Note: Descriptions are shown in the official language in which they were submitted.
~36~;7~ ~
. 2558-185/BBBBBl
TEST PLATE ASSEMBI,Y DEFINING DISCRETE REGIONS ON
A MICROPOROUS MEMBRANE WITH LOW BOUNDARY DISTORTION
This invention relates to an apparatus for bio-
chemical testing and screening procedures involving the
use of a microporous membrane.
A biochemical test plate assembly capable of
handling multiple simultaneous tests involving a single
microporous membrane is disclosed in Fernwood et al. r
U.S. Patent No. 4,493,815, Bio-Rad Laboratories, Inc.,
January 15, 1985. The assembly provides a standard
8-by-12 rectangular array of cylindrical wells, with
the bottom of each well sealed by a common microporous
membrane. The membxane in turn rests above a recess
forming an enclosed chamber from which a vacuum may be
drawn or which may be completely sealed against air
loss thereby providing a static air cushion beneath the
membrane. The device may thus be used for either (a)
drawing a fluid containing bïochemical species through
the microporous membrane, or (b) supporting a static
fluid above the membrane for an indefinite length of
time. The exposed membrane regions collectively provide
an array of discrete test regions with highly defined
boundaries. Accurate automated detections can then be
performed on the membrane after it is removed from the
assembly.
The assembly generally consists of two aper-
tured plates (an upper and a lowex) and a base platecontaining a recess to form the vacuum chamber beneath
the welTs. The microporous membrane and an apertured
gasket are placed between the two apertured plates.
The membrane is thus the only obstacle be-tween the upper
plate apertures and the vacuum chamber, thereby permit
ting both flow-thxough and static contact procedures,
'
.. : ... . . .
, . . :. . . :
.
';' ' ' ' ' "
~L2~
depending on the air pressure in the chamber. The wells
and flow passages are sealed from the surr~unding room
atmosphere by the apertured gasket between the two aper-
tured plates, and a further gasket between the lowerapertured plate and base plate.
It is critical that these seals be perfectly
air-tight so that prolonged tests can be done without
loss of chamber pressure. This requires highly polished
finishes at the surfaces where the seals are made, which
adds considerably to the cost of manufacturing.
Furthermore, since the membrane must be fully
moistened before assembly of the parts, the exposure of
its outer edges to the atmosphere during the test proce-
dures raises another disadvantage - evaporation from
these edges. This induces outward migration of the
biochemical species which have contacted the mer~rane
through the ~utermost wells. ~he result is distortion
of the outermost test regions on the membrane. This is
a serious failing, since the lack of uniform contact
areas obscures the test results in a number of ways.
An improvement over the device described above
is offered by the present invention, in which the lower
of the two apertured plates is fully enclosed by the
remaining two plates. This reduces the number of seals
which have atmospheric contact to a single seal ~etween
the two enclosing plates. The microporous membrane is
thus sealed off from the atmosphere entirely, and evap-
oration from the membxane itself is eliminated as wellas any lateral diffusion driven by the resulting capil-
lary attraction. With these features, the assembly of
the present invention overcomes both of the problems
mentioned above, while still retaining the same versa-
tility of use and function. The result is a test plateassembly which provides even greater accuracy and repro-
ducibility.
~ '
- .; , . . ........... .
: ' . . .
-' .: ' : ''' . ' .. ': '
,
: :
~2~3~57~
The invention is illustrated, merely by way
of example, in the drawings, in which:
FIG. 1 is an expanded view of one embodiment
of a test plate assembly according to the present inven-
tion;
FIG. 2A and FIG. 2B are a plan view and end
view with cutawayl respectively, of the lower apertured
plate shown as one of the components in FIG. l;
FIG. 3A and FIG. 3B are a plan view and a
side sectional view, respectively, of the bottom plate
shown in FIG. l; and
FIG. 4, on the second sheet, is a side view in
partial cutaway of the assembled parts of the embodiment
shown in FIG. 1.
As in Fernwood et al., referenced above, the
test plate assembly of the present invention is inten-
ded to accommodate a multitude of simultaneous biochem-
ical tests, each in one of a series of discrete wells
or reservoirs arranged in a horizontal array. Although
the number, size and spacing of the wells may vary, the
most common and versatile arrangement is one comprising
96 circular wells in an 8-b~-12 rectangular array, with
a center-to-center spacing of approximately 9mm, an
arrangement used by a large variety of associated labo-
ratory eguipment. Other examples include oval or slot-
shaped wells with associated apertures of appropriate
shape. For convenience, the drawings and the remainder
of the description herein refer to a standard 96-well
array.
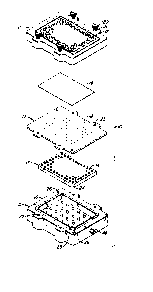
FIG. 1 illustrates one embodiment of the test
plate assembly of the invention. The assembly is desig-
nated by the numeral 10, its primary parts consisting
of an upper plate 11 having a plurality of apertures 12
arranged in the aforementioned array; a middle plate
13, also wi-th a plurality of apertures 14 aligned with
those of the upper plate 11; a lower plate 15 containing
., ~
. - ,. , .: .. ' :. .
. . . : , . : , . . , . :
~~i5~
a recess 16 sufficiently large to receive the middle
plate 13; a gasket sheet 17, having apertures in the
identical array and alignment of the upper and middle
plates 11, 13; and a microporous membrane 19 of suffi-
cient length and width to cover all of the apertures in
the array.
The assembly is held together with foux cap-
tive manually operated screws 20, with coil springs 21
for holding the screws in a raised position until they
are pushed down and screwed into the lower plate 15.
This keeps the screw tips from interfering with the
alignment of the porous membrane and gasket during as-
sembly and disassembly. For convenience, the screws are
captive in the upper plate 11, the threaded ends (not
shown) mating with threaded holes 22 in the bottom plate
15 after passi~g through holes 23 in the gasket sheet 17.
Proper placement of the middle plate 13 inside the lower
plate 15 is ensured by chamfering of the middle plate 13
at one corner 24 to mate with an angled corner segment
25 of the inner wall of the recess 16. Proper orienta-
tion of the upper and lower plates is achieved by a pair
of guideposts 26 along one side of the upper surface of
the lower plate 15 to fit into corresponding holes (not
shown) in the underside of the upper pla-te 11.
The middle plate 13 is shown in detail in
FIGS. 2A and 2B~ Surrounding each aperture 14 is a
boss 30 extending upward from the plate. The upper
surface of each boss is flat and coplanar with each of
the remaining bosses. The result is an even and concen-
trated pressure on the gasket sheet 17 resting on top
of the middle plate (see FIG. 1.) when the asse~bly is
secured together, the pressure being concen-trated around
the rims of the apertures.
The underside of the plate has an array of
protruding ribs 31, which add structural strength to
the plate and also keep the lower opening 32 of each
aperture clear, avoiding stoppage of li~uid. As
~, . . ~ , .
- . ., : . ~ - :
' ' '' ~ ' . '~ ' ' '
'' ',
5~8
mentioned above, one corner 24 of the plate is cham-
fered for purposes of proper orientation.
A detailed look at the lower plate 15 is of-
fered in FIGS. 3A and 3B. It will be noted that therecess 16 is similar in lateral dimensions yet slightly
larger than the middle plate 13, to snugly accommodate
the latter. The angled wall 25 at one corner mates
with the chamfered corner 24 of -the middle plate to
ensure that the middle plate is inserted with the bosses
facing upward.
The recess is surrounded by a ledge 40. When
the parts of the assembly are secured together under
the tension of the tightening screws, the ledge 40 is
forced against the outer edge of the lower surface of
the upper plate 11 through an intervening gasket, thus
sealing the interior of the recess 16 from the labor-
atory environment~ In the embodiment shown, the seal
is achieved by a perimeter gasket (not shown) which
rests in a machined groove 42 (shown in FIG. 3B) which
completely encircles the recess 16. The gasket may
assume any o a variety of conventional f~rms such as,
for example, a large O-ring of circular cross-section
(to mate with the curved groove 42 shown) or rectangular
cross-section. Alternatively, the gasket may be combined
with the gasket sheet 17 (see FIG. 1) as an extension
thereof in the form of a protruding ridge. The latter
is illustrated iIl FIG. 4, discussed in detail below.
Returning to FIG. 3B, the recess has a slant-
ing floor 43 and a series of support posts 44 extendingupward ~rom the floor, on which the downwardly protrud-
ing ribs 31 of the micldle plate 13 rest. This holds
the middle plate above the sloping floor 43, defining
an open space 45 below the middle plate to allow drain-
3S age of any liquids passing through the apertures in themiddle plate. The sloping floor 43 promotes li~uid
drainage toward a port ~6 at one side. The port may be
connected to a vacuum line (not shown) for use of the
~' ' .
-. . . . .
' . ' ' ' ,. ' '' ' ~' '. '.: ~ ' ,', :' ' '
- ' : ' ' ' ' ' '' " ;~ ' '. , . .' '
. .............. ' ' '. ' . ', . ..
, " ', ' ' ' ' ' , ' ' ' '~', ~,~, ' ' . , '
. " ' ' .' ' ~
~L2~36~r;7~3 ~
assembly in performing a filter assay. Alternatively,
the port may be closed by a valve (also not shown) to
close off the inner space 45 of the base plate when a
static blot assay is to be performed.
The assembled structure is shown in FIG. 4.
It will be noted that the peripheral seal is formed by
a protruding ridge 47 on the gasket sheet 17, extending
downward into the groove 42 which has been machined
into the lower plate 15. The microporous membrane 19
lies entirely within the area defined by this protruding
ridge 47. Accordingly, when the entire apparatus is
assembled, and the apertures 12 in the upper plate are
occupied by li~uid samples, the microporous membrane
has no contact with the external atmosphere. Evapora-
tion from the side edges and the resulting distortion
of the test areas in the membrane is thereby avoided.
It is preferred that the range of compression
of the peripheral seal exceed the range of compressio~
of the gasket sheet by the ~osses 30 around each set o~
aligned apertures. The peripheral seal will then be
formed first as the securing screws are tightened, and
less pressure is exerted on the individual aperture
seals at the top of each boss. This minimizes distor-
tion of the contact areas on the microporous membrane.This difference in the range of compression may be
achieved in any of a variety of ways, but is most con-
veniently achieved by selecting an appropriate height
for the ridge 47 and thickness for the remainder of the
gasket sheet 17.
Further features of the drawing show prefer-
red embodiments o~ the structure. The apertures 12 in
the upper plate, for instance, are generally cylindri-
cal, the diameter of each undergoing a reduction from
the upper surface of the plate to the lower surface.
This is useful in concentrating the biochemical species
as it passes through the well and is deposited on the
microporous membrane, improving the ease of detection
.
,. . ~ , .
. - . ~ . , . ., . ., : : . . ' , .
: - . - . ~ ~
.. . ..
... . , - . :
- : . . .
.
. . . : . .
~ 365~ ~
and subse~uent processing steps. To maximize the well
capacity, the tapering portion is located toward the
bottom of the aperture, providing a well capacity rang-
ing from about 100 to about l,000 microliters in volume.
As another feature, the apertures 18 in thesheet gasket 17 are of slightly smaller diameter than
bo-th those of the upper plate (at the narrower end) and
the lower plate. In this way, the define~ test area on
the microporous membrane 19 is slightly smaller than
the diameter of the apertures in the plates, and slight
misaligmnents of either the plates or the gasket sheet
will not affect the size of the test area, since it
will still be in full contact with the liquid either
held in or passing through the wells.
The plates may be constructed of any rigid
inert material, preferably transparent so tha-t the test
fluids may be observed. Conventional materials will
suffice, notably acrylic, polycarbonate, polypropylene
or polysulfone. A convenient means of forming the plates
is by injection molding. Since this avoids the need
for machining of the plates individually, open spaces
or gaps are easily incorporated into the structures to
reduce the weight and the amount of plastic required.
The embodiments shown in the drawings are simplified,
however, for a better understanding of the functional
aspects of the construction.
As in the structure disclosed in Fernwood e-t
al., referenced above, -the test p}.ate assembly of the
present invention may be used for two basic modes of
operation - ~orcibly drawing a fluid through the mem-
brane, and retaining a ~luid above the membrane for a
prolonged period of time. The former may be achieved
by drawing a vacuum through the vacuum port g6, while
the latter is achieved by sealing the port 46 from the
atmosphere and retaining a slight positive pressure in
the recess of the base plate below the middle plate.
These functions may be performed either individually or
:' ' . '. ' ' ' ' ~ ., '': ' ., ,
- ' , . . : :
,
~ .' .
5~8
seguentially in a wide variety of biochemical laboratory
procedures, with improved results in terms of accuracy,
reproducibility, and lack of distortion.
The foregoing description is offered primari-
ly for purposes of illustration. Although a variety of
e~nbodiments has been disclosed, it is not intended that
the present invention be limited to the particular struc~
tures or methods of operation set forth above. It will
be readily apparent to those skilled in the art that
numerous modifications and varia-tions not mentioned
here can st.ill be made without parting from the spiri-t
and scope of the invention as claimed hereinbelow.
- - .
~ - -: . .
~.