Note: Descriptions are shown in the official language in which they were submitted.
~z~
The present invention relates to a process for
decolorizing bulk-dyed paper and self-copying paper (NCR
paper). In many cases the latter paper also contains bulk-
dyed paper layers.
While a plurality of processes are known for recy-
cling ordinary waste paper, as for example, newspapers and
periodicals, a number of unresolved problems are encountered
in the reprocessing of NCR paper.
The flotation processes conventionally used in so-
called de-inking are actually suitable for detachlng printing-
ink particles from the paper fibre and from the fibrous mate-
rial, but it has been found that they are only slightly effec-
tive in the decolorization of bulk-dyed paper.
Additlonal difficulties are caused by the micro-en-
capsulated dyes of copying paper. In the production of
coloured paper problems are encountered, or example, in recy-
cling the waste into the production on changing the colour.
Furthermore, as a ~unctlon of the portlon of bulk-
dyed paper when using waste paper a dlsplacement of the colour20
shade during the productlon cannot be excluded. When uslng
mixed waste paper for producing recycle paper the uniformity
of the production can be endangered by a high portion of bulk-
dyed paper.
The use of sodium hypochlorite for destroying the
dyes, followed by bleaching with sodium dithionite is known
(Tappi, July 1979, Vol.62, Page 27 to 30). However, hypochlo-
rite can be used only when paper ~ualities containing no cel-
lulose are available. This limited applicability of hypochlo-
rite is due to the fact that the hypochlorite reacts with the30
lignin of ligneous subst~nces. Therefore, the increasing por-
tion of ligneous crude paper materials also renders the use of
hypochlorite practically impossible when reusing bulk-dyed
-- 1 --
~X81509
waste paper. Furthermore, the use of hypochlorite as bleach-
ing agent can also result in the formation of chloro-organic
compounds and in the emission of chlorine constituting a bur-
den on the environment.
A process for decolorizing NCR paper is described in
EP-A 0120132. In this process the paper is broken up in the
presence of sodium hydroxide and hydrogen peroxide without
adding sodium tetrasilicate and without being followed by a
flotation. However, in practice these processes did not find
acceptance because of the unsatisfactory results.
According to DE-OS 1 546 252 the use of formamidine
sulphinic acid, preferably in the presence of sodiu~ bisul-
phate, is recommended for bleaching wood pulps having low cel-
lulose contents.
A similar problem is the sub~ect of DE-PS 3,309,956,
wherein the bleaching of crude paper materlals, as for exam-
ple, wood pulp, as well as that of waste paper is d~scrlbed,
involving mixtures of newspapers and lllustrated magazines
which are mixed, in a conv0ntional flotation process, with
formamidine sulphinic acid instead of hydrogen peroxide with-
out any appreciable increase of the whiteness. The present
invention provides a process for decolorizing bulk-dyed paper
and self-copying paper containing micro-capsulated dyes and
frequently also bulk-dyed paper sheets.
The present invention thus provides a process for
decolorizing bulk-dyed paper and self-copying paper ~NCR
paper) in an alkaline aqueous medium in which the decoloriza-
tion is carried out at a pulp consistency of 2 to 20% and at a
temperature of 20 to 80C, preferably 40 to 60C with 0.05 to
2.5% by weight, preferably 0.1 to 1% by weight, of formamidine
sulphinic acid (FAS), re~ative to the amount of paper(atro).
In a particularly desirable embodiment the pH value
-- 2
~X81509
of -the pulp is so adjusted that at the beginning of the reac-
tion it is between 7.5 and 12.0, preferably between 8.5 and
10.0 and on completion of the reaction it is between 6.0 and
8.0, preferably between 6.5 and 7.5.
For this purpose the paper is first broken up
preferably in a pulper (disintegration of the pulp) at a tem-
perature of between 20 and 80C, preferably between 30 and
70C, in an aqueous medium that is alkaline adjusted prefer-
ably with sodium hydroxide. This is then followed by the
decolorization with FAS.
In a favourable embodiment the FAS is not applied
subsequently but it is applied immediately at the beginning or
during this process step.
Since the breaking-up process per se only requires
approximately lO to 30 minutes, the pulp ls subsequently
allowed to react completely, at 20 to 80C, preferably at 40
to 60C while circulating lt further when required.
Applicable crude materlal is bulk-dyed paper which
can be in a printed-on form or blank, without cellulose or
without containing wood. Self-copying paper contalning micro-
capsulated dyes can also be used.
Particularly when using bulk-dyed paper either
printed on or weakly printed on, or self-copying paper or
waste from the production of these paper types further process
steps and further additional chemicals such as soaps can be
dispensed with in the normal case.
For printed-on paper-without cellulose or containing
wood - and/or when requiring a high degree of whiteness it has
been found that it is expedient to have the prior art process,
such as the flotation-de-inking process or the washing-de-ink-
ing process or a sequence of flotation/washing or wash-
ing/flotation or a combination of washing and flotation pro-
1~81509cesses, using hydrogen peroxide, caustic soda solution, sodium
tetrasilicate and other conventional dispersing and flotation
agents, precede the decoloration with formamidine sulphinic
acid. DE-AS 2 303 827, DE-AS 2 813 448; PTS-Symposium
"Altpapier-Aufbereitung", 1981 Munich, w. Auhorn and J. Mel-
ger, Page 57 to 64 relate to de-inking processes according to
the prior art.
A standard formula for flotation de-inking is as
follows:
1% of hydrogen peroxide (% by weight, relative to
atro pulp)
1% of NaOH
0.3% of Na5 DTPA (pentasodium salt of diethylene
triamlne pentaacetic acid or
another known complexing agent)
3% of sodium tetrasilicate
0.6% of sodium oleate
The above-described problem of decolorizing bulk-
dyed paper types can be solved in a simple manner by means of
the process according to the present invention, i.e., by
treating the pulp with formamidine sulphinic acid. Recycle
paper containing dyes from colour capsules of self-copying
paper can also be decolorized by treatment with formamidine
sulphinic acid according to the present invention while the
treatment with dithionite in the acid range frequently even
results in a loss of whiteness.
When rating dyed paper not only is the whiteness
(R457) a criterion, but measuring the colour also is a crite-
rion. Therefore in the test described hereafter this measure-
ment was also carried out (according to DIN 5033). The data
for the amounts of FAS and dithionite relate to atro pulp.
Example 1 (Comparison wi~hout Formamidine Sulphinic Acid)
Waste paper from 80% of bulk-dyed copying paper
yielded on being broken up without chemicals a whiteness of
- 4 -
i
1281509
42-9% remission (R457)- The colour location is at x = 0. 356
and y = 0. 372. By treatment with 0.5~ of H2O2, 1% of solution
of caustic soda, 2% of sodium tetrasilicate and 0.5% of soap
and 0.1% of DTPA at a pulp consistency of 12% and at 40C a
whiteness of 53.9% remission (R457) and a colour location of x
= 0.333 and y = 0. 354 are measured after the flotation.
Example 2
waste paper from 80% of bulk-dyed copying paper (NCR
paper) is sub~ected to a classical flotation-de-inking process
and subsequently bleached by reduction.
For this purpose the waste paper is impregnated for
15 minutes at a pulp consistency of 15% and at 40C, it is
allowed to react for 60 minutes, separated into fibers for 10
minutes at a pulp consistency of 3% in a Frank standard break-
ing-up device and floated for 10 minutes at a pulp consistency
of 1% in a laboratory flotation cell.
The flotation-de~inked pulp ls then bleached by re-
duction for 1 hour at 70C and at a pulp consistency of 4.5%
with FAS at an initial pH value of approximately 9.0 and a
final pH value of approximately 7. 2 and with dithionite at pH
5.5 to 6. The pH values are first optimized with regard to
attainable decolorizations.
The flotation-de-inked waste paper has a whiteness
of 42-9 remission (R457)- The colour location is x = 0. 356
and y = 0. 372. A more or less complete decolorization of the
flotation-de-inked pulp as a function of the amount of reduc-
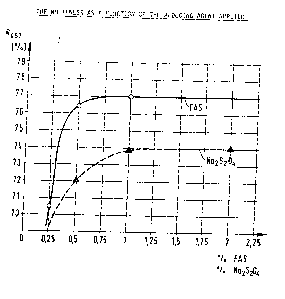
ing agent applied can be observed. (see Fig. 1)
When using 1% of FAS the colour location shifts to x
= 0.322 and y = 0. 333 and when using 1% of dithionite it
shifts to x = 0. 327 and y = 0. 337.
The whiteness increases as a function of the amount
of reducing agent applied increases to 74% remission (R457)
1;~8~509
when using dithionite and to 77% remission when using FAS.
Particularly in the range of lower concentrations the white-
ness obtained with FAS can be obtained only with 2 to 3 times
the amount of dithionite.
Example 3
sulk-dyed waste paper having a high wood content
(more than 40%) is flotation-de-inked in a 120-tato plant and
subjected to subsequent bleaching in a laboratory for 60 min-
utes at a pulp consistency of 15%. For this purpose 0.5% by
weight of FAS and 1.5% by weight dithionite are applied
respectively. The results have been compiled in Table 1.
They show the great bleaching power of FAS even at a
temperature of 37C.
Table 1
Decolorization with Decolorization with
flotation- 0. 5% of FAS1. 5% of Na2S2O4
de-inked (initial pH) - 8. 3
(finalOpH) = 7.1~H = 5.5
R457 temp.( C) R457temp. ( C)
61. 3 50 66.9 50 66.0
61.9 50 65.8 50 64.9
68.6 37 7~. 6 50 71.3
66.3 37 72.0 50 69.7
Example 4
Waste paper is flotation-de-inked according to the
prior art and the material partially dyed as a function of the
origin is reduced with FAS at a pulp density of approximately
12 to 14% in a bleaching toner operated downwardly. The
throughput is 4.5 tons per hour. The aqueous FAS solution is
dosed through a conveyor screw leading to the tower. Samples
of the pulp are taken before it reaches the bleaching tower
(reaction time of 2-3 minutes) and after leaving it (reaction
time of 90 minutes). The results have been compiled in Table
2. It can be seen that as early as after 2 to 3 minutes of
reaction time the pulp has been decolorized substantially.
-- 6
lZ81509
._ ,_
L;
LX)Ll-~ `
r~lr~J ~ r~) ` t
r~J r~ ) r~l r~l
r~ :~ 7 1 77 r 7r r~ 7
~ ~ r~7 - o ~-- Ir~
~ ~ t--Lr~ ~O ~
~ r_ x ," t~7 ~ 7
lo ~ 3 o o o o o o
~1~. O ~7L~l ~i ! .
(1~ tY;(~' 1- `O ~ ~7 ~ $
3 == l
,L-- .~_ I 1`~ r~ r it
n~ :~ ~
~7 ~ o C) o o o o
r~ I . ,
t~ ~ i t-
J~ .,1 1'~ t~7 r~7 ~7 r~7 1`'7
.~ ~ X o o o ~ o o
~ O . _ ......... _._
LL_~ ~ L~r~Jr~lri ~ O` ~ 7
~:t ~ tct7cr~ r~l ~t O Q)
~1 ~ ~ L'K~ O L~ O
~1 ._ =. -- 1~
2 0 ~n u~ Ir~ o o ~ ~t
C Ll 1~7 ~ t' ~t ~t ~ O O
O ~; o o ~ o o o o '~
~ I~ I' - -- -- -- -------
h ~ ~!Ln m ot7 r~J o r~) o~ Ln 00 0 0 5-1 h
O Cl rl o~ o oo r oo ~O 1~ ~O ~ ~ .~ .C,
C O t,E~ E
a o ,~ Lr~ O ,~. r~J O
_ ~ ~ ~J Lr~ rn Lr~ I 0`
_ L~
_ _ a) Q~
~ ~ r7 r7 ~ r7 r7 r7 I I
~ O O O O O O er Ul
a) O ~ ~ O Lr~ o~
~r.. ~ o~) Ln o r~) ~ ~ rl tl
0 x r7 r7 r77 r~7 r7 r7
~ ~ o o o 3 0 0 0
,_~ 0 Ln r'o ~ r~ r7 7 ~ 7 o 7~ a~
.Q ~ ~ ~ ~ Ln ~, r~J 0 o~ O o O r-
E~ ~4 _ li~ O ` ~0 Lr~ 51 Lr~ 0 h ~--I
~ r~ 7 ~J Ln ~O
lZ81509
These tests carried out on a large lndustrlal scale
once more show clearly that bulk-dyed paper (3-6) ls not de-
colorlzed by flotatlon de-lnklng and still shows a distinct
colour cast thereafter.
The flotation was carried out, using the following
additives:
1% of hydrogen peroxlde
0.8% of NaOH
O . 2% of complexing agent (Na5DTPA)
2.0% of sodium tetrasilicate
0.6% of soap
0.1% of dispersing agent (sodium ollnate)
Example 5
A mlxture of approxlmately 70% bulk-dyed waste from
the productlon of self-copying paper is mechanically treated
in a laboratory kneader within 20 minutes at a pulp consis-
tency of 18% and at a temperature of 45C and a pH value of
9 .0
This procedure simulates in an enhanced manner the
conditions in a thick matter pulper and results in a partial
destructlon of the dye capsules with which the paper is
coated. The emerging compounds that are at first colourless
then complete the reactlon to the dyes.
Subsequently the decolorizations were carried out at
a pulp consistency of 5% and at a temperature of 60C with
formamidine sulphinic acid (FAS) and wlth dlthionite. The
results have been compiled in the Tables 3 and 4.
iX8150~
Table 3
colour location pH pH
R457 x Y ~ initial final
~ . ~ . . .... ... , . ......... ._
crude
material 53.1 0.3213 0.3229 53.51
50C in the
kneader 45.1 0.3227 0.3240 46.84
0.5% FAS 60.8 0.30300.3058 51.61 9.0 7.5
0.5% di-
thionite 55.5 0.30600.3083 49.63 6.0 6.3
.,. , . ....._ .. , ... .. ... . . . . .. . . .. .
70C in the
kneader 45.0 0.32080.3207 45.77
0.5% FAS 61.8 0.30150.3039 51.77 9.0 7.4
0.5% di-
thionite 57.8 0.32130.3229 53.51 6.0 6.1
Table 4
colour location pH pH
R457 x Y ~ initial final
crude
maOerial 53.1 0.32130.3229 53.51
50 C in the
kneader 44.2 0.32570.3263 47.60
1% FAS 63.1 0.30600.3100 55.5 9.0 7.3
1% di-
thionite 57.0 0.30870.3119 52.22 6.0 6.0
~ , .. . . ~ ._ _ _ _ _ _ _ _ _ _ _
70C in the
kneader 45.4 0.32090.3212 46.88
1~ FAS 60.8 0.30420.3073 51.95 9.0 7.1
1% di-
thionite 53.1 0.32130.3229 53.51 6.0 6.1
Example 6
A mixture of blank, approximately 70% bulk-dyed
waste (without cellulose) from the production of self-copying
paper is treated at a pulp consistency of 18~ and a tempera-
ture of 70C in a laboratory kneader with FAS ( initial pH
value: 9.0, caustic soda solution and FAS are added simultane-
ously) and with sodium dithionite) initial pH value: 6.0).
The mixture is then diluted to a pulp consistency of 5%, the
pH value is again adjusted to 9.0 (FAS) and to 6.0
(dithionite), whereupon the mixture is stored for 1 hour at
60C. The results have been compiled in Table 5.
_ g --
.
1281SO~
Table 5 Decolorization of blank waste during the dissolution
of the pulp
FAS and 0. 5% by weight 1.0% by welght
dithionite
~ __ _ _
Chromaticies Chromaticies
4 5 7 ~ R 4 5 7 x Y
crude
material 53.1 0.3213 0.3229 53.51 53.1 0.3213 0.3229 53.51
~ ,.. .,. ~ . .. , .. .. .. , .,. _.. ~ .. .
FAS 59.6 0.3008 0.3020 50.96 65.2 0.2969 0.2979 51.87
dithionite 47.5 0.3104 0.3156 46.54 50.3 0.3178 0.3197 51.61
Example 7
A mixture of blank, up to approximately 80% bulk-
dyed waste (free from cellulose) from production of self-copy-
ing paper (NCR paper) is impregnated with the de-inking chemi-
cals at a pulp consistency of 15% and at a temperature of 40C
for 15 minutes, where upon the mixture is allowed to react
completely for 45 minutes at a pulp conslstency of 5% and at a
temperature of 40C. This is followed by separating into fi-
bres for 10 mlnutes in a standard breaking-up device at a pulp
consistency of 3%, washing for 10 minutes wlth 2 litres of wa-
ter per minute and a screen mesh width of 6~,um in the Degussa
20 laboratory washing-de-inking cell at 60C. The washed pulp is
then bleached for one hour at 60C, and a pulp consistency of
5% with 0.5% of FAS/3~ of NaOH and 1% of FAS/0.5% of NaOH.
De-Inking Chemicals:
a. 0.6% of NaOH
0.5% of Na olinate
b. 0.8% of NaOH
0.5% of Na olinate
0.5% of H O
2.0% of sod~um tetrasilicate
0.2% of Na5DTPA
The results have been compiled in Table 6.
-- 10 --
:
1281509
Table 6 Reductive Subsequent sleaching of NCR Waste according
to a Wash-De-lnking Step
a) without H2O2 b) with H2O2
R457chromaticies R457 chromatic'ties
crude
material 40.30.3358 0.3495 52.4 40.3 0.3358 0.3495 52.4
wash-de-
inked 44.30.3513 0.3652 66.7 45.6 0.3508 0.3642 68.7
subsequent bleaching
with 0.5%
of FAS 71.90.3180 0.3272 77.5 73.8 0.3173 0.3266 78.8
with 1%
of FAS 75.20.3139 0.3214 77.8 78.1 0.3143 0.3222 80.8
Example 8
A sample of NCR waste like that ln Example 7 is
treated wlth de-inking chemicals as in Example 7a and 7b,
whereupon lt is floated in the Degussa flotation cell for 10
minutes at a pulp conslstency of 1% and then washed for 10
minutes wlth 2 litres of water per minute at a pulp consis-
tency of 1% and at a screen mesh wldth of 64um in the Degussa
laboratory wash-de-inking cell. The pulp thus obtaln ls sub-
jected to reductive subsequent bleachlng wlth F~S as ln Exam-
ple 7. The results have been compiled ln Table 7.
Table 7 Reductive Subsequent Bleaching of NCR Waste in a
Flotatlon Step and a Wash-De-Inking Step
a~ without H2O~ b) with 0.5~ of H2O2
R457 chromaticities R457 chromaticies
crude
material 400.3355 0.3486 49.8 40.00.3335 0.3486 49.8
flotation
de-inked 40.60.3371 0.3481 56.6 43.4 0.3421 0.3540 56.7
wash
de-inked 44.10.3489 0.3598 67.7 45.2 0.3536 0.3642 69.0
subsequent bleaching
with 0.5%
of FAS 72.8 0.3180 0.3263 77.4 75.2 0.3180 0.3275 81.0
with 1.0%
of FAS 74.20.3182 0~3253 78.8 77.8 0.3156 0.3239 81.9