylphenyl)-5-
methylamino-2,3-dihydrothiophene;
152-allyl-3-oxo-4-(2-methoxy-3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophen~;
2-trifluoromethyl-3-oxo-4-t3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-(2-chlorovinyl)-3-oxo-4-(5-nitro-3-trifluoro-
20 methylphenyl)-3-oxo-5-methylamino-2,3-dihydrothiophene;
2-methyl-3-oxo-4-(2-methoxy-3-chlorophenyl)-S-
methylamino-2,3 dihydrothiophene;
2-ethyl-3-oxo-4-(2-chloro-3-fluorophenyl)-5-
methylamino-2,3-dihydrothiophene;
252-vinyl-3-oxo-4-(3-propoxyphenyl)-S-methylamino-
2,3-dihydrothiophene;
2-methyl-3-oxo-4-(3-difluoromethoxyphenyl)-5-
: methylamino-2,3-dihydrothiophene;
2-allyl-3-oxo-4-(3-butylthiophenyl)-5-methyl-
30 amino-2,3-dihydrothiophene;
2-trifluoromethyl-3-oxo-4-(3-trifluoromethyl-4-
bromophenyl)-5-methylamino-2,3-dihydrothiophene;
2-(2-chlorovinyl)-3-oxo-4-(3-chloromethylthio-4-
methylphenyl)~3-oxo-5-methylamino-2,3-dihydrothiophene;
352-methyl-3-oxo-4-[3-(4-fluorobutyl)phenyl]-5-
methylamino-2,3-dihydrothiophene;
. 2-ethyl-3-oxo-4-(3-chlorophenyl)-5-methylamino-
2,3-dihydrothiophene;
2-vinyl-3-oxo-4-~3-butylphenyl)-5-methylamino-
2,3-dihydrothiophene;
~2a~7s2
01 -42-
2-allyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
methylamino-2,3-dihydrothiophene;
05 2-trifluoromethyl-3-oxo-4-(3,4-difluorophenyl)-
5-methylamino-2,3-dihydrothiophene;
2-(2-chlorovinyl)-3-oxo-4-(3-bromophenyl)-3-oxo-
5-methylamino-2,3-dihydrothiophene;
2-cyclopropylmethylene-3-oxo-4-(3-trifluoro-
10 methylphenyl)-5-methylamino-2~3-dihydrothiophene;
2-cyclopentylethylene-3-oxo-4-(3-trifluoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-methyl-3-oxo-4-(2-methoxy-3-chlorophenyl)-5-
methylamino-2,3-dihydrothiophene;
2-ethyl-3-oxo-4-(2-chloro-3-~luorophenyl)-5-
methylamino-2,3-dihydrothiophene;
2-vinyl-3-oxo-4-(2-nitro-3-butoxyphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-allyl-3-oxo-4-(2-methyl-3-trifluoromethyl-
20 phenyl)-5-methylamino-2,3-dihydrothiophene;
2-(trifluoromethyl)-3-oxo-4-(3-trifluoromethyl-
4-bromophenyl)-5-methylamino-2,3-dihydrothiophene;
2-(2-chlorovinyl)-3-oxo-4-(3-nitro-3 propyl-
phenyl)-3-oxo-5-methylamino-2,3-dihydrothiophene;
2-hexyl-3-oxo-4-(3-fluoromethylthiophenyl)-5-
methylamino-2,3-dihydrothiophene;
2-propyl-3-oxo-4-(3-iodophenyl)-3-oxo-5-methyl-
amino-2,3-dihydrothiophene;
2-isopropyl-3-oxo-4-(2-chloro-3-fluorophenyl)-S-
30 methylamino-2,3-dihydrothiophene;
2-cyclohexyl-3-oxo-4-(3-tri1uoromethylphenyl)-
5-methylamino-2,3-dihydrothiophene;
2-(2-trifluoromethylbenzyl)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(beta-naphth-1-ylethyl)-3-oxo-4-(3-trifluoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene;
. 2-(2-fluoro-3-2',2'-dichloroethylbenzyl ! -3-oxo-
4-(3-trifluoromethylphenyl)-5-methylamino-2,3-dihydro-
thiophene;
2807~
01 _43_
2-(2,3-dichloro-6-methylbenzyl)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
05 2-(beta-phenethyl)-3-oxo-4-(3-tri~luoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-[3-(2-bromophenyl)propyl]-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-[1-methyl-2-(phenyl)ethyl]-3-oxo-4-(3-tri-
10 fluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-naphth-1-ylmethylene-3-oxo-4-(3-trifluoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(2-fluoronaphth-1-ylmethylene)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(3-butylnaphth-1-ylmethylene)-3-oxo-4-(3-tri-
1uoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(5-methoxynaphth-1-ylmethylene)-3-oxo-4-(3-
trifluoromethylphenyl~-5-methylamino-2,3-dihydrothiophene;
2-(6-nitronaphth-1-ylmethylene)-3-oxo-4-(3-tri-
20 fluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(7-trifluoromethylnaphth-1-ylomethylene)-3-
oxo-4-(3-trifluoromethylphenyl)-5-methylamino-2,3-dihydro-
thiophene;
2-(2-chloro-8-methylnaphth-1-ylmethylene)-3-oxo-
25 4-(3-trifluoromethylphenyl)-5-methylamino-2,3-dihydro-
thiophene;
2-[beta-(8-~luoronaphth-1-yil)ethyl]-3-oxo-4-(3-
trifluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-[1-(7-methoxynaphth-1-yl)ethyl]-3-oxo-4-(3-
30 trifluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-inden 1-ylmethylene-3-oxo-4-(3-tri1uoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(2-fluoroinden-1-ylmethylene)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-methoxymethylene-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-propoxymethylene-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
2-ethoxymethylene-3-oxo-4-(3-trifluoromethyl-
~ phenyl)-5-methylamino-2,3-dihydrothiophene;
01 ~44~ ~ Z ~7 5~
2-(2-methoxypropyl)-3-oxo-4-(3-trifluoromethyl-
phenyl)-5-methylamino-2,3-dihydrothiophene;
05 2-methylthiomethylene-3-oxo-4-~3-trifluoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene; and
2-(1-propylthioethyl~-3-oxo-4-(3-tri1uoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene.
2-(3,4-dichlorophenyl)-3-oxo-4-(3-trifluoro-
10 methylphenyl)-5-methylamino-2~3-dihydrothiophene;
2-naphthyl-3-oxo-4-(3-trifluoromethyl-4-
bromophenyl)-5-methylamino-2,3-dihydrothiophene;
2 (3-methylbenzylphenyl)-3-oxo-4-(3-methoxy-4-
methylphenyl)-3-oxo-5-methylamino-2,3-dihydrothiophene;
2-(3-fluorophenyl)-3-oxo-4-(3-chlorophenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(2-fluorobenzyl)-3-oxo-4-(3-methylphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(3-chlorobenzyl)-3-oxo-4-(3-butylthiophenyl)-
20 5-methylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-beta-chloroethylphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-methoxy-3-oxo-4-(3-trifluoromethylphenyl)-5-
methylamino-2,3-dihydrothiophene;
: 25 2-n-butoxy-3-oxo-4-(3-trifluoromethylphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(1-naphthyl)-3-4-(3-methoxyphenyl)-5-
methylamino-2,3-dihydrothiophene;
2-(3-chloro-8-fluoronaphth-1-yl)-3-oxo-4-(3-
30 trifluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(2-tri1uoromethyl-3-methyl-8-methoxynaphth-1-
yl)-3-oxo-4-(3-trifluoromethylphenyl)-5~methylamino-2,3-
dihydrothiophene;
2-inden-1 yl-3-oxo-4-(3-triEluoromethylphenyl)-
35 5-methylamino-2,3-dihydrothiophene;
2-(2-fluoroinden-1-yl)-3-oxo-4-(3-trifluoro-
methylphenyl)-5-methylamino-2,3-dihydrothiophene;
2-(2-chloro-3-propylphenyl)-3-oxo-4-(3-tri--
fluoromethylphenyl-5-methylamino-2,3-dihydrothiophene;
: 40
01 -45- ~28~7~
2-(2-nitro-3-methoxyphenyl)-3-oxo-4-(3-tri-
fluoromethylphenyl)-5-methylamino-2,3-dihydrothiophene;
05 and
2-(3-methoxy-5-nitro-7-fluoromethylnaphth~l-yl)-
3-oxo-4-(3-trifluoromethylphenyl)-5-methylamino-2,3-
dihydrothiophene;
Similarly, by approximately doubling the amount
10 of dimethylsulfate and increasing the reaction time, the
corresponding 5-dimethylamino homologs thereof can be
prepared. By using diethylsulfate in place of dimethyl-
sulate the corresponding 5-ethylamino and 5-diethylamino
homologs of the above compounds can be prepared.
Example 5
2-(2-Fluorophenyl)-3-oxo-4-(3-trifluoro-
meth~lphenyl)-5 allylamino-2,3-dihydrothiophene
This example illustratas a general procedure
which can be used to prepare 5-substituted amino compounds
20 of the present invention.
One gram of sodium hydroxide in 4~0 ml of water
is added to a mixture of 4.0 g of 2-(2 fluorophenyl)-3-
oxo-4-(3-trifluoromethylphenyl)-5-amino-1,2-dihydrothio-
phene in 80 ml of methylene chloride at room temperature
25 followed by the addition o~ 1.37 g of allyl bromide and
0.27 g of benzyltriethylammonium chloride. This will
result in a two-phase mixture. The mixture is stirred at
room temperature for about 18 hours after which time it is
washed three times with water, dried over magnesium
30 sulfate and concentrated in vacuo. The residue can be
purified by chromatography over silica gel to yield the
title compound.
Similarly, by applying this procedure to the
products listed in Examples 1 and 2, the corresponding
5-allylamino analogs thereof can be prepared. Similarly,
by approximately doubling the amount of allyl bromide and
sodium hydroxide, the corresponding 5-diallylamino analogs
thereof can be prepared.
~0
-46- ~ ~ 8075~
In a like manner, by usin~ ethyl bromide in
place of allyl bromide, the corresponding 5-ethyl and
05 5-diethyl analogs can be prepared.
Similarly, by following the same procedure by
respectively using methoxymethyl bromide, ethylthiomethyl
bromide, methyl bromoacetate, methyl 2-bromobutyrate, 1,5-
dibromopentane, and cis-1,4-dibromobut-1,3-diene in place
10 of alkyl bromide the corresponding 5-methoxymethylamino,
5-ethyilthiomethylamino, 5-methoxycarbonylmethylamino, 5-
(l-methoxycarbonyl propylamino), 5-piperidin-1-yl and 5-
pyrrol-l-yl analogs of the products llisted in Examples 2,
3 and 6 can be prepared ~or example:
2-phenyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
methoxymethylamino-2,3-dihydrothiophene;
2-methyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
methoxymethylamino-2,3-dihydrothiophene;
2-ethyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
20 methoxymethylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
ethylthiomethylamino-2,3-dihydrothiophene;
2-methoxy-3~oxo-4-(3-trifluoromethylphenyl)-5-
ethylthiomethylamino-2,3-dihydrothiophene;
2-methyl-3-oxo-4-(3-trifluoromethylphenyl)-S-
ethylthiomethylamino-2,3-dihydrothiophene;
2-ethoxymethylene-3-oxo-4-(3-trifluoromethyl-
phenyl)-S-ethylthiomethylamino-2,3-dihydrothiophene;
2-ethyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
30 ethylthiomethylamino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
methoxycarbonylmethylamino-2,3-dihydrothiophene;
3-methyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
methoxycarbonylmethylamino-2,3-dihydrothiophene;
2-methylthiomethylene-3-oxo-4-(3-trifluoro-
methylphenyl)-S-methoxycarbonylmethylamino-2,3-dihydro-
thiophene;
2-ethyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
methoxycarbonylmethylamino-2,3-dihydrothiophene;
01 ~47- ~2 ~O ~S~
2-phenyl-3-oxo-4-(3-trifluoromethylphenyl)-5-(1-
methoxycarbonylprop-l-yl)amino-2,3-dihydrothiophene;
o5 2-methyl-3-oxo-4-(3-trifluoromethylphenyl)-5-(1-
me~hoxycarbonylprop-1-yl)amino-2l3-dihydrothiophene;
2-fluoro-3-oxo-4-(3-trifluoromethylphenyl)-5-(1-
methoxycarbonylprop-l-yl)amino-2,3-dihydrothiophene;
2-ethyl-3-oxo-4-(3-trifluoromethylphenyl)-5-(1-
10 methoxycarbonylprop-1-yl)amino-2,3-dihydrothiophene;
2-naphth-1-yl-3-oxo-4-(3-trifluoromethylphenyl)-
5~ methoxycarbonylprop-1-yl)amino-2,3-dihydrothiophene;
2-inden-1-yl-3-oxo-4-(3-trifluoromethylphenyl)-
5-(1-methoxycarbonylprop-1-yl)amino-2,3-dihydrothiophene;
2-phenyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
piperidin-l-yl-2,3-dihydrothiophene; and
2-phenyl-3-oxo-4-(3-trifluoromethylphenyl)-5-
pyrrol-l-yl-2,3-dihydrothiophene, etc.
Similarly, by applying the above procedures
20 using the 5-methylamino products of Example 4 as starting
materials, the corresponding 5-(N-methyl-N-allylamino), 5-
(N-methyl-N-ethylamino), 5-(N-methyl-N-methoxymethyl-
amino), 5-(N-methyl-N-ethylthiomethylamino), 5-(N-methyl-
N-methoxycarbonylmethylamino), and 5-(N-methyl-N-l'-me-
5 thoxycarbonylpropylamino) analogs can be prepared.Example 6
2-Phenyl-3-oxo-4-(3-trifluoromethylphenyl)-
5-amino-2,3-dih~drothiophene oxide
CF3 ~
11~
In this example 1.75 g of 2-phenyl-3-oxo-4-(3-
tri1uoromethylphenyl)-5-amino-1,2-dihydrothiophene was
dissolved in 35 ml of methylene chloride at room tempera-
ture. To the resultiny solution was admixed dropwise a
4 solution of 1.53 g of 80~ m-chloroperbenzoic acid in 35 ml
01 -~8~ 80752
o~ methylene chloride. The reaction mixture was stirred
overnight (about 18 hours) at room temperature after which
05 time it was washed three times with aqueous sodium
thiosulfate solution, one time with lN hydrochloric acid,
one time with water, one time with saturated aqueous
sodium bicarbonate and one time with brine. The organic
phase was dried over magnesium sulfate and concentrated ln
10 vacuo to give 1.86 g o~ brown foam which was chromato-
graphed on silica eluted with 50/50 petroleum ether/ethyl-
acetate yielding 1.03 g of the title compound.
Similarly, the corresponding dihydrothiophene
oxides o~ the thiophene products listed in Examples 1, 3-5
15 can be prepared via the same procedure but using the
corresponding dihydrothiophenes of Examples 1, 3-5 as
starting materials.
Example 6A
2-Phenyl-3-oxo-4-(3-trifluoromethylphenyl)-
5-methylamino-~,3-dihydrothiophene dioxide
CF3
/ N ~ S
CH3
The title compound can be prepared via the
starting material described hereinabove in Example 4,
30 using the procedure described in Example 6, but doubling
the amount o~ m-chloroperbenzoic acid.
The corresponding dihydrothiophene dioxide
analogs oE the products llsted in Examples 1, 3-5 can be
prepared via the same procedure using the corresponding
35 dihydrothiophene starting materials.
`; ~ 7~;~
- 49 - 61936-1682
Example 7
Lithium salt of 2-phenyl-3-oxo-
4-(3-trifluoromethylphenyl)-
5-methylamino-2,3-dihydrothiophene
Thls example illustrates a procedure which can be used
to prepare the cation salts of the present invention.
In this examp].e, 6.6 ml of 1.6M n-butyllithium in hexane
is added dropwise to a stirred solution containing 2.86 g of
2-phenyl-3-oxo-4-(3~-trifluoromethylphenyl)-5-methyl-amino-2,3
dihydrothiophene in 25 ml of tetrahydrofuran at -30C. The
resulting mixture is stirred for 20 minutes and then concentrated
in vacuo to afford the title compound.
Similarly, by following the same procedure, the
corresponding lithium salts of the compounds of Examples 1, and
3-5 can also be prepared.
Example 8
The compounds listed in Tables A and B hereinbelow were
prepared using the appropriate starting materials in procedures
described in the Examples hereinabove. A number of comparison
compounds were also prepared using similar procedures. These
comparison compounds include among others the reference compounds
5-amino-3-oxo-~-pheny]-2,3-dihydrothiophene, 5-amino-3-oxo-4-(2-
fluorophenyl)-2,3-dihydrothiophene and 5-amino-3-oxo-~-(2-chloro-
phenyl)-2,3-dihydro-thiophene. The compar:ison compounds are
reported in Table C here~nbelow.
~,~
~L2~3075~
, ~ * ~C
o
~ 00 ~ ~ ~ ~ I _I W
v ,, O u:, i I , In ~ I . I cn I I c~
,1 o I r o u~
a) p~ ~ ~ cO ~ o ,~ r~ ~ .~ ~ o co Ln ~ o
X ~ ~ o ~ ~ o ~ ~ co ~ ~ cs~
~ ::1 ~I O CO 1" 00 ~ --1 OD ~ ~r ~ ~ t~ ~ N
Col O U~ In ~ d' d' ~ u~ d' U'~ ~r d' d' ~ ~ d'
.,.1 V ~7 o~ IJ Il~ ~ CO U~ ~ d' U') 1~1 L~l el~ In
U~ Z ,1 ~ oo ~ ~o Cl~ et~ 00 ~ er ~ t~ ~I ~ el'
t_) Il'~
¢ ~a
Z ~ ~ ~ ~ I`
:: :~ ~I r~ ~ o o ~ ~ ~ r~ m o r o o u~
~ ~o1 ~
Z ~ .
E3 ~ ~ D 0~ U~ U~ _I ~ 0~ ~ ~ ~ ~ CO ~ _I
:~: m _, ~D _, ~o ~ _, ~ ~ ~ _, ~ ~ u~ ~ ~ In
~; ~ ~ ...............
u~ ~ ~ ~o ~ ~ r- ~ ~ cs~ ~ ~ ~ ~
/ ~ er o _I ~ o a~ ~ ~ er o er u~ ~ ~ O
_/ ~ ~ . . O
. O O
o ~ Z ~ E~ u7 Ln In Ln ~
U~ a3 /~ ~ / ~ ~ . u~ O ~ ~ ~r d' o ~ ~r
O / c~
0 ~ ~ r
~ -,, r~
m ~m 3: m ~m
c~ ~ ~ ~ ~ ~ ~ ~ ~ ~
m m m ^~ m m ~ m m ~C
m
~ ~m m C~ m ~m X m ~ m ~m
m m C~
u c~ l l l l o l l l l l ~ l l
~ co
~; m ~ m t~ m m m m m m m m~
o
D: ~
p:; t`l t`l ~ O
m :C
~ ~ m m
m m ~ X ec ~
ll
o
Z ~ ~ ~ ~ ~ ~D 1` co ~ O ~ ~ ~ ~ U~
~ o ~n o
o o ~
~ D
er ~ I
,i o I I 00 I r~ o~ ~ I I
æ o o o o o o o ~ o o
~1 ~ a~ ~ r ~ n o
~ ~ CO Lrl _1 0 0 0 ~ ~ ~ ~ ~ ~ ~ ~ ~ ~r ~ a~ ~
q~ o
O ~ ~r ~ ~ ~ ~ ~ ~ ~ ~r ~r ~ ~r
h t~ r u~ Ln co ~ ~`1 co o ~ ` o r~
H Z _I ~ ~ ~ l O O a~ I ~r O ~ \ r~l ~ O O C~
~I r~ r ~
c: :~ a~ O
~ O
~ LO~ :~
z ~ . a~
El~ ~ C) ~ ~1 ~ ~ 0 ~ O O t~ ~ S
æ $ ,1 ~ ~ u, u~ ~ o ~ In r cn ,~ ~ o co ~ p.
~ ~ ................... ~,
_ ~ O ~ U~ U~ ~ Ll~ U~ ~ ~ ~ er r~ ~r ~r
~ W
In 9 1~ r~ ~ ~ oo cn ~ ~ ~D 0~ t` ~ ~ ~ a~
~ ~ ~ ~ ~ o ~ ~ ~ c~
O ~ :~ ................ ~ ....... O
o O O ~ ~ ~ ~ I` ao ~ ~ _I ~o ~ 0 o ~ ~ ~o ~ ~ ~ 5~
u ~ ~ O
,~ . o ~ o o o ~ o o a~
c~ ~ oo _~ ~ ~ ~ ~r ~ ~ ~ u~ a~ r ~ ~ ~ 1~ co ~ 1--
_l n 1` a~ co ~ a~ o ~ o r~ ~ o ~ r~
u~ Ul U~ U~ ~ U~ ~D ~ ~ ~ ~ Ul ~ ~D In ~ ~ U~
E~
m ~ m 3: :~ m m m m m
--m ~ ~ I m
!T! ~ U C~ ) * I I I I I
~ U~
r ~ 3
r~ r~) r~ r~ r~) r,~l rr) r~
.~, ~ m m m m :d :c m m o
P~ ~ c~ ) ~ m m ec ~ C ~ ~ m ,1 h ::~
~ O ~
~1 ~u o
u~ h
O
,~ m'~
~ ~) o
~ D: m m ~ m m m m m :c s ~
Il 11 11
r~ rJ~ o ~-1 r,~l r~ D 1` CO ~ O r~ ~ ~ ~ is.
r~l r~ ~ r~ ~ ~ r,~ r~l ~ ~ r~l r,~l ~ ~ r~) r~ ~ r~1 r~) r'~
~ u~ o ~ o u~
o o ~ ~l ~ ~
31 ~81~)7~Z
~ ~ .
o~ ~ ~r 1-- ~ ~ ~
er o ~ ~r ~ ~ o
~D ~ ~ ~ ~ ~ C~ U~ ~ ~ ~ O
,, o 1_,,,,, ~ CO ~ i I
o ~ o ~ ~ ~
r~ d' O In ~ ~ ~ u~ ~ o ~ ~1
:~: r _l ~1 ~ ~ ~ ul a~ u~ ~ ~ a~ o
~ D ~ ~ ~ In
c :~ t-- o ~r ~. ~ o _I 1` o~ ~ ~ o
a) o
~ ~ ~ ~ ~n In d' ~ ~r ~ ~ ~ er ~r r~
o
~ .
,~ v ~ a~ 1` ~ O
u~ Z _~ ~ r~ ~r ~ o co co co ~ o ~r
u~ O ~ ~ In I
., ~ cl 5~ r co ~D o~
~¢ ~: :~ O er C~ I~ ~ I~
~ Co~l ~0 ~
Z ~ O co c~ ~ o
5 5 ~_1 O~ G~ O ~9 0 d' ~r d' 1-- 0 U~
~ ~
_ ~ ~ ~ ~ r~
_ ro ~ ~1 ~ Ir~ I` ~ ~ ~r ell In O!~
~ ~::
O ~ ~ ..... -
VO O u~ u~ ~ er ~ ~ ~ O ~ u~ ~ In 1-
--~ ~ In U~
. ~ ~ CO ~ O ~ _l ~D ~ O o _l
~1 V O ~ J O 1~ 00 00 0;1 01:) D a~ 1~1 ~ co
. 1 ~5 Il~
C) It) ~ 9 W ~D ~D 1~ Lrl Itl
~ ~Q~
~ ~ ~ ~ ~ ~ ~
m
v m m m m
m ~
C
m m o
m m m :c m m c~ m m m m m c~ ~
rl
o
~ r~
,~
P; O ~ o
~ ~ ~ ~ ~ ~ ~ r~ t)
m m m c~ c~ c~ c ~ o m m m
.1
Z u~ co ~ o ~ ~ ~ ~
o O ~ o
~2~7~
~ U~ ~ ~ ~
C ~ ~ ~ L~
.,, ~ C~ ~ _, _,
~1 0 I I I I
O ~ o u~
a) ~ ~ ~ ~ u~ o 1--
~u t--
~1 , ",. co ~o ~D
~ ~ ~ ~I CO ~ ~ U~
O O
~s C~
U~ Z ~1 N ~
C.)
Z ~ In ~ ~ CO
O ~D CO ~O CO CO
t~' EL~ d' ~
~0
Z ~ ~) co co ~ ~ ~D O
~ :C _I ~ ~1 0 er OD 1`
O, ~ E~ ~ .......
v~--O ~ a~ o er
a~ ~ ~ er r- ~ o co u~
r~/ \ O O ~ 00 d' 00 U~ ~D
/ Z Q E-- Lr~ In ~ ~ In In
/~ ~ / \~ . ~ ~ o~ ~ r ~
E~ ~O~) ~ C~ V
~ a~
\~ ~ u~
~ ~ r~
m D: m
I
C~ C~ ~.
~ o
o O o ~
o
o
~ c~
I I I I I I a
o
Z 0~ ~ O ~ ~ ~
~r ~ In ul n In
o O _I o
I
= ~ - 54 - 61936-1682
~ 28~7S~
OD
n ~ ~ r-
o ~ ~ I~
.,, O ~ a) Ln ~ ~ ~ I
a o O u~ ~ 1--~ ~ ~ u~ ~ 0 ~ ~9
~ ~ ~ 9 0 ~
~ o .
~ ~ O ~ ~ ~1 ~ ~
~C ~ ~ O
E~
~ ~ ~1 ~ ~9 ~ l` ~ ~ ~D ~ d' co In ~
3 ~ o ~ r o ~
O
~ .
~ U ~ ~ ~ u~ ~ oo ~ ~ ~ CO C~ OD O
tn $ ~ ~ D ~ o o ~ ~ ~r
Z; C)
O ~ ~ ~ l ~ ~D ~ ~ 1--~ C~ ~r co
t~ ~ ) o Ln r~
8 ~ 0
r ~ 1:4 ~9 ~s~ u) u) ~r u) ~9 ~9 ~ u~ Is) ~n
a ~i h
O O ~ I d . N ~ ~ r~) ~ ~) CS~ ~ 0~ t~ ~1 ~1 1
H ~ / CA) U CO (r1 ~r t~ ~1 1
~ ~ ~ ~ ~ I~ ~ ~ ~ ~ ~ ~ U~ O O
O /~--
r~
~> ~
m ,,~ ~ ~ ~
O rl,--1
$
~1 $ ~ $ ~ $ C) ~ d' ~ ~ r~
I $ $ ~ $ ~ ~ $ $ $ $ ~ ~ ~
o
;~
~I$ d~`~ C ~ O
a)
0 ~ O a
o ' ' ' ' ' ' l l ' ' l ' l
Z~ ~ ~
01 ~55~ ~'~ 8~ 75
Example 9
In this example, the compounds of Tables A and B
05 and the comparison compounds of Table C, hereinabove, were
respectively tested using the procedures described herein~
below for pre-emergent and post-emergent activity against
a variety of grasses and broad-leaf plants including one
grain crop and one broad-leaf crop~ The compounds tested
are identified by compound number in Tables A, B and C
hereinabove.
Pre-Emergent Herbicide Test
Pre-emergence herbicidal activity was determined
in the following manner.
Test solutions of the respective compounds were
prepared as follows:
355.5 mg of test compound was dissolved in 15 ml
of acetone. 2 ml of acetone containing llO mg of a non-
ionic surfactant was added to the solution. 12 ml of this
stock solution was then added to 47.7 ml of water which
contained the same nonionic surfactant at a concentration
of 625 mg/l. In the case where the test material used is
not essentially pure compound the amount of material used
is adjusted to provide the desired concentration of com-
pound.
Seeds of the test vegetation were planted in apot of soil and the test solution was sprayed uniformly
onto the soil surface either at a dose of 27.5 micro-
grams/cm2 or in some instances as indicated in Table 1
hereinbelow, certain of the compounds were tested at a
lower dosages. The pot was watered and placed in a
greenhouse. The pot was watered intermittently and
observed for seedling emergence, health o emerging
seedlings, etc., for a 3-week period. At the end of this
period, the herbicldal ef~ectiveness of the compound was
rated based on the physiological observations. A 0-to-100
scale was used, 0 representing no phytotoxicity, 100
representing complete kill. The results of these tests
are summarized in Table 1.
-56~ 75~
Post-Emergent Herbicidal Test
The test compound was formulated in the same
05 manner as described above for the pre-emergent test.
This formulation was uniformly sprayed on 2 similar pots
containing plants 2 to 3 inches tall (except wild oats,
soybean and watergrass which were 3 to 4 inches tall)
(approximately lS to 25 plants per pot) at a dose of
10 27.5 micrograms/cm2 or in some cases at lower dosages as
footnoted in Table 2. After the plants had dried, they
were placed in a greenhouse and then watered intermit-
tently at their bases as needed. The plants were observed
periodically for phytotoxic effects and physiological and
15 morphological responses to the treatment. After 3 weeks,
the herbicidal effectiveness of the compound was rated
based on these observations. A 0-to-100 scale was used,
0 representing no phytotoxicity, 100 representing complete
kill. The results of these tests are summarized in
20 Table 2.
~LZ~31)7~2
~Ioo~oo~oo~oU~oooo~o
~1 ~ D O O ~ O ~ o o o ~ o
~ ~ _t ~ _l ~ ~ ~
U~
O oO In Oo o Oo Oo oO O O
_, _, _, ~ ~ ~ ~ ~
~a ~ .
~0 a~ x u~
c u~ U'
O S~ o o ~n ~ o o o o o o o o o o o o
., ~ s~ o o ~ o~ o o o o o o o o o o o
d~ 3
O
U? o o ~ o o o o o o o o o o o
V C ~ o o ~ CO I o o ~ o o o o o o C~ o
¢ ~ .
,~ ~ ~ o o o o o ~ o n o In n In
¢ ~ U O u~ o~
. ~ o o o U~ o U o o o o o o o o o ~
u~ 3 rs~ Ir1 u~ r~ r5~ o o o O O O o O O ~`
~1 U~ ~
~ r~ ~5 ~ .
a~ .. ~ ~c ~
4~ O r~ OOOOOOOOOOOOOOOO
~0~ ~J J.) O000~nooooooooooo u~
O ~ ~
o ~/ ~4 U
O O O O r~ O O O O O O O O O O O d'
U~ OOOO~OOOoOoOoOoO
U1
o~ o ~ ~ r~ ~ r` r~ r~ o ~ ~ r~
~ æ ~ ~
~ Ul o U~
o
.
~ Z8075X
o I O o o o u~ o ~ Lr~ o o co u~ In o o o
.~ I O O u~ ~ ~9 ~ ~ ~ O O C~
U2
v o o In ~ u~ o L~ o o o o u~ ~ co u~
o o o~ ~ ~ o o o c~
V~ 3
~ O U~
U~ ~J U~
O ~ o O o O o O o o o o o o ~o o CO O
5:~ h O o o o o ~ ~ IJ~ o O O o co d' a~ o
P~ ~
d~ ~
SJ O O O O O CO a~ o o o o o o o c~ o
C~ o o o o o cr. ~ o o o o o o ~la ~ o
., I O ~ o u~ In O O O O O ~ 00 0 ~ O O O U~
co r~ ~ ~ d'
. ~ ~ U~
~ ta ~ o o o o o o o u~ o o ~ o o In o o
~ ~ 3 u~ a~ o o o ~ ) o o ~ o o a~ o o
~ ~ .~
~ x ~
4~ o ra
0 o s~ o o o o o u7 u~ ~ o o o o co o o o
o o o o o ~ a~ co o o o o a~ ~ o o
r
o s 3
m dP s~
, ~ o o o o o ul ~ I` O O ~ O 00 a) o o
~ o o o o o ~ o~ o o a~ o ~ a~ o o
o~ o 1~ O ~ ~ ~ er U~ ~D t` CO Cr~ O .
~ Z ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
o
~ o U~ o Ln
o o
, .. ..
s~
I o u~ n o o Lr~ O O o o
o co ~ 9In O CO
u~
O In 00 0 0 0 0 0 0 0~ U~ O O O O
u~ :~ ~
a) o ut
(n ~ ~n
~ o ~
C~ ~ ~ InOO~OOOInoooooo
~ S~ Ll~ o o a~ ~D O O 0 00 0 0 ~ O O
~ o o g o ~ o oo o o o o o o o o
~ c)
~, ~1 o o u~ o ul o u~ o o ~ u~ o o o o
o co r~ o oo a~ Lr o ~
~nu~ O O O ~ O o o a:~ o o o o o o
E~ v :~ 3~ o o o u~ o o o a~ o o o o o
~: ~ ~I _I _I _I _I _I ~1 ~ ~ ~ _I
,1 .~
~ o ~a
O ~o o o o n o o o Ln o o o o o o u~
J-d' O O O 00 0 0 0 CO O O O O O
~I ~ ~) ~ ~I ~I ~I ~ ~I
~a ~ :E: 0~) '
h e
~ r~ o o ~ 0t~ O ~ O O O U) a~ o
.. .a ~ ,-, ~ ~ ,~ ~,
o ta
~ o ~ ~ In ~ ~ ~o ~ o ~ ~ ~ et' I
o Z r~
C) ,
_I ln o Ln o
O O ~
7~
~ I o o o Lr o o o o o o o o o o o o
~1 ~ o
~o
o o o o u. o u. o o o o o o O o O o
a:~ o _~
:~ ~
u~ 3
a~ x u~
o ta
S~ o o o o o o o o o o o o o o o o
r~ ~ co o ~
" d~ ~
~ O O O Co o U~ o o o o o o o o o o
4~ ~ o
_ ~
o ~J ~ ~ O~oOOOOO 00000
o~ ~ ¦ oooooInoooooooooo
~ ~ .~ ~ ~
P~ X P~
4J O
S~
O ~ oooooooooooooooo
J-) 1- L~ n ~1 o o o
l ~ U7 _I
ro C
O ~:4 ~:
h ~ ~1
~ U~ oo o o o o o o o o o o o o
~,1 ~D ~ ~ O O
~~1
~r Ln ~ ~ a)
O~o cr o ~ ~ ~ l l l l l l l l l l
~ æ ~ u c~ o o c~
~ o u~ o
o o I I
7S2
a)l o o o o o o
.
o oooooo
U) ~ .~
a~ o u~
U~
o ~
h ~) h
W .~ t:J O O o O o o
dP ~
U~
~ oooooo
oooOoO
J- ~ ~ 000000
~ X ~ ~
~U O ~ ~
t~ ~0 ~ O 000 oo
~a L: 3 S l
1~ ~ ~ 0~)
S~ d~
m ~ c
~ .~
,~ 3 O O O o o o ~D
.a ~a
~1
'~
O
~0 l l l l l l
O ~) C) C) C~ C.) C.) 11
O o Ln O
752
~1 o o 1~ In O O In o o o
~ ~ ~ ~ ~ ~r ~ ~ ~
~ o oooooooo ~U~oL~ooo
o 0 ,, 3
0 ~ 0
U~ ~ O ~J O O O O O O O 1~1 0 0 0 0 U~ Lt Il~ O
3 c: ~
.Co d~ ~:
a~ ~ o o o o o o ~ o ~ o o oLr~ u~ o o
~ C: Q -~ o ~
~` ~
C o o o o o u~ o o ~ o
0~ Q
s:: ~ ~ 3 1 0 0 0 0 o 1-~) o o t~ o u~ u~ o ~r, o o
S~ ~ P~ X .
o ' ~a ~ s~ ~
~ ~ O ~ o In o o u~ O co O ~0 0 ~ O ~ f" ~ ~
~ ~ I ~ u~ ~ O CO O r~ o o~
h P~ ~
,., m h
Il~ ~ C~
~1 :~ O O O O O L~ o ~ Lr~ o o 11~ 0 l~l
0 ~ ~ oo r a~ Lt~ co :o ~ co
~ ~a
3C ~J
O ~ Q L~
O ,~ ,1 ~ ,~ -~ ~ ~ 11
C)
~ o U~
O O ~ l N
~'~8~52
a)
~, , o o o o o o o o U~ o o o o o o o o
P; ~ ~ ~
U~
O Iu~o~u~OOOooInOOOOOOU~
~ ~ ~ ~ I` ~ ~ ~
J~ ~
C) 3
U~ .~
a) O I~?
U~ ~ U~
0 O 0
I ~ o o o o o o ~ o In O O O O O O L~
I`
~ o)
dP ~
~a
~ I o o o In In O O In u~ o o o o o o o o
QCI~
~ ~.)
V ~
o a I O O u~ u~ o ut o o o In Ln o o ~n o n ~
O ~
J ~ 31 1 ~ o o I U c:~ o ~ o o u~ o Ltl
~: ~ C~ Cl~ ~ U~ ~ ~ OD 0~ Ln U~ ~ ~ ~r ~ d' ~t'
~ ,~ .,
_l ~ P~
1:~, .,~
~U O ~
O 0 I o ~ O o u~ o o L~ O o o
v ~ o ~ ~ ~ ~ ~ o cr~ o~ U~ co ~ ~ ~ ~
l ~ U~ ~ ~
~:) ~ :~
O P~ ~:
h ~
~ a~
~ I o o o ~ o U~ o Lr~ o U~ O o ~ L~ o U~ U~
Q
E~
,
P~ O t-- 0~ ~ O ~ ~ ~ ~ L~ o
Oæ
C~
~ ~ O U~ O U~
O O --I
7S~
a~
.~, o o o u~ o o o In O O o o o o o o
~ ~r ~ ~ ~ ~ ~ r~
U~
O o o o o u~ O o o In O O In o o
u~ In ~ ~ o o o
~ ,,
~ ~1
U~ .~, ~
X
U~ o Ul
U~ ~ ~q
o ~
h
o o ~ o u~ O In In O O O O O O O O
5~ h u~ co ~r ~r ~ 00 ~ t` ~`I
~ a)
dP (a
U~
~O
In O O ~ U~
~O er O O O U~ O O O O O O O O
~ ~r In lSl ~ 1~ ~1
_ h
o ~ u~ In o c~ In O U~ O O O O In o o o o n
~J Q t~
3~ o o o ~ o o o o ~ o o o I
~J I
~l ~
t)
~ . E~
o ~a
a ~ s~
o ~ O O u~ O u~ In ~ o o o o o o o o ~ o u~
~1 ~ J~ ~o ~ ~1 o ~ a~ ~ o o ~ ~ o a~
~d ,C ~ 1 . o
h dl~ h h
~ .~
h ~o
u~ ~ O r~In o u~ o o o o o o o o
~o ~ ~ ~ oo 1~ ~ cn ~ ~ Ln a~
~ v
Ql O Ln ~o ~1 ~ ~ ~ In ~ r~ oo cn o ~ E~
a
~ o ~ o u~
o o ~
~ Z8~7`~'Z
a)l
.~1 o o o o o o o o o o o o o o o
U~
,~
o ooooooooooooooo
~ ro r~
U~ ~ 3
~ Xo U~
u~ ~ ~n
~ o a
5~ ~ ~
c~ ~ ~:r u~oooooooooooooo
dP ~
U~
SJ
~ OOoOOOoOOOOOoOO
.a
h
c ~ ~ n o o O o o o o o o ~ o o o o
3~ O C::\ u7 o o o o o o o o o o o
~: ~) U t--
.,~ ,~
~1 C~ P~
P~ X
o ~a
O ~ OOOOOOOOOOOOOOo
~J 1~ ~
O ~
dP
~ OOoOOOOOOOoOoU~o
t~ 1~
C
O
o ~ ~ ~ ~ O r co a~ ~ ~ ,
O ~ r7 t
E~ Z ~ u c~
o
~ o U~ o
o ~ _I _I
.~1 o o o
~ .
o
o o o
~ _,
0 ~') '3
a) x
~ O U~
u~ ~ a
0 ~ 0
O O O
~:4 a)
d~ ~2
,, u~l
~ O O O
ro c)
~0 ~ ~ o O
31 O
~J
~ ~
_~ O
X N
O
t~ ~) ~J
~~ O 0 00 0
0 .C ~ O
h d!~ ~1
:Q ~ t~
a) .~
D
o O O
~a
U)
er u~
O
~ O l l l
O Z C,) C_) C.) 11
, C) t~
~ o
O O 1~
, , ~ .
01 -67~ 8~75~
As can be seen from the above Table 1, the com~
pounds oE the invention generally exhibit a broad spectrum
05 of excellent pre-emergence phytotoxic ac~ivity and espe-
cially so Compounds Nos. 7-9, 13-18, 38, 39 and 46. Also
certain of these compounds exhibited reduced phytotoxicity
with respect to soybean while retaining excellent pre-
emergent phytotoxicity with respect to both broadleaf and
10 grassy weeds. As shown by Table 2 a number of the
compounds also exhibited from modest to very good post-
emergence phytotoxicity but are primarily pre-emergence
herbicides. In contrast to this it can be seen that none
of the comparison compounds exhibited any pre-emergence
herbicidal activity whatsoever and only three of the
comparison compounds (and none of the reference compounds)
exhibited any post-emergence activity and this was at a
very low level. Also, as can be seen from Table 1 the
compounds of the invention having a 4-t3-trifluoromethyl-
20 phenyl) substitutent and/or a 5-methylamino exhibited
substantially superior phytotoxicity.
Obviously, many modifications and variations of
the invention described hereinabove and below can be made
without departing from the essence and scope thereof.
~5
~0