Note: Descriptions are shown in the official language in which they were submitted.
TISSUE-IMPLANTABLE FLUID CONDUCTING DEVICE
FIELD OF THE INVE~TION
The invention relates to the field of
ophthalmology, and particularly to devices and methods
for conducting fluids within the eye.
BACKGROUND OF THE INVENTION
In a variety of ophthalmologic procedures,
treatments, and research, it is desirable to conduit
fluids into, away ~rom, or a~out the eye or spaces
within the orbit of the eye. For example, it is
frequently desirable to provide repeated and/or
prolonged drug delivery into or around the eye. In
the past, drug delivery techni~ues have involved
implanting in the eye osmotic mini-pumps attached to
silastic tubing. See, e.g., Miki, "A Method for
Chronic Drug Inusion into the Eye", 28 Jap. J. of
Ophthalmol., 140 (1984); Eliason, ~'An Ocular Perfusion
System", 19 Invest. Ophtholmol. Vis. Sci., 102 (1980);
Michelson, "Experimental Endophthalmitis Treated with
an Implantable Osmotic Minipump", 97 Arch.
Ophthalmol., 1345 (1979); and Miki, "Intraocular
Cannula for Continuous, Chronic Drug Delivery", 103
Arch. Ophthalmol., 712 (1985).
All of these devices, however, require the
implantation of a mini-pump which must be designed and
)7~
prepared to deliver a specific predetermined drug
desired. These devices are large and cumbersome to
attach to the eye for even a few days. They give only
a slow, constant infusion, being incapable of
delivering a bolus, or of delivering selectively
different drugs over an intermittent time period.
Furthermore, such devices depend upon the presence of
a pumping pressure to prevent reflux of fluid out of
the cavity into which the drug is being infused. If
the pump becomes detached, either purposely or
accidentally, there is no mechanism to prevent
extrusion of ocular fluids, potentially causing loss
of pressure in and damage to the eye.
If the discharge end of a drug delivery tube is
placed in a subconjunctival space but not
intraocularly, healing of the tissue around the end
frequen~ly results in scarring, rendering the space
non-a~sorbent or substantially less abs~rbent of
fluid. This problem is also encountered with devices
designed to drain excess aqueous humor from an eye
having glaucoma (elevated intraocular pressure),
thereby inhioiting fluid flow and causing ocular
pressure to rise to potentially dangerous levels. An
excellent account of the history of glaucoma surgery
is found in Bick, Use of Tantalum for Ocular Drainage,
Arch. OPhthalmol., 42:373-388 (1949).
In one prior art device, the exterior end of a
tube extending through the wall of the eye is provided
with a pressure relief valve in the form of small
slits made through the wall of the tube at its end.
Reference is made to Krupin, T., et al, Valve Implants
in Filtering Surgery, Am J. Ophthalmol., 81:232-235,
(1976). It is reported that fairly close control over
the pressure needed to open the valve may be
obtained. If the exterior or distal end of the
~ (37~;
tube is inserted beneath a flap of conjunctiva or the
like, of course, the valved tube is subject to the
same drawbacks as the other tubes described above.
Glaucoma surgeons have discovered that when
surgery fails it is usually because the "bleb", the
subconjunctival drainage space created by the surgeon,
has become fibrosed, causing it to shrink and become
non-absorbing.
One device that has been somewhat successful in
maintaining the fluid absorbency of the bleb during
the healing process was described by Molteno in 1969.
Molteno, "New Implant for Drainage in Glaucoma",
British J. of Ophthalmol., Vol. 53, p. 161 (1969).
Molteno's device was made from a "stellon" brand
acrylic monomer. The device consisted of two parts--a
flat plate fashioned to conform to the sclera and a
gutter incorporated at the point where a drainage tube
met the plate to assure an even spread of drainage
into the bleb. In 1979, Molteno disclosed a new
device that had a biconc~ve base plate and a long
silicone tube, which served the same function as the
first device. Reference is made to Chapter 11 of
Glaucoma SurqerY by Luntz, M.H., Harrison, R., and
Schenker, H.I. (1984), for a description of this
device.
A need exists in the medical field for a
tissue-implantable device which would operate
substantially on a continuous basis to permit fluids
to be infused and/or drained into and around the eye
but would not be subject to the drawbacks associated
with healing and scarring of tissue or the
complications associated with prior implantable pumps.
i
~ 407fj
SUMMARY OF THE INVENTION
The invention, in one embodiment, provides an
infusion cannula for delivering fluids to various
cavities ~f the eye. The cannula comprises a tube
having first and second ends and a check valve
therebe~ween for allowing fluid flow only toward said
first end. Means is provided for securing the first
end within the eye, and means is provided at the
second end for attaching the cannula to a fluid
source, which may be a glaucoma alleviativn device, a
drug infusion device, a hilt for receiving a tube from
an infusion pump, or even a rubber plug for receivlng
a hypodermic needle or similar device. The cannula
may also include a compressible reservoir and/or a
plurality of one-way check valves. The reservoir
further may be adapted to receive a hypodermic needle
directly therein.
In another embodiment, the invention relates to
a method of delivering fluid to various cavities in
the eye, comprising the steps of providing an infusion
cannula having first and second ends and a check valve
therebetween to allow fluid flow only toward said
first end; surgically inserting the first end of the
cannula into the eye; attaching a fluid source to the
second end of the cannula; delivering fluid into the
cannula; and detaching the fluid source from the
second end of the cannula, whereby the check valve
will prevent escape of any fluid through the cannula.
In yet another embodiment, the invention
provides a tissue-implantable, fluid dissipating
device enabling fluid from a source to be delivered to
and absorbed by tissue of the eye. The device
includes a housing having walls defining an interior
4~7s;
cavity, the housing walls having s~ffiaient rigidity
to prevent substantial collapse of the cavity when the
device is implanted, orifice means communicating with
the cavity to allow fluid transfer from within the
cavity to surrounding fluid resorptive ~issues when
the device is i~planted, and tube means communicating
with the cavity to conduct fluid from a source to the
cavity, the cavity having an inner diameter
substantially greater than any inner diameter of the
tube means. The device preferably generally "floats"
within the tissue pocket within which it is embedded.
That is, the majority of the outer surface of the
device confronts, but is not bound to, tissue which is
absorptive of liquid PsCaping from the orifice.
When the latter embodiment of the invention is
surgically implanted in the eye wall, one end of the
tube means communicates with the cavity, the other end
communicates with a fluid source such as an infusion
drug source, or an apparatus receiving aqueous humor
from the antérior chamber of the eye. A fluid
re~ervo~ ar example, may collect the excess eye
fluid released from the anterior chamber, and
reference is made to my United States Patent No.
4,554,918 4
~- - In operation, fluid flows
through the tube means into the interior of the cavity
and thence outwardly through the orifice to be
absorbed by fluid resorptive tissue facing the
orifice, and desirably also tissue generally
enveloping the implanted device.
The ho~sing of the latter embodiment may be
provided with a variety of configurations. In the
preferred embodiment, however, the housing comprises a
plate member and a hood member peripherally connected
to the plate member to define a fluid-collecting,
.,
1~407-j
outwardly open cavity or pocket therebetween.
Desirably the plate member is curved to fit the
contour of the eye wall. An edge of the hood member
typically extending arcuately over the plate member
forms the orifice or mouth of the pocket opening. The
radius of curvature of the hood member is less than
the radius of curvature of the eye wall in order to
form the pocket therebewteen.
BRIEF DESCRIPTIO~ GF DRAWINGS
Figure 1 is a cross-section of an eye showing a
partially broken-away view of the device of the
invention implanted in the eye;
Figure 2 is a perspective, somewhat schematic
view of an eye showing an infusion device of the
invention implanted therein;
Figure 3 is a broken-away view of a modified
embodiment of the invention;
Figure 4 is a broken-away view of another
modified embodiment of the invention;
~ igure 5 is a broken-away view of yet another
modified embodiment of the invention.
Fi~ure 6 is a broken-away, schematic
representation of an eye showing the positioning
therein of a device of the invention;
Figure 7 is a broken-away cross-sectional view
taken along line 7-7 of Figure 6;
Figure 8 is a broken-away cross-sectional view
of another embodiment of the invention;
Figure 9 is a broken-away cross-sectional view
taken along line 9-9 of Fi.gure 8;
Figure 10 is a plan view of another device of
the invention;
Figure 11 is a broken-away cross-sectional view
taken along line 11-11 of Figure 10;
~ 07~i
Figure 12 shows another embodiment of the
invention;
Figure 13 shows a valve in cross-section and
broken away, useful with a device of the invention.
BEST MODE OF CARRYI~G OUT THE INVENTION
Fig. 1 shows somewhat schematically a
cross-section of the human eye including a device of
the invention implanted therein. In that figure the
cornea is designated "C"; the iris as "I", the lens as
"L", the sclera as "S", the retina as "R", the pars
plicata of the ciliary body as "PT", the pars plana of
the ciliary body as "PN", the vitreous cavity as "V",
and the limbus as "M". An infusion cannula device
(10) of the invention is shown inserted through the
pars plana of the ciliary body "PN" into the vitreous
cavity "V".
One embodiment of the device is shown more
fully in Fig. 2 . The cannula (10) includes a ~ube
(11) having a first end (12) inserted through the pars
plana "P~" of the ciliary body into the ~itreous
cavity "V". As shown in Figs. 2, 4 and 5, the first
end (12) may comprise a relatively rigid tube
connectable to a more flexible tube (11). The rigid
portion (16) facilitates implantation and affixation
of the devi.ce. The second end (13) of the tube (11)
includes a hilt (22) for connection to an infusion
fluid source.
A one-way valve (14) is disposed within the
tube between its first (12) and second (13) ends.
This valve is a chec~ valve to allow fluid flow only
in a direction toward the first end, in this case
being in the vitreous cavity "V" of the eye. The
1~4()7~
device may further include a flange (17) which may be
secured to the sclera "S" by sutures or other
conventional means.
In one surgical procedure for implanting the
device (lO) the conjunctiva and Tenon's capsule is
opened down to bare sclera. An incision is made
through the sclera into the vitreous cavity, avoiding
the retina if over the pars plana or more anterior.
Following this, the first end (12) is threaded through
the scleral wound, and the flange (17) is secured with
sutures placed in the sclera. The conjunctiva is then
closed about the tube with sutures, allowing the
second end (13) to come out of the conjunctiva into
the cul-de-sac and exiting between the eyelids to be
taped or sutured to skin of the temple.
Preferably the device is implanted through the
pars plana of the ciliary body, generally about 3mm
from the limbus "M". Under appropriate circumstances,
however, the device could also be implanted at the
limbus or cornea into the anterior chamber, or through
the retina into the vitreous cavity. As previously
noted, and as will be described later in greater
detail, the device may also be adapted to deliver
fluid into any portion of the orbital spaces,
subconjunctivally, subtenonally, or retrobulbularly..
Fig. 3 shows an alternate embodiment in which a
rigid trochar (20) is first implanted through the pars
plana of the ciliary body, and a flexible tube is
thereafter inserted through the trochar (20). The
trochar (20) may then be removed, and the tube
connected to the balance of the infusion cannula
device (10). This technique facilitates use of
entirely flexible materials, reducing the likelihood
of irritation to or erosion of the conjunctiva.
(J7~
_ g _
For patients requiring use of the device for
only a relatively short period of time, (such as
several days or a few weeks) the second end (13) of
the device may be position forwardly, exiting the eye
between the eyelids and temporarily affixed, for
example, by adhesive tape to the temple or forehead of
the patient. The second end (13) may include a hilt
(22) which can be selectively connected to or
disconnected from complimentary tubing from an
infusion fluid source. Alternately, the second end
(13) of the tube may comprise a solid rubber plug (23)
adapted to receive fluid therethrough by injection
from a hypodermic needle. In this embodiment, the
tube may be small enough to position the second end
(13) within the cul-de-sac of the conjunctiva. Such
embodiment would be more suited to administration of
infusion fluids over longer periods of time or when
~he patient is not hospitalized. This embodiment also
has the advantage of being more aesthetically pleasing~
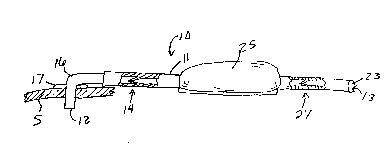
Fig. 5 shows another e~'oodiment in which the
device further includes a reservoir (25), and an
optional second check valve (27). This embodiment is
particularly suited to long term application of ~he
device (for example, several weeks or months). When
implanted, the second end (13) of the tube, which
includes a rubber plug ~23) for receiving a hypodermic
needle, is positioned within the cul-de-sac of the
conjunctiva, When administration of infusion fluid is
desired, the physician inserts a hypodermic needle
through the rubber plug (23) and fills the reservoir
(25) with infusion fluid. The reservoir itself may be
attached to the sclera "S" of the eye in a position to
allow convenient digital manipulation to express the
infusion fluid outwardly from the reservoir toward the
first end (12) of the device (10)~ Alternately, the
.
40~5i
-- 10 --
reservoir may be elastic, exerting a relatively
contact compression force to slowly deliver the
infusion fluid. The check valve or valves prevent
reflux of the infusion fluid.
The device may be manufactured by any of a
variety of well known suitable materials. Rigid
portions of the device may be made from
polymethylmethacrylate (PMMA~ or other suitable
materials such as biologically acceptable metals. The
flexible portions of the tubing may be made from
silicone rubber or other similar materials. The
one-way check valve may be any of a variety of well
known designs which need not be described in detail,
but include, by way of example, well known "duck
valves".
In use, the physician may surgically implant
the device o~ the invention, as previously described,
at the appropriate location in the eye. The second
end (13) of the tube (11) may be draped outwardly
between the eyelids and affixed for example, to the
temple by adhesive tape. When infusion treatment is
desired, a suitable infusion fluid source, such as an
infusion pump, may be connected to the second end (13)
of the tube (11), and the appropriate fluid infused.
The fluid source may then be detached from the second
end, the check valve preventing escape or reflux of
the fluid. After an appropriate period of time,
treatment may be repeated. If long term treatment is
desired, the device may be provided with the
appropriate structure as previously described to allow
the entire device to remain within the eye and the
cul-de-sac of the conjunctiva. In either case, the
device of the invention provides a means for
repeatedly introducing appropriate fluids into the eye
without causing repeated physical invasions of the
~ 07~i
eye. The device further provides the flexibility of
varying treatment from one time to the next, both as
to amount and type, without replacing or disturbing
the device.
Referring now to Fig. 6, another embodiment of
the invention is depicted. In this drawing, sclera is
designated as "S"; the overlying conjunctiva and
Tenon's Capsule together is designated "T"; the
anterior chamber, "A"; and the cornea "C"~ For
clarity, other structural portions of the eye have
been omitted.
This embodiment of the invention provides for
fluid dissipation, and includes a housing (130) having
walls (135) defining an interior cavity (133)
positioned against or adjacent to the outer surface of
the sclera "S" beneath the conjunctiva and Tenon's
Capsule "T". The housing ~130) is desirably made of a
pliant material such as silicone rubber, a more rigid
p~lymeric material such as polymethylmethacrylate, an
inert metal such as gold, or any other convenient and
~iologically accepta~le material. The housing (130
is typically oval or disk-shaped with a length and
width typically in the range of a centimeter or
smaller. The housing walls ~135) defining the
interior cavity (133) have sufficient rigidity to
prevent substantial collapse of the cavity when the
device is implanted. Located in the housing walls
(135) are orifice means (145), such as small holes
with diameters of at leas~ 0.2Smm that permit fluid
transfer from within the interior cavity to fluid
resorptive tissues (116) of the eye. The fluid
resorptive tissues (116) include the sclera "S" as
well as conjunctiva "T" and other adjacent tissues of
the eye.
V7~
- 12 -
The orifice means (145~ described above may
include any of a variety of openings that may be
located in the housing walls (135). It may be the
mouth (146~ of the cavity, a hole in the housing wall,
(135) or mul~iple holes in the housing walls (135).
When the device is implanted the conjunctiva and
Tenon's Capsule "T" lying over the upper walls (135)
of the housing do not attach to the housing (130).
For this reason a spa~e (114) exists between the
tissue and the housing (130) so that, ideally, fluid
flowing out of the housing (130) can envelop the
housing (130), maximizing the surface area of
resorptive tissue (116) available for contact with
fluid. The fluid must be able to flow from the cavity
to be absorbed by the tissue.
The tissue of the eye will grow around the
edges of the opening. If the distance between the
closest inner edges of the opening measures less than
0.25mm, the tissue growing into the opening around the
edges may be able to contact other tissue and grow
together, clogging the orifice means, thus ef~ectively
blocking the flow of fluid from the cavity into the
surrounding tissue. In order to provide maximum
drainage the orifice means should have an effective
diameter of at least 0.25mm, preferably at least
0.5mm. (For purposes of this application, an orifice
has an "effective diameter" of at least 0.25 mm when
the orifice defines an opening extending at least
0.125mm in all directions from a point within the
opening; i.e., a circle having a diameter of 0.2smm
can be inserted through the orifice.)
Tube means (120) having an inner diameter
substantially less than the inner diameter of the
cavity (133) communicates at one end (121) with the
cavity (133), and at the other end with a fluid
source. The fluid source may be an artificial device
attached to the eye (such as that shown as (122) in
Fig. 6), the anterior chamber (15) of the eye i~self,
an internal or external drug infusion apparatus or
other external devices. The orifice includes a rim
having a surface adapted to contact fluid resorptive
tissue along a locus of points defining a surface
representing the closest approach of fluid resorptive
tissue into the cavity. The rim should be located at
least approximately 0.25mm, preferably l.Omm, from the
housing end of the tube means.
In a preferred embodiment shown in Figs. 6 and
7, the housing (130) comprises a plate member (134)
and a hood member (132) peripherally connected to
define an interior cavity having an inner diameter
substantially greater than any inner diameter of the
tube means therebetween. The plate member (134)
desirably is curved to fit snugly against the eye
wall. The hood member (132) extends arcuately over
the plate member (134) and has a radius of curvature
less than the radius of curvature of the eye wall
(about 12-15mm) of a human eye, preferably less than
about 8-lOmm. The arcuate edge (136) of the hood
member (132) typically is rounded so that the tissue
of the eye that contacts the hoad member, when the
device is implanted in ~he eye, will not be injured by
the edge of the hood member. In this embodiment, the
orifice means is defined by the space between the
plate member and the arcuate edge of the hood member
and has an effective diameter of at least about 0.25mm
to prevent closure by tissue The device is implanted
so that the plate member (134) contacts the sclera
"S", and the exterior of the hood member (132)
contacts the conjunctiva and Tenon's Capsule "T".
-- 14 --
The plate member (134) desirably is cemented or
otherwise attached to a peripheral flange (131). The
flange (131) may be of silicone rubber,
polymethylmethacrylate or other acceptable polymers,
or other convenient biologically acceptable material,
and may be fastened to the scleral wall by sutures or
other means. The flange (131) may have perforations
(138) t~ receive sutures or to permit tissue ingrowth
or both.
The tube means (120) preferably includes a
unidirectional check valve as shown in Fig. 13 to
permit fluid to flow only toward the interior cavity
(133) of the housing (130) only, In the device shown
in ~igs, 6 and 7, the tube means (120) can e~tablish
communication with the interior cavity (133) from any
position as long as the end (121) ~i.e., the last
portion of the tube means that has an inner
cross-sectional area that is substantially equal to
the inner cross-sectional area of the rest of the tube
means) remains at least a~out 0,25mm away from the
orifice rim.
In another embodiment of the device shown in
Figs. 8 and 9, the housing (130) comprises a hood
member (132) having a maximum radius of curvature less
than the radius of curvature of the eye wall of the
human eye. The hood member ~132) will thus be concave
and will define an interior cavity (133) having an
inner diameter substantially greater than any inner
diameter of the tube means which extends between the
scleral wall (112) and the interior walls of the hood
member, The peripheral edge of the hood member (132)
will be attached to the scleral wall, The orifice
means (145) in this embodiment is defined by the inner
peripheral edge (147) of the walls of the hood member,
the sclera "S" of the eye providing the resorptive
07si
- 15 -
tissue interface to allow fluid absorption. A
peripheral flange (121) may be peripherally cemented
or otherwise attached to the edge of the hood member
(132), or the hood member (132~ may itself have a flat
surface around its peripheral edge so that the hood
member may be attached to the scleral wall "S" of the
eye forming an interior cavity (133) within the walls
(135) of the hood. Attached to an inner wall of the
hood member (132) may be a plate member (134) that
would permit the housing end of the tube means (120)
to communicate with the cavity (133) and yet be spaced
at least about 0.2smm from the sclera "S" or any other
resorptive tissue in any direction. Although a plate
member is shown, it would not be necessary if the tube
means end communicates with the housing from the top
or sides as long as the end of the tube means is kept
at least about 0.25mm from the sclera.
In yet another embodiment of the invention
shown in Figs. 10 and 11, the housing (130) comprises
a plate member (134), preferably oval or disk-shaped,
having a perip~eral edge to which a plurality of
support structures ~155) are attached. The support
structures extend arcuately over the plate member and
meet in a common point, defining an interior cavity
(133). This device resembles a cage. The support
structures (155) are of sufficient rigidity to prevent
substantial collapse of the cavity when the device is
implanted, i.e., although the housing may undergo some
deformation, the cavity and orifice means remain open
and the 0.25mm limitation with respect to the orifice
means are continuously met. The open space between
support structures (155) must have an effectlve
diameter of at least about 0.25mm to prevent tissue
overlying the support structures (155) from forming a
bridge between the support structures (155) thereby
7S;
- 16 -
clogging the drainage area. The tube means (120)
should communicate with the housing in such a way
that the support structures (155) will protect the
tube means (120) from contacting resorptive tissue of
the eye, thus preventing tissue from growing over the
tube end and preventing fluid flow. In other words,
the rim of the orifice means (the space between
support structures) must be at least about 0.25mm away
from the housing end of the tube means.
- In still another embodiment of the invention
shown in Fig, 12, the housing may be an extension of
the tube means. In this device the housing has walls
that define a cavity having an inner diameter
substantially greater than any inner diameter of the
tube means and orifice means, the cavity and the
orifice having a cross-sectional area available for
fluid flow substantially greater than that of the
interior of the tube means. The housing walls
extending from the walls of the tube means must have
sufficient rigidity to prevent substantial collapse of
the cavity when the device is implanted. Orifice
means located in the housing walls must have an
effective diameter of at least about 0.25mm, and the
rim must be at least about 0.2smm away from the end of
the tube means.
The fluid dissipation device depicted in
Figures 6-13 may be used with any ~luid delivery
system in the eye, including infusion systems and
ocular pressure relief systems such as that shown in
my U~S. Patent 4,554,918.
Other types of housings may also be used so
that the dissipation device serves the same purpose of
pro~ecting the end of the tube means from being
clogged through contact with tissue of the eye. Such
variations may include a device in which the tube
~I ~r~07~
- 17 -
means separates into two separate portions at the
housing end so that there are two or more ends located
within the housing. Typically the two ends will form
a "T" within the housing of the device. Other
variations will be apparent to the skilled artisan.
While a preferred embodiment of the present
invention has been described, it should be understood
that various changes, adaptations and modifications
may be made therein without departing from the spirit
of the invention and the scope of the appended claims.