Note: Descriptions are shown in the official language in which they were submitted.
S433
This invention relates to a procèss and an
apparatus for coating particles such as ceramic and metal
cores, with fine powder, such as metal or ceramic powder.
More particularly, the present invention is concerned with
a process of preparing cores coated with fine powder, the
resulting product having a more uniform microstructure and
higher bonding strength than a product resulting from a
conventional mixing of the components.
In the production of composites containing a
dispersion of particles, a uniform dispersion of particles
and a strong adhesion of these particles to the matrix are
required for the making of high performance composites.
Various techniques have been reported for the
production of metal or ceramic coating on metal or ceramic
cores:
1. Physical or chemical vapor deposition,
2. Electro- or electroless plating,
3. Precipitation of salts or hydroxides from
solutions and their reduction or decomposition:
4. Surface reaction of particles, and
5. Solid state sintering of particles.
Vapor deposition gives tight coatings of metals
and ceramics (oxides, carbides, nitrides etc.), however,
thick coatings are difficult to make or take a long time.
Furthermore, these kinds of coatings are limited to some
materials having appropriate evaporation or reaction rate.
Plating is a popular method but the type of coating is limited
to metals such as Ni, Co, Fe, Cr, Cu, etc., and the coating
is usually thin. Precipitation based on decomposition
techniques is applicable only to coatings of oxides which
~2~S433
can be reduced by hydrogen. Surface reaction leads to
protective layers on the surface of particles by oxidation,
carburization, nitridation and so on. However, these
techniques are also limited by the kind of coating material
and the thickness. Sintering is based on the adhesion of
powders on the surface of the particles. In this case the
uniformity of coatings seems to be a problem because it
depends on the condition used for the mixing of the particles
and on the diffusion of the constituents.
U.S. Patent No. 3,492,379, issued January 27, 1970,
inventor G.B. Redding discloses particles of nuclear fuel
which have been coated with a thermosetting resin having
powdered graphite incorporated therein. Before coating,
powdered graphite is mixed with the resin powder to make the
mixed powders flowable without difficulty. The coating is
essentially based on the set resin having carbon particles
dispersed therein and there is no possibility to remove the
resin while retaining only the carbon particles on the
particles of nuclear fuels.
It i8 an object of the present invention to provide
a process in which there is no limitation in terms of cores
and coating materials.
It is another object of the present invention to
provide a process for coating particles which enables to
achieve a very thick coating of fine powder.
It is another object of the present invention
to provide a coating process which is simple and at the
same time gives tough coatings, consolidated around cores.
It i8 another object of the present invention to
provide coated cores which are not only useful for making
~285433
composites containing a dispersion of particles, but may alqo
be used to make porous composites and plasma or flame spray
composite powders.
In accordance with the present invention, there is
provided a process for coating particles with fine powder
which comprises providing a mixture comprising the particles
and a binder which is capable of slowly melting to viscous
state. Then, the mixture is tumbled while slowly heating
same to enable the binder to reach the viscous state while
allowing the particles to be substantially covered with the
binder. The next step includes cooling the mixture
substantially to room temperature and thereafter breaking
up particles that may have agglomerated during the tumbling
and heating, to give individual particles covered with the
binder. As a final step, the fine powder is added to the
individual particles covered with the binder and the mixture
is tumbled and heated again to the viscous state under
conditions effective to provide a coating of the fine powder
on the particles.
In accordance with a preferred embodiment of the
present invention, the coated particles could be heated
further to remove the binder leaving the particles exclusively
coated with the fine powder.
The particles preferably consist of plaqtic or
ceramic cores, for example, they are particles of A1203
and SiC or any suitable pla~tic material, or consist of
metallic cores, such as particles of Fe, Ni, Co, Cr, Cu,
Al, and alloys thereof.
The particles have a preferred mean diameter of
about 40-750 ~m.
In accordance with a preferred em~odiment of the
~28S433
invention, the fine powder includes fine metal powders, a
ceram~c powder, carban powder, or plastic powder.
They are preferably selected from the group
consisting of Al, Cr, Fe, Ni, Co, Mo, Ti, Si, Cu and W powders
and the like and have a preferred diameter of 1-10 ~Im.
In accordance with a preferred embodiment of the
invention, a surfactant is added to fine powder to inhibit
its agglomeration when coating the particles with the fine
powder. The preferred surfactant comprises aluminum stearate.
Although any suitable binder can be used the
preferred binder is polyethylene glycol or paraffin, such as
CARBOWAX (Fisher Scientific Polyethylene Glycol 3350
technical grade) and PARVANTM 52 and 67 (Esso Imperial oil
paraffin).
In accordance with a preferred embodiment of the
invent-ion tumbling--is preferably carried-out in a rotating
cylindrical container which is tilted with respect to the
horizontal axis.
The ranges of ingredients may vary to a large
extent. However, it has been found suitable to add about
5 to about 30 volume percent of the fine powder with respect
to the volume of particles, preferably about 10 to about 15
volume percent.
On the other hand, the mixture of particles and
binder may comprise about 0.5 to about 5 weight percent
binder, preferably about 1 to 2 weight percent binder.
Both heating steps are usually carried out up
to a temperature of about 80C, preferably about 60C.
Although any cooling means may be used, it is
preferred that cooling be carried out by air blowing while
the cylinder is rotating.
~ 285433
Whenever a surfactant i9 used, it is preferably
added in an amount less than about 0.5 weight percent, most
preferably less than about 0.1 weight percent.
According to the inventi~n, an apparatus for coating
particles with fine powder may comprise a frame, a rotating
cylinder mounted on the frame tilted with respect to the
horizontal axis, an inlet in the cylinder for introducing
material therein, means to cause rotation of the rotating
cylinder, means for slowly heating the rotating cylinder, and
means for cooling the heated content of said rotating
cylinder to room temperature.
The invention is illustrated by means of the
following drawing, in which:
FIGURE 1 is a schematic illustration of an apparatus
adapted to coat particles according to the invention:
~ ~ ~FIGURE 2 shows~eight A12O3 cares coated with
different metal powders,
FIGURE 3 shows two Ni-coated A12O3 cores and two
Ni-coated SiC cores.
Various experiments were made using different
materials, conditions and apparatus and these will f;rst be
discus~ed. It is understood however, that this invention is
not to be restricted by the examples and that it should only
be limited by the appended claims.
MATERIALS
A12O3 (Matfer Inc.: CorundumTM ~D grit)~ and Sic
(Norton: CarborundumTM 24 grit, Fisher Scientific:
CarborundumTM 150 and 320 grit) particle~ were used as cores.
These cèramic cores have an irregular shape.
Al, Cr, Co, Mo and W (Cerac Inc.), Al tAlcan
Aluminum Corp., Grade 105~, Ni (Sherritt Gordon Mines Limited,
Grade NF-lM) and Fe (Q~ebec Metàl Powd~rs, Atomet 95) powders
_ 5 _
~ 285a~33
were used as coating constituents. Al (Cerac) powder
contains flake. Al (Alcan) and Fe powders are nodular.
Cr, Ni, Co, Mo and W powders are granular.
Carbowax (Fisher Scientific, Polyethylene glycol
3350 Technical grade) and paraffin (Esso Imperial Oil,
Parvan 52 and 67) were used as binders. The melting point
of CarbowaxT is 58C and its freezing point is 48C, according
to the measurement made by the inventors. Paraffins (ESSO
Imperial Oil: ParvanT 52 and 67) have a melting point of 52C
and 67C, respectively. Al-stearate (Sargent-Welch Scientific,
technical grade) may be added to fine metal powders in order
to prevent their agglomeration.
APPARATUS
A cylindrical container, made from acrylic glass,
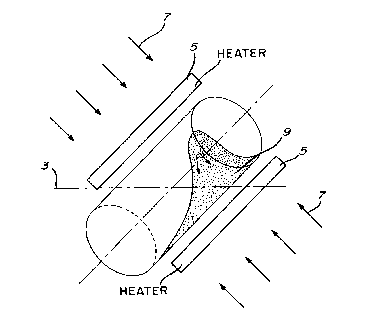
was used for coating. The apparatus is schematically
illustrated in Fig. 1. It consists of a cylinder which is
tilted with respect to the horizontal axis 3. Heating means
5 are provided to heat the content of the cylinder to a
suitable temperature and air cooling means 7 enable to cool
the heated content to room temperature. During the rotation,
the powder mass 9 move~ in both circular and axial directions
along the length of the cylinder which reduces the tendency
of powder accumulation at the ends of the cylinder, often
causing the formation of large agglomerates.
Two cylinders with different sizes were used:
cy~inder I (45 mm I.D. x 95 mm) and cylinder II (50 mm I.D. x
125 mm). The cylinder I was heated by means of an air blower
and cylinder II by means of an electric heating band. The
temperature inside the cylinder was measured with a Cu/Fe-Ni
thermocouple.
- 6 _
~85433
COATING PROCESS
The general procedure consists of adding particle~ -
to be coated and the binder to container 1. The container
is first rotated followed by heating until the binder becomes
viscous. Then the mixture is cooled and the agglomerated
particles are broken up by adding balls to the container.
The fine powder is then added to the particles in the
container and the mixture is heated to provide a coating of
the fine powder on the particles. If particles coated with
fine powder, without binder is required, binder could be
removed by heating. In some cases, Al-stearate i~ added to
prevent the agglomeration of the fine powder.
Binder-coated ceramic cores and metal powders were
charged into the cylinder. The cylinder was then heated and
rotated simultaneously. The following process parameters were
used:
Content of binder : 5 wt% (based on caramic cores).
Charge of metal ~ icleos 20 to 60 vol.% (Me/Me ~ ceramics).
Filling of cylinder : 5 to 10 vol.%.
Tilt angle : 30 or 45.
Rotation speed : 40 rpm.
Temperature : 60 to 85C.
Heating time: : 300 s for cylinder I: 600 s for
cylinder II.
Agglutination time : 300 s for cylinder I, 600 s for
cylinder II.
Cooling : Air blowing while cylinder
rotating.
Metal-coated ceramic cores were separated from free
metal powders by sieving. The proportion of the coating was
determined by weight measurements.
~.285433
The size distribution of ceramic cores wa~ measured
by sieving. The ~ize of metal powders was determined with the
HIAC particle size analyser PA-720 (Pacific Scientific).
Particle shape and microstructure of coatings were observed
by SEM.
A1203 (60 grit) cores were coated with different
metal powders. The particle size of metal powders and the
proportion of the coating are shown in Table 1. The volume
mean diameter of metal particles was between 5 and 10 ~m.
The quantity of the metal powder charge was kept constant on
a volume basis in order to make comparisons on the efficiency
of the coating process. The typical appearance of the
resulting coated cores is shown in Fig. 2.
Al (Cerac) powder gave perfect coating on A1203
;; cores. An increase of the Al powder charge slightly increased
the amount of the coating at the expense of its quality since
it became fluffy owing to the poor adhesion of Al powders. The
eoating with Al (Alcan) particles was also perfect and all
~ ~ of th~e AI charged was conQumed to form the coating. Cr
; 20 powder gave a fairly large amount of coating but some A12O3
~cor s had defects. Ni powders yielded a very small amount of
coating with many defects. Co and Mo powders showed non
~uniform coating growth: some A1203 cores had defects whereas
;the others became spherical with a thick coating, growing up
to about 2 mm in diameter. W particles gave a thick coating
which was nearly perfect.
~: :
:
,
: ~ .
~ - - 8 -
,
3S433
TABLE I RESULTS OF AGGLUTINATION COATING ** ON A1203
CORES WITH VARIOUS METAL POWDERS.
Metal Volume Charge Metal content
Powder mean(g) Me/(Me + A1203)
Diameter (Vol. %)
(ym) A1203~ Metal* Charge Product
.
Al 10.4 ZO 3 19 11
(Cerac)
Al 10.4 20 6 31 14
(Cerac)
Al 8.3 20 6 31 31
(Alcan)
Cr 6.1 20 16 32 25
Cr* _ 20 16 32 24
Ni 8.7 20 20 32 5
Co 7.1 20 20 32 16
Mo 7.1 20 23 32 18
W 4.6 20 43 31 2S
* Cr particles above 38 ym were removed by sieving.
Sample : Al O (60 grit, Carbowax 5 Wt%) .
Me~a~ particle3 (no binder).
* Container : cylinder II, Tilt angle: 45.
Rotation speed : 40 rpm, Temp.: 70C, Time : 10 min.
~.285433
The coating efficiencies of metal powders are
in the following sequence: Al (Alcan), Cr = W, Co = Mo, Al
(Cerac), Ni. Al (Cerac) powders yielded perfect coating in
spite of its low amount which probably resulted from their
flaky particles. Al flakès were agglutinated in lamellae on
A12O3 surface. Since Cr powder contained may large particles,
the surface of the coating was rough. Even if large metal
particles above 38 um were removed, the amount of Cr coating
changed little (Table 1). It has been observed that ~r
particle of about 50 ~m diameter can be agglutinated on
250 ~m A12O3 cores.
The effects of paraffin and Carbowax binders were
studied with the A12O3-Al (Alcan) system. The results are
shown in Table II. The proportion of coating produced during
the agglutination i8 about the same for both binders. Most of
Al powder was agglutinated onto A12O3 cores forming a perfect
coating. A similar effect was also observed with Co and Mo.
A12O3 cores coated with paraffin have lower flowing charac-
teristics.
TABLE II EFFECT OF PARAFFIN AND CARBoWAX IN
THE A12O3-AL SYSTEM.
BinderMeltingMetal content
pointAl/(Al + A12O ) Ob~ervation
(C) (vol. %) 3
Charge* Product
Paraffin 52 48 47 Perfect coating
(Cerac) including large
Paraffin 67 48 47
(Cerac)
30Carbowax 58 48 47 Perfect coating
* Sample: A12O3 (60 grit, binder 5 wt%) 20 g, Al (Alcan,
no binder) 12 g.
The other conditions were the same as in Table 1.
-- 10 --
~285433
TABLE III EFFECT OF THE SIZE OF CERAMIC CORES ON THE
AGGLUTINA~ION OF Al AND Ni.
Ceramic Core Temp. Charge * Metal content
(g) Me/(Me+ Ceramic)
Core size (Vol. %)
(ym) (~C) A123 SiC Ni Al Charge Product
(60 grLt1 180- 70 20 _20 _ 32 5
SiC 500_ 60 _ 10 12_ 31 .19
(24 grit) 1000
SiC 500- 65 _ 10 _ 5 38 38
(24 grit) 1000
SiC 500- 65 _ 10 10 55 55
(24 grit) 1000
SiC 75- 65 _ 10 _ 10 55 49
(150grit) 106*
SiC 75- 80 _ 10 _ 10 55 53
(lSOgrit) 106*
SiC 32- 65 _ 10 _ 10 55 39
(320grit) 45*
: SiC 32- 85 _ 10 _ 10 55 35
(320grit) 45 _
* Sample : A1203 (60 grit) and SiC (24, 150, 320 grit)
(Carbowax 5 wt%). Ni and Al (Alcan) (no
binder).
Container : cylinder I for SiC (24 grit) - Ni system,
cylinder II for the others.
Tilt angle : 45, rotation ~peed: 40 rpm, Time: S-10 min.
-- 11 --
~Z85433
The coating of nickel is one of the most difficult
to perform. The effects of paraffin, Carbowax and Al-stearate
added to the Ni powder were thus investigated with A1203 cores.
The results are shown in Table IV. For a 1 wt% addition of
paraffin or Carbowax to Ni powder, the increase in the mass of
the Ni coating was small for paraffin-doped A1203 cores. The
contents of paraffin and Carbowax in the Ni powder were
increased up to 5 wt% for Carbowax- doped A1203 cores. In this
case, Ni coating apparently increased, but the Ni coa~ing
contained defects and the product included agglomerates of Ni
powder. The addition of Al-stearate to the Ni powder increased
the thickness of the coating. However, the Ni coating on
paraffin-doped A1203 cores was imperfect. On the other hand,
Ni coating on Carbowax-doped A1203 cores became nearly perfect
for Ni coating above 9 vol.%.
~85433
'c æ~ ~ ~ a O
8 ~: ~ ~ _
~Z~o ~ C
V__ bO ~ O ~0 0~0 00 0 ~
:~: Z ~ _ _ _ _ ~ o
_._ ,0,
~ . . ~
~ Z ~ ~ ~o c~ o ~
C~ ~,',
O tC _ _ E
0 ~ ~ ~ ~ ~ O O O ~ ~ ~ O ~_,
zi ~ 0~ _ _ __ _c~ ~
e
V ~ U~ O U~ O O U~ U~ ~ o 4~ U
a -- ~ ~ e
_ .
C .~. . . . ;* * .~ *
E ~ O o o o O O o O o o o o e
S ~ ~ ~ ~ ~ ~ o `D ~O r~ O
_ 8~
i~ e ~ I _ _ _ ~ _ e
O æ ~ .
g V
.. . O
C K ~ ~ K ~ ~U
P~ ~e: ~ ~ ^ 0 ~ ~--
_~ ~ c~ 3 0 ~ ~ 3
c v I a~ I ,.0 10 I ~ 0 1 Q ~ 0 ~0 0
~ ~ ~ a ~ ~ ~ ~ e V :;! 0 e " ~ c
~ _ _ _ .__.__A___ ___ __ E
E~ C e~ o
i~ ~ 3 u~ ~ u~ u,
': ~o ~
.~ ~ . .. ~ -
_~ ~ ~_~ ~ ' ~c
la c o ~
*
~ ~8S4~3
With no addition of Al-stearate many coating defects
were observed on the edges of irregular shape cores, owing
to the fact that edges are subjected to collisions and are
left uncoated. On the other hand, Al-stearate-doped Ni powder
perfectly covered irregular A12O3 cores, even their edges.
When undoped Fe particles were used, the resulting coating
corresponded to 24 vol. % and defects were observed on the
edges. On the other hand, the addition of Al-stearate to Fe
powder reduced the Fe coating to 15 vol.%, but Al-stearate
doped Fe powder perfectly covered SiC cores.
The addition of Al-stearate to Ni powder is
effective only up to 0.2 wt% and no further increase of the
~i coating is observed above this value.
The effect of the addition of Al-stearate to metal
powder is noteworthy. Al-stearate is a surfactant and, when
the molecules are adsorbed on metal particles, the long
carbon chains inhibit the agglomeration of the metal powder.
In the coating according to the invention, therefore, Al-
stearate may improve the dispersion of metal powder giving a
perfect coating on ceramic surface of cores including
particle edges.
''il'
- 14 -