Note: Descriptions are shown in the official language in which they were submitted.
8;~
Thi~ is a divisional application of copending application
~erial no. 470,058, filed December 13, 1984.
~EL COMPOSITIONS
ColnpressLon ignition Ellel compositions and
acl(lil:ive mictures oE orgallic nitrate ignition
accelerator and hydrocarbyl-subsl:ituted succinlmi.de, in
5 amoullts suEEicient to resist the coking ten-lencies oE
compression ignition fuel compositions when u.sed in the
operation oE indirect injection diesel enyines.
Throttling diesel nozæles have recently come into
widespread use in indirect injection automotive and
lO liyllt-duty diesel truck engines, i.e., compression
ignitioll en-Jines in w11ich the ~uel is injected into and
; iynited in a prechamber or swirl chamber. In this way,
the ~lame front proceeds Erorn the prechamber into the
laryer compression chamber where the combustioll is com-
15 pleted. Engilles designed in this manner allow Eor
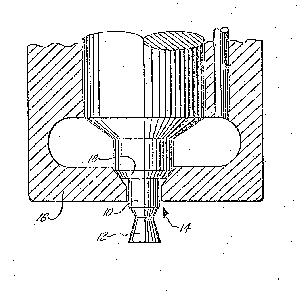
quieter and smoother operation. The Figure oE the
Drawill~J illustrates the yeometry oE the typical
throttling diesel nozzle (oEten reEerred to as the
"pintle nozzle").
UnEortunately, the advent o~ such en~ines has
yivell rise to a new problem, that o~ excessive colciny on
~3~3
t1~e critical surfaces oE the injectors that inject fuel
into t11e precha1nber or swirl chamber o the engine. In
paLticular and with reference to the Figure, the carbon
tell(ls to Eill in all oE the available corners and
5 surEaces oE the obturator lO and the Eorm 12 until a
smooth pro~ile is achieved. T11e carbon also tends to
bloc~ t11e drilled oriice l~ in the injector body 16 a1ld
fill up to the seat l8. In scvere cases, carbon builds
u~ on the form 12 and the obturator lO to such an extent
lO that it interferes with the spray pattern of the fuel
issuing from around the perimeter oE orif1ce 14. Such
carbon build up or coking oEten results in such
undesirdble consequence~s as delayed fuel injection,
increased rate oE uel injection, increased rate of
15 co1nbustion chamber pressure rise, and increased engine
noise, and can also result in an excessive increase in
emission from the engine of unburned hydrocarbons.
~ 1hile low fuel cetane nu1nber is believed to be a
rnajor contributing factor to the coking problem, it is
20 not the only relevant Eactor. Thermal and oxidative
stability (lacquering tendencies), fuel aromaticity, and
such ~uel characteristics as viscosity, surface tensio
and relative dens1ty have also been indicated to play a
role in the coking problem.
An important contributio1l to the art would be a
fuel composition which has enhanced resistance to cokin~
~L2~
tendellcies when employed in the operation of indirect
injection diesel engines.
In accordance with one of its embodiments, this
invention provides distillate fuel for indirect injection
compression ignition engines containing at least the
combination of (a) organic nitrate ignition accelerator, and
(b) hydrocarbyl-substituted succinimide or succinamide, or
the combination of (a) organic nitrate ignition accelerator,
(c) hydrocarbyl amine having from 3 to 60 carbons and from 1
to 10 n:itrogens and (d) N,N' disalicylidene-1,2-
diaminopropane, or the combination of (b) hydrocarbyl-
substituted succinimide or succinamide, (c~ hydrocarbyl amine
having from 3 to 60 carbons and from 1 to 10 nitrogens and
(d) N,N'-disalicylidene-1,2-diaminopropane, said combinations
being separately present in an amount sufficient to minimize
coking, especially throttling nozzle coking, in the
prechambers or swirl chambers of indirect injection
compression ignition engines operated on such fuel.
Another embodiment of the present invention is a
distillate fuel additive fluid composition comprising (a)
orgallic nitrate ignition accelerator, and (b) hydrocarbyl-
substituted succinimide or succinamide, or (a) organic
nitrate ignition accelera-tor, (c) hydrocarbyl amine having
from 3 to ~0 carbons and from 1 to 10 nitrogens and (d) N,N'-
rn/
~3~ '3
disalicylidene-1,2~diam.inopropane or tb) hydrocarbyl-
substituted succinimide or succinamide, (c) hydrocarbyl amine
havirlg from 3 to 60 carbons and from 1 to 10 nitrogens and
(d) N,N'-disalicylidene-1,2-diaminopropane~in an amount
suEficient to minimize the coking characteristics of such
fuel, especially throttling nozzle coking, in the prechambers
or swirl chambers of indirect compression ignition engines
operated on such fuel.
Since the invention also embodies the opera-tion of
an indirect injection compression ignition engine in a manner
which results in reduced coking, a still further embodiment
of the present invention is a method of inhibiting coking,
especially throttling nozzle coking, in the prechambers or
swirl chambers of an indirect injection compression ignition
engine, which comprises supplying said engine with a
distillate fuel containing at least the combination of (a)
organic nitrate ignition accelerator, and (b) hydrocarbyl-
substitutsd succinimide or succinamide, or the combination of
(a) organic nitrate ignition accelerator, (c) hydrocarbyl
amine having from 3 to 60 carbons and from 1 to 10 nitrogens
and (d) N,N'-disalicylidene-1,2-diaminopropane or the
combination of (b) hydrocarbyl-substituted succinimide or
succinamide, (c) hydrocarbyl amine having from 3 -to 60
carbons and from 1 to 10 nitrogens and (d)
~,
rn/
;
33
-- 5
N,~'-disalicyclidene-1,2-diaminopropane, said combina-
tions being separately present in an amount sufficiel-t
to millimize such coking in an enc~ine operated on such
Euel.
A Eeature of this invention is that the
combillation oE additives utilized in its practice .is
capable oE suppressing cokiny telldencies oE Euels userl
to operate indirect injection compression ignition
ellgilles. Such bellavior was exhibited in a series oE
stalldard engine dynamorneter tests conducted as des-
cribed in Examples I, II and III hereina~ter.
A wide variety of organic nitrate ignition
accelerators, componellt (a), may be ernployed in the
fuels of this invention. Pre~erred nitrate esters are
the aliphatic or cycloali.pllatic nitrates in which the
alipllatic or cycloaliphatic group is saturated, con-
tains up to about 12 carbons and, optionally, may be
substituted ~ith one or more oxygen atoms.
Typical organic nitrates that may be used are
20 methyl nitrate, ethyl nitrate, propyl nitrate, isopropyl
nitrate, allyl nitrate, butyl nitrate, isobutyl nitrate,
sec-butyl nitrate, tert-butyl nitrate, amyl nitrate,
isoamyl nitrate, 2-amyl nitrate, 3-arnyl nitrate, hexyl
nitrate, I-leptyl nitrate, 2-heptyl nitrate, octyl
25 nitrate, isooctyl nitrate, 2-ethylhexyl nitrate, nonyl
nitrate, decyl nitrate~ undecyl nitrate, dodecyl
~f
, ~
' , ' " '
~3~83
nitrate, cyclopentyl nitrate, cyclohexyl nitrate, methyl-
cyclohexyl nitrate, cyclododecyl nitrate, 2-ethoxyethyl
nitrate, 2-(2~ethoxy-ethoxy)etllyl nitrate, tetra-
hydro~uranyl nitrate, and the like. Mixtures of such
materials Inc~y also be used. The preEerred i.gllitiOIl
accelerator Eor use in the Euels oE this invention is ;
mixture o octyl nitrates available as ~n article oE
commerce Erom ~thyl Corporation under the trade m~rk
DII-3 ic;nition improver.
The hydrocar~yl-substitu~ed succinimides,
colnponellt (b? oE the Euels oE this invention, are well
kno~ll. They are readily made by Eirst reacting an
oleEinically unsaturated hydrocarbon oE the desired
moleclllar weight with maleic anhydride to form a
hydrocar~yl-substituted succinic anllydride. Reaction
temper~tures oE 100-250C are used. l~ith higher hoiling
oleEinically-unsaturated hydrocarbons, good results are
obtailled at 200-250C. This reaction can be promoted by
the addition oE chlorine Typical oleEins include
cracked wax oleEins, linear alpha oleEins, branclled
ch~in alpha oleEins, polymers and copolylners oE lower
oleE;Ils. These include polymers oE ethylene, pro-
pylelle, isobutylene, l-hexelle, l-clecene and the like.
UseEul copolylners are ethylene-propylelle copolymers,
ethyleile-isobutylene copolymers, propylene-isobutylene
copolylners, ethylelle-l-decene copolymers an~ the like.
~' f'~ 3
-- 7
llydrocarbyl substituents have al$o been l-nade Erom
oleEin terpol~mers. Very useEul products have been made
~ ylene-c3 12 alpha ole~in ~ C5-12
conju(~ated diene terpolymers; SIICh as ethylene-
propylelle-1,4 hexadiene terpolymer; ethylene-propylell2-
],5-cyclooctadielle terpolymer; ethylene-propylelle--
norboLI)elle terpolymers and the like.
OE the Eoregoing, by ar the most useEul hydro-
car~l substituents are derived Erom butene polymers,
especially polymers o isobutylene.
The molecular weight of the hydrocarbyl sub-
stituent can vary over a wide ran~Je. It is desirable
that the liydrocarbyl group have a molecular weit311t of at
least 500. Although there is no critical upper limit, a
preferred range is 500-500,000 number avera~e rnolecular
~ei~ht. The more preEerred avera~e molecular weight is
700-5,000 and most pre~erably 900-3,000.
Hydrocarbyl-substituted succinimides and
succinami~es are made by reaction oE the desired
hyt3rocarbyl-substituted SUCCi.lliC anhydride ~ith an amine
havin(~ at least one reactive hydrot3en atom bonded to an
arnine nitrot3en atom. Examples oE these are methyl
a-nine, dimethyl amine, n-butyl amine, cli-(n-dodecyl)
alni.ne, I~-(alninoethyl) piperidine, piperazine, ~-(3-amino-
prol~yl) piperazine, and the like.
- ' ' ~ .
PreEerably, the amine has at least one reactive
prilnary amine group capable of reacting to ~orm the
pr~erred succinimides. Exaln~les of such primary alnin~s
are n-octyl amine, N,N-dimethyl-1,3-propane diamitle,
I~-(3--alninopropyl) piperazine, 1,6-hexane dialnine, and
the like.
llydroxyalkyl amines can also be use-3 to make the
suc_lni;nide-succinaJnicle components of the invention
wllicll contain some ester grouus. These amines include
ethallo]. amine, diethanol amirle, 2-hydroxypropyl amine,
~l-h;~roxyeth~l ethylenedialnine and the like. Such
hydroxyalkyl a-nines can ~e made by reacting a lower
alkylene oxide, such as ethylene oxide, propylene oxi(3e
or l~utylene oxide with a~nmoni.a or a primary or secondary
ainine such as ethylene diamine, dethylene triarnine,
trietilylene tetrarnine, tetraethylenepentalnille and the
like.
A more preferred class oE primary amines used to
make the succinimide, succinamide or rnix~ures thereoE
are tlle polyalkylene am.ines. These are polyamines and
mixtures of polyamines which have the general. Eormula
~12N-~ R - Nll~ n
~2~383
whereill R i~ a divalent aliphatic hydrocarbon group
hclvinc~ 2-4 carbon atoms and n is an integer from 1-10
includillg mixtures of such polyalkylene amines.
In a highly preEerred eml)odiment, the poly-
5 a] kylene amlne is a polyethyleneamine containillg 2-6
e~hylelleamine units. These are represented by the above
l~orlnula in WiliCIl R is the group ~C112C112- and n has a
value oE 2~6.
The amine used to make the succinimide,
10 succinamide or mixture thereoE need not be all alnine. 7
mono or poly-hydroxyalcohol rnay be include~ in the
reaction. Such alcohols can be reacted concurrently
with the amine or the two alcohol ~and amine may be
reacted seyuentially. Use~ul alcohols are methallol,
15 el:hallol, n-dodecanol~ 2-ethyl hexanol, ethylene ~lycol,
propylene glycol, diethylene glycol, 2-ethoxy ethanol,
trilnethylol propane, pentaerythritol, dipentaerythritol
and the like.
Use~ul arnine-alcohol products are described in
20 U.S. 3,1~4,~17~; U.S. 3,576,7~13; U.S. 3,632,511; U.S.
3,~0~1,763; U.S. 3,836,471; U.S. 3,936,480; r~.s.
3,948,B00; U.S. 3,950,341; U.S. 3,957,354; U.S.
3,957,855; U.S. 3,991,098; U.S. 4,071,548 and U.S.
4,173,5~0.
rrhe reactioll between l:he hIdrocarbyl-sub~l:ituted
succinic anllydride and the amine can be carried out by
.
: ~ , . ~; : . -
.
.: ~ . . . :
~- .; . .
., , . ' :
3 L~
- 10 -
mixing the compollellts and heating the mixture to atemperature high enougll to cause a reaction to occur but
not so high as to cause decolnposition of the reactants
or products or the anhydride may be heated to reaction
t rnperature ~nd the amine added over an extended
period. A useful temperatllre i9 100-250C. ~est
resull:s are obtained by conducting the reaction at a
temperature high enough to distill out water Eormed in
the reaction.
~ preferred succini.nide~succinamide component is
availal~le as an article o comlnerce froln the Edwin
Cooper Company under the trade mark l-IITEC E-6~. This
product comprises a mixture of active ingredients ancl
solvent. Thus, when ~ rEc~ E-644 is used as componellt
(b) in formulating the ~uels of this lnvelltion, the
product as received should be used at a concent~ation oE
at least about 40 PTB (pounds per thousand barrels) -
O. 11~36 grams per liter - to insure that the Einished
blend contains an adequate quantity of tlle foregoillg
; 20 succini!nide-succinamide ingredient although sm~ller
amounts may be success~u]ly employed
The nitrate ignition accelerator--component
(a)-~sbould be present in an amoullt of at least 100 to
lOOO p~rB (pounds per thousan~l barrels3 - 0.2859 to 2. 859
gr~s ~e litrr oE the ~se ~1. PreE-ra 1~, the
~ ~488~3
concelltration oE the ignition acce1erator is 400 to 600
PTB tl.1~36 to 1.715~ grams per liter~.
It is not be1ieved that there is anything
critical as regards the maximum amoullt oE compollellts ~a)
and (b) used in the Eue1. Thus; the maximum amount oE
tllese components ~ill probably be ~overned in ~ny qiven
situatioll by matters oE choice and economics.
The coking-inhibiting components (a) and (b) oE
the invention can be added to the Euels by any means
known in the art Eor incorporating small quantities oE
additives into distillate Euels. Components (a) and (b)
can be added separately or they can be combined and
ac~ded together. It is conveniellt to utilize addikive
1uid mixtures ~/hich consist of orgallic nitrate ignition
accelerator and hydrocarhyl-substituted~succi~nimide-
.
succinamide agent~s. These additive E1uid mixtures are
~ added to disti11ate uels. In other ~ords, part oE the
; present invention are coking inhibiting 1uids whicl
coml)rise oryanic nitrate ignition accelerator and
llydrocarby1-substituted succinimide-succinamide.
U.se oE such 1uids in addition to resulting in
great convenience ln storage, hand1ing, transportation,
blelldillg with fue1s, and so Eorth, also are potent
concelltrates ~Ihich serve the Eunct~ion oE inhLbiting or
minirni%in~7 the coking char~cteristics oE compressio
,
:~ :
.
- -
.
,"" ,
. : . .
~ 8~3
ignition distillate fuels used to operate indirect
compres~sioll ignition engines.
In these ~luid compositions, the amoullt oE eom-
ponellts ~a) and (b) can vary widely. In general, the
flu;d compositiolls contain 5 to 95~ by weight Oe the
organic nitrate ignition accelerator co~nponellt and 5 I:o
95?~ by weight oE the hydrocarbyl~substitut~3
succinitnide-succinamide componellt. rrypically~ Erom .01
by ~eight up to 1.0% by weight oE the colobination will
be suEicient to provide good coking-inhibiting proper-
ties to the distillate uel. ~ preEerred distillate
fuel composition contains Erom 0.1 to 0. 5% by weight of
the combination containing ro~n 253 Lo 95% by weight of
the organie nitrate ignition accelerator and Erom 75~ to
5% by weight of the hydrocarbyl~substituted succinimide-
succinamide component.
The additive fluids, as ~ell as the distillate
fuel compositions o the present invention may also
contain other additives such as, corrosion inhibitors,
antioxidarlts, metal deactivators, deteryents, cold Elow
improvers, inert solvents or diluents, an~l the like.
Accordillgly, a more preferred distillate fuel
composition includes a hydrocarbyl amine in combinatio
with the present additives.
. . .
. ~ ' ' ~ '
- 13 -
~ hile a variety oE hydrocarbyl amines may be used
in the uel compositions o this lnvention, a primary
alipllatic amine, the aliphatic group of which iS
tertiary, e.g., an amine oE the forlnula:
R N~l2
wllereill R is one or a mixture of tertiary aliphatic
yroups containiny 8 to 18 or mo~e (preEerably 12-16)
carbon atorns is preferred. Most pre~erably, these
tertiary aliphatic yroups are tertiary alkyl groups. It
10 is al~so preEerred that hydrocarbyl amine component tc~
inclu~e in addition to the above-depicted amine one or
more hydrocarbyl amines differint3 therefrom.
U.s. Pat. ~o. 3,909,215 gives a description oE
the various hydrocarbyl amines having from 3 to 60
ca~bons and ~rorn 1 to 10 nitrogells which may be ernployed
in the fuels oE this invention. A few additional
exaln~)les of desirable amines include 2,6-di-tert-
butyl-~-dirnethylamino-~-cresol, N-cyclohexyl-
~dimethylalnine, and N-alkyl,N,N-dimethylamines in which
20 tlle alkyl group is one or a combination o~ alkyl groups
preEeral~ly llavin~3 8 to 18 or more carbon atolns.
~ particularly preEerred hydrocarbyl amine is
a~ailable commercially ~rom the Rohm and ~laas ~ompany
ulltler the trade mark Primene 81R. The Primene ~lR*is
* trade maFk
;'. ' :
~ . .
' . . . .
believe~i to be a mixture of primary aliphatic arnines in
t/hich ~he aliphatic groups are predomilIalltly C12 an-l
C14 tertiary alkyl groups.
~rhe Euels oE this invention should contain at
lea.c;t 1.5 to ~0 PTB (0.00429 to 0.11~3 gra,ms/liter Oe
compolIent (c), tlIe hydrocarbyl allline.
~ ccordingly, another embo-iirnent oE ~ e present
invention is distillate Euel for indirect injection
com.oression ignition engines containilIg at least tlIe
colnbination oE (a) organic nitrate ignition accelerator,
(b) hydrocarbyl-substituted succinimide, a~nd (c)
hydLocarbyl amine, said combination being present in an
amount suEEicielIt to rninimiæe coking, especially
throttling nozzlP coking in the prechambets or swirl
chainbers in indirect injectiolI compresslon ign;tion
engilIes operated on such fuel.
Also included as a ieurther emhodirnent of the
; invelItion is a distillate euel additive composition
comprisilIg (a) organic nitrate ignition accelerator, (b)
hydrocarbyl-substituted succinimide and (c) h~dcocarbyl
amilIe in an arnount sueEicient to minimize the coking
; characteristics of such Euel, especially throttling
nozzle coking in the prechambers or swirl~chambers in
in(3irect injection cornpression ignition engines ol~erated
on such l~eI.
:. :
- :
~8~ 3
In general, these additive Euel compositions will
contain as mllch as 50~ by weight of the combination oE
organic nitrate ignition accelerator and hydrocarbyl-
substituted succinimide and up to 50~ of the hydrocarbyl
amine or other additives when they are present.
In a still Eurther embodiment of the inv~ntio
there is provided a method o~ inhlbiting cokin~3,
especially throttling nozzle coking in the prechambers
or swirl chambers o an indirect injection compressio
10 ignition engine which comprises supplying said engine
~ith a distillate Euel containin~3 at least the co~-
bination oE (a) oryanic nitrate ignition accelerator,
(b) hydrocarbyl-substituted succinlmide and (c)
hydrocarbyl amine, said combination being present in an
; ~moullt sufficient to minimize such coking in an engine
operated on such fuel.
~ nother additive which can be used to advantage
in the present invention is a metal deactivator.
I~xamples oE these are salicylidene-o-aminophenol,
20 disallcylidene~ethylenediamine and disalicylidene
propylenediamine. ~ particularly pre~erred metal
deactivato~ is N~,N'-disalicylidene-1,2-di~minopropalle
(80 weight percent active in 20 weight percent toluene
solvent) wnich is available as an article of colnmerce
~rom Ethyl CorpO~Ition under tbe tr~de mark . "Ethyl' MDA.
.
- ~ . ' ' , ',:
, ' '
: . . ,: . , .
~x~
- 16 -
The fuels of this invention should contain at
least 0.2 to 5 PTB (0.00572 to 0.012 grams per liter) o~
comL)onent (d~, the metal deactivator, preferal~ly N,~'-
disalicylidene-1,2-diaminopropane.
~ccordin~ly, another ernbodiment oE the present
inve!ltiorl is distillate Euel Eor illdirect injection
coml?res3ioll ignition en~ines containing at least the
cornbination of (a) organic nitrate i~nition accelerator,
(b) hydrocarbyl-substituted succinilnide, (c) hydrocarbyl
10 a~ nd (d) N~N~-disalicylidene-l~2-diainillopropane~
said combination being present in an amount su~icient
to minimize coking, expecially throttling nozzle coking
in the prechambers or swirl chalnbers in indirect
injection compressioll ignition engil~es operated on such
15 fuel.
~ lso included as a Eurther embodiment of the
invelltion is a distlllate Euel additive colnposition
comprising (a) organic nitrate ignition accelerator, (b)
hydrocarb,y~l-substituted succinlmide, (c) llydrocarbyl
20 amine, and (d) NtNl-disalicylidelle-lt2-diaminopropalle in
an al;~oullt sufficient to minimiæe the coking charac-
,
teristics of such Euel, especiaily throttling nozzle
coking in the precharnbers or swirl chambers of indirect
injection compression igllition engines operatcd on such
25 fuels.
; In general, these additive Euel compositions will
~ contain as Inuch as 50~ by weight of the combin.~L all ~f
..
- 17 -
organic nitrate ignition accelerator and hydrocarbyl-
substituted succinimide-succinamide and up to 504 of the
combination of hydrocarbyl amine and N,N'-disalicylidene-
1,2-diamino2ropane or otner addilives when ~hey are
5 presellt.
In a still further ernbodi!nellt oE the invention
there is provided a metllod of inhibiting coking,
especially throttling nozzle coking in tlle prechambers
or swirl chambers in an indirect injectioll colnpression
i~nitioll engine which comprises supplying said engine
with a distillate fuel containin~ at least the com-
binatioll oE (a) organic nitrate ignition accelerator,
~b) hydrocarbyl-substituted succinimide, (c) hydrocarbyl
amine and (d) N,N'-disalicylidene-1,2-diaminopropane,
15 said cornbination being present in an amount to minirnize
such coking in an en-3ine operated on such Euel.
In another embodiment of tllis invention, the
coking-inhibiting components (a), (c) and (d) of the
invention can be added to the uels by any means known
in the art for incorporating small ~luantitie.s oE
; additives into disLillate ~uels. Compollents (a), (c)
and (d) can be added separately or they can be combined
and added togetller. It is convenient to ~itilize
additive flllid mixtures wllic}l consist oE orgallic nitrate
ignition accelerator, hydrocarbyl amine alld met.~l
deactivator agents. T}l~se additive fluid rnixtures are
.
'' ' ' '' ' '''' '" ' '
.
. ' '' '
- 18 -
adde(l to distillate fuels. In other words, part of the
presellt invention are coking inhibiting Eluirls which
col~prise organic nitrate ignition accelerator,
hy~lrocarbyl amine having Erom 3 to 60 carbons and Erom 1
to l0 nitrogens and metal deactivator, preEerably N,N'-
disalicylidene-l,2-dia~nilloprol?ane.
In these Eluid cornposition.s, the amollllt oE
coinL~ollents (a), (c) and (d) can vary widely. In
general, the Eluid compositions contain l0 to 97~9~ by
10 weiyht of the organic nitrate ignition accelerator
component, 2.0 to 75~ by weigllt oE the hydrocarbyl arnine
and 0.l to 15~ by weight metal deactivator. ~ypically,
Erom 0.0l~ by weigllt up to l.0~ by weigllt oE the
combination of the cornponents (a), (c) and a(d) will be
lS suEEicient to provide good coking-inhibitillg properties
to the distillate fuel. A preEerred distillate Euel
col~osition contalns Erom 0.l to 0.5~ by weigllt of the
colnbination containing from 50 to 97.9% b~ weight of the
organic nitrate ignitioll accelerator, Erom 2.0 to ~S~ by
20 ~eight of the hydrocarbyl amine and Erom 0.l to 5.0~ by
weigllt of the mctal deactivator cornpo1lent.
; In another embodiment of this invention, the
co~;in~J-inllibiting components (b), (c~ and (d) of the
invention can be added to the Euels b~ any means kno~/n
2S in the art or incorporating slnall quantitie.s oE
addi~ives into distillate Euels. Componellts (b), (c)
~'' . .
~.......................................... . . .
' ~ ~
4~
- 19 -
alld (d) can be added separately or they can be combined
alld ~dded to(3ether. It is co~venient to utilize
additive Eluid mixtures which consist oE hydrocarbyl-
substituted succinimide-succinamide agents, hy~rocarbyl
5 amine and ~I,N'-disalicylidelle-l,2-dialninopropane. rrhese
additive Eluid mixtures are added to distillate ~uels.
In otller words, part oE the present invention are coking
inhibiting fluids which comprise hydrocarby1-subst~tuted
succinimide-succinamide, hydrocarbyl amine having Erom 3
- lO to ~0 carbons and l to lO nitrogens, and metal deacti-
vator, preferably N,Nt~disalicylidene-1,2-diaminopropanP.
In these 1uid compositions, the amount OT'
components ~b), (c) and (d) can vary widely.~ In
general, the Eluid compositions contain lO to 97.9% by
15 weigl1t of the hydrocarb~l-substituted succinimide-
succinamide component, 20 to 75% by weight o~ tlle
hydrocarbyl amine and O.l to 15% by weight Tnetal
deactivator. Typically, from 0 01% by weight up to 1.0
by weigllt oE the combination will be suf~icient to
20 provide good coking-inhibiting properties to the dis-
tillate uel. A preEerred distillate fuel composition
contains ~rom O.l to 0.5% by we1yht of the combination
containing Erom 50~ to 97.9% by weight o~ the hydro-
carbyl succlnlmide-succ1namide coïnponent and ~om 2.0~
25 to 45% by weight oE the hydrocarbyl amine and ~rom O.l
to 5.0~ by weight oE the metal deactivator, prefera`oly
N,~'-disalicylidene-1,2-diaminopropane.
'
, ~' --
"-..: . ' '
- 20 -
The practice and adv~ntages o~ this invention
will become still further apparent from the Eollowing
illu<,trative example.
EX~MPLE l
In or~er to determine the eEfect of the Euel
col:lpositions o the present invention on the coking
tcndency o~ diesel injectors in indirect injection
compression ignition engines, use was rnade o[ a com-
mercial diesel engine operated on a coking test cycle
developed by Institute Francais Petrole and as prac-
ticed by Peugeot S. ~. Tlle amoUnt of coking togetl-er
with a quantitative indication oE the adverse conse-
quences of such coking was determined by means of (i)
injector air flow perCormance, (ii) emission o~ unburned
hydrocarbons, (iii) engine nolse, and (iv) injector
deposit ratings. The engine employed in the tests was a
.
19~2 Peugeot 2.3 liter, 4-cylinder, turbo-charsed XD2S
diesel engine connected to a MidWest dynamometer through
an engine clutch. This engine is equipped with Bosch
injectors positioned within prechambers, an(1 is deemed
representative of the indirect injection compression
i9nitiOIl engines widely used in automobiles and lLght-
du~y trucks.
The base Euel employed in these engine tests was
a commercially-avai:Lable diesel fuel having a noninal
cetane rating of 42. FI~ analysis indicated the Euel
.., ~ ',
,
~:''' ' ' '
; ~ ,
~3~ 33
- 21 -
was composed by volume oE 31.5~ aromatics,~3.0% olefins
and 65.5~ saturates. Its distillation range (ASTM
~-158) l"as as Eollows:
~arometer 29.46 incnes oE llg (0.9987 ~ars)
-5 Initial 406F - 207.78C
~vaporated at F - at C
~39 226.11
~50 232.22
~56 235.56
~63 239.
480- 2~8.89
499 259.44
521` 271.67
:
-60 545 2a5.0
572 300.0
~ 80 ` 603 317.22
; ~5 621 327.22
643 339.44
G78 ~ 358.89
Final 678F 358.89
Recovery 97.5%
Residue 2.5
1. o s s ; N o n e
'
' ~ ~
: : :::
: . . .
. ' : .
~: .' '., ' ~'
- 22 ~
Otller inspection data on the base Euel were as
Eollows:
Kinematic Viscosity, (~STM D-445) . . . 3.50 Centi~
sl:o~ s, ~0 C
Pour Point (~slrM D-97)................ -26C
Cloud Point (~STM D-97) ............... 33C
Flash Point (ASTM D-93) . . . . . . . . 91~C
Steam Jet Gum . . . . . . . . . . . . . 2.4 ng/100 ml
~niline Point (AslrM D-611). . . . . . . 143.4F (61.89C)
10 Total Sulur. . . . . . . . . . . . . . 0.41 wt. ~
~amsbottom Carbon, % (AslrM D-524) . . . 0.1~60 on 10%
Residuum
Gravity (~STM D-287). . . . . . . . . . 31.8 ~PI
SpeciEic Gravity @ 25C . . . . . . . . 0.~86
Cetane rating . . . . . . . . . . . . . 41
A test blend was prepaced Erom this base Euel
(Fuel A). Fuel A contained a combination oE (i) 506 PTB
(1.~47 grams/liter) of mixed octyl nitrates (a com-
mercial product availahle ~rom Ethyl Corporation under
20 tlle trade mark DII-3 Ignition Improver), ~ 1 PT3
(0.117 grarn/liter) of HITEC E-644, a product oE Edwin
:~Cooper, Inc., believed to be a hydrocarb~l
succinimide-succinamide made by reacting two moles oE a
polyisobutenyl succinic anbycdricle (PIBSA) lith one mole
oE a polyethylene amine rnixture having an average
composition corresponding ~to tetraetllylene pentamine,
.'~ ' . ,
~.
3,3
- 23 -
(iii) 14 PTB (0.0~ grams/liter) oE a hydrocarbyl amine
available commercially from Rohm and IIaa.s Colnpany un-ler
the trade mark Primene 81~ an(l ( iv~ 1. 7 PT13 ( O. on486
grams/liter) oE Rthyl* Metal neactivatOr, a product oE
Ethyl Corporation, the active ingredieIlt oE whicII is
N,~'-disalicylideIle-1,2-diaTnilloproparle. The manu-
factu~r gives the Eollowing typical properties for its
HIT~C~ E-G4~ product:
.
Appearance . ~ark brown viscou.s
].0 liquid
Nitrogen, wt. ~ 2.0
Specific Gravity
at 60/60F 0.928
: Viscosity at 210F, cs 340
(98.~9C)
The Prlmene 81R*is believed to be a mixture oE
prilnary aliphatic amines in which~the aliphatic grouDs
are predominantly~C12 and C14 tertiary alkyl groups.
~ The manufacturer gives the ~ollowing typical
: 20 properties-.Eor its Ethyl* metal I)eactivator:
. ~
Form Li~uid
Color Amber
Density, at 68~
g/IIIl 1.0672
lb/gal ~.91
Active ingredient~ wt % 80
* trade mark
:
, ' ' ' ,
,:
. : ' . ' -
: ~ . , .
8~3
. - 2~ -
Solvent vehicle (toluene), wt ~ .20
Elash point, open cup, ~ 8~ (2~.89C)
Fire point, F 100 ~37.78C)
Solubility
In ~asoline (rrypical) Saturated solut:ion
contains 9
In water, wt. % 0.04
Sllell Rotella T* an SAE 30, SF/CD oil ~as used
as the c.rankcase lubricant.
BeEore starting each test, new soscll DNOSD -
1510*nozzles were.installed USill9 new copper gaskets and
flame rings.. The ~uel line was ~lushed with the new
test uel composition to be tested and the .Euel Eilter
bowl and ~uel return reservoir were emptied to avoid
additive carry-over roln test-to-test.
~ t the start o each test, the en~ine was
operated at 1000 rpm, light load [or 15 minutes. AEter
this warm-up, the eng:ine was subjected to the following
autolnatic cycle:
.
~ 20 Event llPM Beam Load Minutes EGR
1 750 0 4 oEE
2 2750 12.0 6 on
. . .
3 1500 G.2 6 on
4 ~000 16.2 4 oEf
* t:rade ma~k
... .
~ 3
The above 20-minute cycle was eepeated fiO times and tlle
test was completed by running the engine at idle ~or
another 30 minutes. The total elapsed time was thu:,
20.5 hours per test.
When passing Erom one event to the next evellt in
the above cycle, some time, oE course, ~as re~ui~ed to
enal)le the engine to accelerate or decelerate ~rom one
speed to the next. Thus, more specifically, the above
cycle was programmed as Eollows:
10SegmentSeconds rprn ;~eam Loa-3
1 2 750 ~0
2 200 750 ~ 0
3 3* 2500 12
4 7* 2750 ~ 12
` 15 5 35~ 2750 ~ 12
6 3* ~ 2275 6.2
: ~ :
7 7* 1500 6.2
8 330 1500 6.2
9 3* 3500 ` 16.2
7* ~~000 16.2
; 11 230 ~000 16.2
12 3* ~ 2000 0
13 7* 750 ~ 0
14 30 750 0
: :
:
25 * i~epresents two mode periods Eor acceleration or ~eceler~tion
to the next condition.
. ~' '
, ; .. ' ~ ' : '
`' : '
~2f~38.3
. - 26 -
'Iydrocarbon exllaust emissions were measured at
the start o each test (aEter the Eirst 20-minute
cycle), at the 6-hour test interval and at the end o~
the test. These measurements were ma-~e at 750, 1000,
and 1400 rptn idle. Noise level rea-lings were made at a
location tilree ~eet Erom the engine exhaust side. Thc
measurelllents were ma~e at the start and at the end oE
the test while operating at three idle speeds, viz.,
750, 1000 and 1400 rpm.
After the test operation, the injectors were
care~ully removed Eroln the engine so as not to disturb
the deposits Eormed thereon. Measurements were made oE
air ~low through each nozzle at diELerent pintle liEts,
.
and pintIe deposits were rated using the CRC deposit
rating system.
The most signi~lcant test results are given in
Table I, in which air Elow is expressed as~cc/min and
: ~ :
hydrocarbon emissions as ppm.
TABLE I
Air Flow Pintle Obturator llydrocarbon
20 @ 0.1 mm Deposits ~oise, l)B Emissions
Fuel LiEt(10 = clean) EOT* INCT~. EOT* Incr.
,
Base 36 8.0 83.8 3.0 577 406
A 38 8.6~ 81.4 1.9 275 143
* Value at end OT- test; the increase (Incr.) shown is in
comuarison to the valile at start oE test.
I
', : - :
: '
.,; ~ . :
:
. .
-.: - . . -
,
.
-, ' . .
~.2~8~3
The results uresented in Tabl~ I show that there
were less coking deposits (higi~er air 10w rate and
Ee~er deposits), less engine noise and less hydr-ocarbon
emissions Witil Fuel A, the fuel oE the invention, as
col-npared to the Base Fuel.
E:~AMPLE [I
A test blend was prepared Erom the base fuel of
Example I (Fuel B). Fuel 3 contained a coml~ination oE
( i) 506 PTB ( 1.447 grams per liter) of mixed octyl
10. nitrates (a commercial product available ~rom Ethyl
Corporation under the trade mark DII~3 Iynition
Improver), (ii) 13.2 PTB (0.0377 grams per liter) o a
hydrocarbyl amine available commercially from Rohm and
~aas Company under the trade mark Pr~imene 81R and (iii)
1.7 PTB (0. 00486 grams per liter) of Ethyl* Metal
eactivator, a product of Ethyl Corporation, the active
ingredient of which is ~,N'-disalicylidene-1,2-diamino-
propane .
The test engine was operated under the same con-
ditlons as those of Exa!nple I.
The most signi~icant test results are given inTa~le II, in which air flow is expressed as cc/min and
hydrocarbon emissions as ppm.
* trade mark
'
,
. , : . , :, '
. ~ .
' : ' ' : '' '; ,. ' : . ' '
~.2~34~383
- 28 -
T~BEE II
Air Flow Pintle Obturator Hyclrocarbon
@ O. 1 mln Deposits Noisel ~N Emissions
Fuel Lift (10 = clean) EOT* INC~. EOT* Incr.
_ _ __ __
Base 36 8.0 ~3.8 3.0 577 406
~3 49 8.~ ~1.3 2.2 282 51
* Value at end oE test; the increa.se (Incr.) shown is in
coml7arison to khe value at start of test.
The results presented in Table I~ show that there
were less coking deposits (higher alr Elow rate and
Ee~er deposits), less engine noise and less hydrocarbon
e.nissions with Fuel ~, the Euel oE the lnvention, as
com~ared to the Rase Fuel.
:
ExAMprlE III
A test blend was prepared Erom the base fuel oE
xa;nple I (Fuel C). Fuel C contained~a combination oE
(1) 41 PTB (0.117 grams per liter) of NITEC E-644, a
product oE Edwin Cooper~ Inc.~ believed to be a hydro-
carbyl succinimide-succinamide made by reacting two
moles oE a polylsobutenyl succlnlc anhydrlde t~I~SA)
with one mole of a polyethylene amine mixture having an
average compositlon correspon~.llng to tetraethylelle
pentamine, (ii) 14 PTB (0.04 grams per liter) aE a
hydrocarbyl amlne available commercially from ~ohm and
Haas Cornpany under the t~ad~ mark Primene 81R, and
: :
,, -: i
.: .
:: : . - . :
.
.
.
33
- 29 -
liii) 1.7 PTl3 (0.00~8~ grams per liter) o~ Ethylltl~etal
l)eactivator, a product o Ethyl Corporation, the active
inc3redient o~ wllicll is N,N'-disalicylidelle-1,2-di~millo-
p r opa ll e .
The test engine was operated unde the same con-
ditions as those oE Example I. The most si~niEicallt
test results are given in Table III, in wllich air Elow
is e~pressed as cc/min and hydrocarbon emissions as ppm.
' ' ,
T~BLE III
~ir Flow Pintle Obturator Hydrocarbon
~ 0.1 mm Deposits Noise, Ds Emissions
Fuel Lit (10 = clean) EOT* INC~, EO'r* Incr
. __
~ase 36 8.0 83.8 3.0 577 406
C 40 8.5 83!2 3.0 513 278
* Value at end oE test; the increase (Incr.j shown is in
comparison to the value at start oE test.
The results presented in Table III show that
there were less coking deposits (higher air Elow rate
and Eewer deposits), less en~ine noise and less
h~clrocarbon emissions with Fuel C, the uel oE the
invention, as compared to the Base Fuel.
~ ' ' ..
~ lt tr~de m~k
~.,
: . .
'' . ~ ~ .
' ~