Note: Descriptions are shown in the official language in which they were submitted.
~33~
Preparation of high_fructose syrup from inulin containing
naturally occurring materials
This invention relates to a high fructose syrup and,
more particularly, to a high fructose-glucose syrup
obtained by hydrolysis of inulin.
Sugar (sucrose) is an extremely important food in-
gredient. It is consumed in vast quantities in the
industrialized countries and, for instance, annua} con-
sumption in the United States is in the order of 25
billion pounds. Whatever may be the virtues or draw-
backs of high sugar consumption, there is no doubt that
people who can afford to buy sugar will consume it in
large quantities~
Dextrose and corn syrups have been widely used as
sweeteners and have unique properties of their own~
However, they suffer in competition with sucrose because
of their lower sweetness value. Dextrose is only 0.74
times as sweet as sucrose. It has long been recognized
that if a significant proportion of the glucose in a
glucose syrup could he converted to fructose, the re-
sulting syrup would have a much greater sweetness
tapproximately 1.5 times the sweetness of sucrose).
It was already well known in the 1800's that glucose
can be isomerized to fructose by treating with alkaline
catalysts at high pH. However, alkaline isomerization
.. . .
~213;3~
has never been practised commercially. The main problems
are the instability of the glucose in warm alkaline solu-
tion and the non-selectivity of alkaline catalysts, both
resulting in the formation of objectionable by-products.
Costly and complicated refining techniques are needed to
remove the objectionable by-products and undesirably high
ash level.
It is also known to hydrolyze sucrose with mineral
acids to give a mixture comprising equal parts of fruc-
tose and glucose. Ho~7ever, this method su~ers from thehigh cost of the starting material.
The most widely used method of producing high fructose
syrup today is enzymatic isomerization of glucose syrups.
Various enzymes have been developed for this purpose and
one such method is described in British Patent 1,103,394.
This method typically produces a syrup containing reducing
sugars in the weight proportions of about 42~ fructose,
50% glucose and 8~ higher saccharides. This syrup can
then be enriched and refined by fractionation to yield a
syrup containing reducing sugars in the weight proportion
o 90% fructose and 10% glucose.
There are three types of high ructose corn syrup
available today, the 42 wt.% fructose syrup mentioned
above, the 9~ wt.% fructose syrup obtained from the 42
~5 wt.% syrup as mentioned above and a 55 wt.% fructose
syrup which is produceci by blending the 42 wt~ ructose
syrup with the 90 wt.% ~ructose syrup in the required
proportions.
~et another known method o producing high Eructose
syrup is the hydrolysis of inulin. This material is a
polysaccharide obtained Erom the roots of certain plants,
typically Jerusalem artichoke. Hydrolysis of inulin found
in Jerusalem artichokes typically yields a syrup contain-
ing about 65 to 80 wt.% ~ructose.
Jerusalem artichoke tubers are rich in inulin and
.
~2B3~
related fructans, which are fructose polymers contain-
ing one terminal glucose unit. They range in size ~rom
sucrose, consisting of 1 glucose and 1 fructose molecule,
up to true inulin consisting of one glucose molecule and
up to 35-40 fructose molecules.
In studies conducted at the University of Manitoba
by E. Hoehn and reported to the 1982 Manitoba Agronomists
Annual Con~er~nce, the procedure for preparing high ~ruc-
tose syrup rom Jerusalem artichoke tubers included three
major steps, namely: (1) extraction, (2) puriEication and
(3) hydrolysis. It was ound that puriication proved to
be the most difficult step in the processing of Jerusalem
artichoke tubers. Purification procedures including pre-
cipitation of impurities with lime, application of ion
exchange resins and treatment of extracts with activated
carbon were evaluated and syrups with tolerable amounts
of ash and N-containing compounds were obtained, using
a combination of all of these procedures. However, the
approach proved to be lengthy and expensive.
Another system that was evaluated relied on ultrafil-
tration and on enzymatic hydrolysis of inulin and related
fructosans. This involved washing and slicing the tubers,
and then extracting them with hot water. The resulting
extract was subjected to ultrafiltration in which inulin
and related fructosans were retained and low molecular
weight nitrogen-containing compounds and minerals were
removed from the extract. The extract obtained rom the
ultra~iltration was then submitted to enzymatic hydroly-
sis and inulin and related ructosans were hydrolyzed to
glucose and fructose. The hydrolyzate was thereater sub-
jected to ultrafiltration to separate ructose and glu-
cose rom high molecular impurities which were retained
by the membrane. This also represented a complex and
expensive procedure.
In traditional acid hydrolysis o~ inulins, the
~?~
4 --
terminal fructose-glucose bond has been difEicult to
hydrolyze. Since depolymerization causes an increase
in terminal frllctose-glucose bonds, intense conditions
are required for complete hydrolysis. However, since
fructose is easily degraded by acid through the process
of enolization and dehydration, flavored and colored
compounds, which are highly undesirable in a syrup, are
produced in large amounts under intense hydrolyzing con-
ditions, e.g. low p~. Accordingly, it has not been
possible to achieve complete hydrolysis without signi-
ficant fructose destruction.
With the above background and the problems involved
in the production of high fructose syrups from inulin,
the present inventors have attempted to provide a simpler
and less expensive technique for producing high fructose
syrups from inulin-containing materials.
According to this invention a greatly simplified
procedure has been discovered for producing high fruc-
tose syrups from inulin-containing naturally occurring
materials. Typical of such inulin-containing materials
are tubers of Artichoke, Dahlia and Chicory.
~ n the process of the invention, the inulin-containing
tubers are first processed to separate a fluid or juice
therefrom containing inulin and related fructans. This
inulin liquid at a pH of about 3 to 5 is subjected to
hydrolysis to produce a hydrolyzate consisting of a high
fructose syrup containing reducing sugars in which fruc-
tose constitutes at least about 70 wt.% of the reducing
sugars. The hydrolyzate contains a considerable quantity
of ash and a large portion oE this ash is removed by sub-
jecting the hydrolyzate to ion exclu.sion chromatography.
Thereafter, the hydrolyzate is subjected to ion exchange
to obtain the Einal product.
During the hydrolysis, the reaction may be contin-
ued to a high degree oE completion, e.g. a hydrolyzate
~2~
containing at least 95% monosaccharides and less than 5%disaccharides, or it may be continued to a lesser degree
of completion, e.g. at least 80~ monosaccharides with the
balance disaccharides and trisaccharides. When the hy-
drolysis is stopped at the less complete stage, there isan advantage that ~ewer reversion products are produced
during hydrolysis. If a strong acid ion exchange is car-
ried out on the hydrolyzate at an elevated temperature,
e.g. above 70C, the hydrolysis is completed during ion
exchange without any production of any undesirable rever-
sion products.
While various hydrolysis systems may be used, a pre-
ferred system is the "SLT Reactor" as described in
Assarsson and Nagasuye, U.S. Patent 4,469,524. With the
SLT Reactor, inulin liquid is continuously moved through
a confined tubular preheat zone where heat is rapidly
transEerred to the inulin liquid. A hot free-flowin~
liquid is formed and this hot liquid at a temperature
above about 100C and below 170C, preferably 130 to
150C is then immediately forced through a restrictive
opening and into a conEined tubular reaction zone ac~
companied by a sudden decrease in pressure whereby the
inulin contained in the artichoke is made highly reac-
tive. This highly reactive material at the above pH is
continuously moved through the tubular reaction zone to
produce a high fructose syrup containing reducing sugars
in which fructose constitutes at least about 70 wt.~ of
the reducing sugars.
The process is operated at superatmospheric pressures
and the hot llquid immediately beEore the restrictive
opening preferably has a pressure oE at least 300 psi,
while the pressure drop across the restrictive opening
is preferably at least 100 psi.
As a source of inulin, it is particularly advanta-
geous to use tubers o~ Jerusalem artichoke. A typical
-- 6
composi~ion of Jerusalem artichoke tubers is given in
Table 1 below:
Table 1.
Proximate Composition of Jerusalem Artichoke Tubers
Composition
Fresh Wt. % Dry Wt.
Water 80
Carbohydrate 15.2 - 28.8 68.4 - 83.1
Lipid .1 .5
Nitrogenl) .29 - .311.45 - 1.55
Ash .4 - 1.25 2 - 5
Cellulose
and Hemicellulose 2.62 13.1
1) 50% of nitrogen associated with proteins
These tubers are first sorted and washed and an inulin-
containing liquid is extracted from them. This can be
done by macerating the tubers, with or without removal of
the skins, and separating the liquid from the remaining
pulp in a press. The liquid or juice obtained typically
has a solids concentration of about 18 to 20 wt.~ and,
in addition to inulin and related fructans also contains
amino acids, peptides, minerals and other contaminants of
lower molecular weight than inulin. This liquid ~ay be
further filtered and at a pH of 3 to 5 Eed directly to a
tubular hydrolyzer.
The product directly from the hydrolyzer is a high
fructose syrup in which at least 80 wt.~ of the reducing
sugars are monosaccharides, with Eructose constituting
at least 70 wt.% of the monosaccharides and the balance
being glucose. The high eructose syrup from the hydro-
~33~
-- 7 --
lyzer contains less than 20 wt.~ dimers and trimers andsubstantially no higher saccharides.
This syrup is filtered and, if necessary, the solids
content is increased. It is then passed through an ion
exclusion column, e.~. Dowex Monosphere resins, to
remove salt and ash. The syrup is then passed through
a two- stage ion exchange which includes a strong acid
stage, followed by a weak base stage. An important fea-
ture of the process is that if the strony acid stage is
~aintained at an elevated temperature, e.g. above about
70C, further hydrolysis of inulin will take place.
Also in the ion exchange stage a portion Oe the dimers
are removed along with any remaining salts or ash and
approximately half of the protein. The dimers which are
extracted are materials other than disaccharides and are
polar materials derived from some of the contaminants in
the inulin liquid The disaccharides remain as part of
the final product. The product obtained is a high fruc-
tose syrup in which at least 97 wt.% of the reducing
sugars are monosaccharides of which at least 70% is fruc-
tose and the balance is glucose and containing less than
about 3 wt.~ disaccharides. The product from the ion
exchange can be passed through a carbon filter to remove
color and thereafter concentrated by evaporation in the
usual manner.
An important feature of this invention is the com-
pleteness of the hydrolysis Oe the fructans to monosac- !
charides. Thus, even though the reaction is beiny car-
ried out at a mild pH Oe about 3-5 and no enzymes are
used, complete hydrolysis oE high-molecular-weight
fructans occurs. The mild pH, together with very short
reaction time, has the very important advantayes oE
avoiding degradation oE fructose as well as decreasing
the Eormation of color and other byproducts normally
associated with acid hydrolysis.
-- 8
According to another ~eature of this invention, it
has been observed that only about 70-80% by weight of the
inulin in Jerllsalem artichoke tubers is soluble in water
at 50C. It has now been found that substantially 100%
of the inulin can be placed in soluble ~orm by masticat-
ing the tubers to form a pulp slurry, adjusting the pH of
the slurry to about 4.5 to 5.5 and holding the slurry at
a temperature of ahout 45-55C for several hours. It is
believed that the natural inulinases containing in the
tubers are effective under these conditions to convert
the insoluble inulins into soluble form. It is parti-
cularly advantageous to use SO2 for the pH adjustment
since this minimizes ash formation.
After the inulin solubiliæation, the pulp slurry is
passed through a press to separate the juice from the pulp
and the pH of this juice is adjusted to about 3-5. The
juice at that pH is then hydrolyzed in a SLT reac~or as
described above. Complete hydrolysis occurs within a very
short time of about 1-2 minutes at a temperature oE about
160C.
Certain pre~erred embodiment of the present invention
are illustrated in the attached drawings in which:
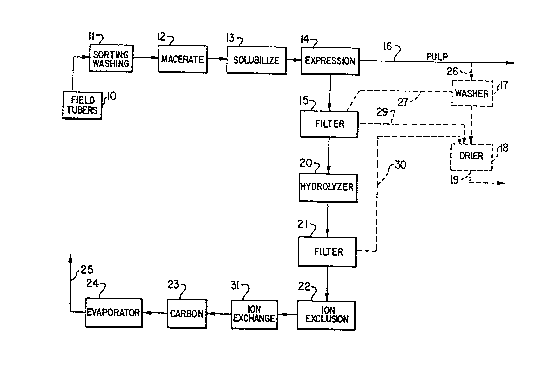
Figure 1 is a schematic flow sheet of one preferred
embodiment of the complete process sequence;
2~ Figure 2 is a schematic representation of one embodi-
ment hydrolyzer that may be used; and
Figure 3 is a plot showin~ separation of salts and ash
Erom sugars during ion exclusion~
Field tubers of ~erusalem artichoke are collected and
placed in storage 10. As a ~irst stage in the process
they are subjected to sorting and washing 11. The clean-
ed tubers, with skins either present or removed, are then
macerated in a macerator 12 and the liquid and pulp mix-
ture obtained is held Eor several hours at about 45-55C
in vessel 13 to solubilize the inulin. This solubilized
.
~Z~3~
g
material is pressed in a screw press 14 to express a juice
~rom the pulp 16. The juice is filtered in ~ilter 15 and
the ~ilter juice with pH adjus~ed to about 3-5 becomes a
hydrolysis feedstock.
The hydrolyzer 20 is shown in greater detail in Figure
2 and includes a juice holding tank 31 which feeds by an
outlet line 32 to a positive displacement pump 33. The
juice is pumped through line 34 at high pressure and into
a heating tube 35.
The main reactor of this apparatus is a closed and
insulated vessel 36 which is essentially a steam vessel
being supplied by a steam inlet line 37 and a steam out-
let line 38. A steam control valve 43 is provided in the
steam inlet line.
The tube 35 is the preheater for the reaction and the
juice passing through tube 35 ~orms into a hot free-flowing
liquid. The outlet of preheat tube 35 feeds into a first
restrictive opening or orifice 39 having a much smaller
diameter than the diameter of tube 35. The outlet of the
orifice 39 connects to a further tube 40 which forms the
tubular reaction zone. This tube passes back through the
steam vessel 36 or a separate heater and the reaction
occurs during the travel of the hot liquid through the
tube 20.
In order to control the pressure within tube ~0 a
second restrictive opening or ori~ice 41 may be provided
at the outlet. The reaction product is then collected
through outlet line 42. Further details of the above
apparatus are described in Assarsson and Nagasuye, U.S.
Patent No. 4,469,524.
The product obtained through line 42 is an inulin
hydrolyzate in which at least 80 wt~ oE the reducing
sugars are monosaccharides, oE which at least 70 wt.~ is
~ructose. This hydrolyzate contains less than 18 wt.
dimers and less than 2~ trimers. It also contains a
-- 10 --
certain amount of protein and ash as well as some color
bodies.
The hydrolyzate i~s filtered in filter 21 and then
passed through an ion exclusion column 22 to remove salts
and ash. The product from ion exclusion is subjected
to ion exchange 31 and the product from ion exchange is
passed through a carbon bed 23 to obtain a product con-
tAining in the reduciny sugars at least 97 wt.% monosac-
charides and less than 3 wt.% disaccharides. The product
is subjected to evaporation to increase the solids con-
tent to 70-80% in evaporator 24 and stored in storage 25.
The pulp 16 from the screw press 13 may be recovered
and according to an alternative embodiment, a pulp portion
26 is washed with water in a washer 17. The liquid efElu-
ent 27, containing some recovered Artichoke juice from the
pulp, is recycled to the main juice stream. The pulp from
the washer is trans~erred to drier 18 and the dried product
19 is of commercial value as an animal feed. Other sources
o pulp may be collected from filters 15 and 21 and trans-
ferred via lines 29 and 30 to drier 18, to form part of
the dried product 19.
The ~ollowing examples are further illustrative embodi-
ments of this invention. All percentages are by weight
unless otherwise specified and all pressures are guage
pressures:
Example I
Tubers of ~erusalem artichokes were used as a source
oE inulin. These tubers have a high moisture content in
the order of 70 to B0% and reducing sugars constitute
about 60-80~ of the dry matter. They typically contain
about 1.4 to 2% nitrogen, about 1 to 5% ash and about 13%
cellulose and hem;cellulose. Only about 50% o~ the nitro-
gen is associated with protelns.
Fresh Jerusalum artichoke tubers were sorted and
.
washed and then macerated in a "Comitrol~' mill made by
Urschel JJabs. Inc. The liquid and pulp mixture obtained
is held for about 2 hours at 50C and then pressed in a
manual screw press to separate artichoke juice and pulp.
The juice contained about 20 ~ dry solids.
The juice obtained was filtered and the pH was ad-
justed to 3.7. This juice with pH adjusted became the
feedstock for a continuous hydrolyzer.
In the hydrolyzer the tubes 35 and 40 were made from
1/2 inch O.D. stainless steel with the tube 35 having
a length of 120 feet and the tube 40 having a length of
240 feet. The first orifice had a diameter of .062 inch,
while the second orifice was in the form of a pair of
adjacent openings each having a diameter of .062 inch.
The bath temperature was maintained at 167C and the
temperature of artichoke juice at the inlet to the first
orifice was 166C, the pressure of the artichoke juice
at the inlet to the first orifice was 540 psi and the
pressure after the orifice was 220 psi, representing a
pressure drop across the orifice of 320 psi.
The residence time within the reactor was 126 seconds,
giving a flow rate of 2.8 feet per second or 1.7 gallons
per minute.
The hydrolyzate obtained contained 12.5% saccharides,
4.7% proteins and 8.7% salts on a dry solids basis, along
with color bodies.
The saccharide component included 92.9% monosaccha-
rides (fructose and ~lucose) and 7.1% disaccharide.s.
This hydrolyzate was Eiltered, the solids content
increased to 30%, and then passed through an ion exclu-
sion colurnn containing Dowe~ Monosphere resin, wherethe ash was separated Erom the sugars. The results of a
separation are shown in Figure 3. The hydrolyzate from
the ion exclusion wa~s then subjected to an ion exchange
chromatography~ Preferably, two ion exchange stages are
~3~0
- 12 -
used, the first being a strong acid cation exchange resin,
e.g. DOWEX 88, and the second being a weak base anion ex-
change resin, e.g. DOWEX 66. The strong acid stage was
operated at a temperature o~ 70C and under these condi-
tions the hydrolysis of the inulin was comple-ted during
ion exchange. About half of the protein was also removed
during ion exchange and the content of the dimers was
decreased from 4.4~ to 2.9%. The product contained sac-
charides as 12% oE the dissolved material therein and
these saccharides included 2.9% disaccharides, 72~5%
fructose and 27.5% glucose.
This product was then evaporated to about 71% solids
to produce a colorless, ash-free syrup which showed no
tendency to form color on storage.
This invention is intended to cover all changes and
modifications oE the examples of the invention herein
chose for purposes of the disclosure which do not con-
stitute departures from the spirit and scope of the
invention.