Note: Descriptions are shown in the official language in which they were submitted.
~ 2~37Z77
-- 1 --
STEEL ARTICLE HAVING HEAVY-DUTY ANTICORROSIVE COATING
sACKGRoUND OF T~E INVENTION
~1) Field of the Invention
The present invention relates to a steel
material or article having a novel heavy-duty coating,
which is capable of assuredly preventing corrosion and
subsequent destruction of a pipe, a vessel, a con-
struction or the like even when it is used under the
ground or in a harbor or river for a long time.
(2) Description of the Related Art
In the field of steel materials or articles
having a heavy-duty anticorrosive coating, wide use was
made of steel materials or articles coated with asphalt,
fiber-reinforced asphalt or coal tar enamel, and steel
materials coated with a coal tar epoxy resin. Recently,
polyethylene-coated steel pipes, which are cheap and
have excellent corrosion resistance (see Japanese
Unexamined Patent Publications No. 50-2072, No. 50-143114
and No. 47-34657) have been used for pipes and steel
pipes for construction work. As the heavy-duty anti-
corrosive coating for steel materials other than steelpipes, there have been proposed a polyurethane mastic
coating (Japanese Examined Patent Publication No.
59-20077), an epoxy mastic coating (Japanese Unexamined
Patent Publication No. 57-133117) and a glass fiber-
reinforced plastic coating (Japanese Unexamined PatentPublication No. 58-29916). Coating materials such as asphalt, fiber-
reinforced asphalt and coal tar are defective in that,
at high temperatures, they soften and become difficult
to handle, and at low temperatures, they become brittle
and are readily cracked. When these coating materials
are used for coating, they must be heated to be fused.
This coating operation requires a great deal of skill
and there arise problems such as the generation of
stimulative gas and smell and the risk of self-ignition.
B ~
.
.
~ ;2 J~72~7
Furthermore, coal tar epoxy resin-coated steel
materials have problems in that the curing speed of the
coating is low, the operation adaptability after the
coating operation is poor and since the strength of the
coating is insufficient, deep cracks or penetrations are
readily formed in the coating during transportation or
working and peeling of the coating or pitting is readily
advanced from these cracks or penetrations. Moreover, a
glass fiber-reinforced plastic coating is disadvantageous
in that the coating cost is high.
The polyethylene coating has problems in that
the coating equipment is large and cumbersome and a
steel article havlng a complicated shape such as a steel
pipe sheet pile cannot be coated.
Moreover, the polyurethane mastic coating or
epoxy mastic coating is unsatisfactory in that since an
epoxy resin or a conventional polyurethane resin has
hydrophilic groups in the molecule, the coating has a
large water absorbing property and in a corrosive
environment, the coating layer absorbs water to cause a
reduction in the electrical insulating characteristics.
SUMMARY OF THE INVENTION
It is therefore a primary object of the present
invention to eliminate the defects of conventional steel
articles having a heavy-duty anticorrosive coating,
namely, to provide a steel article having a heavy-duty
anticorrosive coating, which has excellent corrosion
resistance, water resistance, impact resistance and
electrical insulation resistance.
In accordance with the present invention, there is
provided a steel article having a heavy-duty anticor-
rosive coating, which is obtained by subjecting all or a
part of the surface of a steel article to a pretreatment
and forming thereon a coating of a polyurethane resin,
~5 wherein the polyurethane resin coating is formed by
reacting a mixture comprising, as the main components,
(a) a polyol having the main chain composed solely of
~37Z~7
-- 3 --
carbon atoms and hydrogen atoms, containing at least two
hydroxyl groups in the molecule and preferably having a
hydroxyl value not larger than 120 mg KOH/g, and (b)
an organic polyisocyanate compound.
According to a preferred embodiment of the present
invention, the polyurethane resin comprises (a) a polyol
compound having a hydroxyl value not larger than 120 mg
KOH/g, (b) an organic polyisocyanate, an organic
compound, which is reactive with the organic poly-
isocyanate (b) and is, preferably, (c) a polyol having a
hydroxyl value not larger than 120 mg KOH/g, other than
the polyol compound (a), and/or (d) a compound having at
least two hydroxyl groups and/or amino groups in the
molecule and a hydroxyl value and/or amine value larger
than 120 mg KOH/g and, optionally, a catalyst, an
extender, a plasticizer and a hygroscopic compound. The
composition of the respective components is such that
the amount of the polyol (a) is (100-x) parts by weight
(in which x is from 0 to 50), the amount of the polyol
(c) is x parts by weight (x is as defined above), the
amount of the compound (d) is 0 to 300 parts by weight,
the amount of the compound (b) is such that the molar
ratio NCO/(OH + NH2) of the isocyanate groups of the
compound (b) to the sum of hydroxyl and amino groups of
the polyol (a), the polyol (c) and the compound (d) is
in the range of from 0.85 to 1.5, the amount of the
catalyst is 0 to 10 parts by weight, the amount of the
extender is 0 to 500 parts by weight, the amount of the
plasticizer is 0 to 100 parts by weight and the amount
of the hygroscopic compound is 0 to 30 parts by weight.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a perspective view of a steel pipe having
a heavy-duty anticorrosive coating, obtained in Example
l;
Fig. 2 is a perspective view of a steel pipe sheet
pile having a heavy-duty anticorrosive coating, obtained
in Example 2; and
lZ~3~277
Fig. 3 is a perspective view showing a steel sheet
pile having a high-duty anticorrosive coating, obtained
in Example 3.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The steel article having a heavy-duty anticorrosive
coating according to the present invention will now be
described in detail.
As the polyol (a) of the polyurethane resin used in
the present lnvention, there can be mentioned a hydroxyl-
terminated liquid polybutadiene having at least two
- hydroxyl groups. In view of the elasticity of the
coating, it is preferred that the hydroxyl value
of the polyol (a) be not larger than 120 mg KOH/g.
Preferred polyols are marketed under the tradenames Poly
bd R-45HT~i9, Poly bd R-45(~) and Poly bd CS-15(3 ( supplied by
Idemitsu Petro. Chem. Co.), Nisso PBG-2000 and Nisso
PBG-3000 ~supplied by Nippon Soda Co.), and Polytale
(supplied by Mitsubishi Chem. Ind. Ltd.).
As the compound (b), there can be mentioned tolylene
diisocyanate (hereinafter referred to as "TDI"), crude
diphenylmethane diisocyanate (hereinafter referred to as
"crude MDI"), liquid diphenylmethane diisocyanate
(hereinafter referred to as "liquid MDI"), hexamethylene
diisocyanate, isophorone diisocyanate, methylene-
bis(cyclohexyl isocyanate), xylylene diisocyanate,hydrogenated tolylene diisocyanate and hydrogenated
xylylene diisocyanate.
As the polyol (c), there can be mentioned poly-
oxyalkylene polyols, polytetramethylene ether glycol and
3Q polyester polyols, each having a hydroxyl group value
not larger than 120 mg KOH/g.
As the compound (d), there can be mentioned
compounds having at least two hydroxyl groups, such as
ethylene glycol, propylene glycol, butane-diol, neopentyl
glycol, hexane-diol, octane-diol, hydroquinone, bisphenol
A, trimethylol propane, glycerol, pentaerythritol,
triethanol amine and bis(2-hydroxypropyl) aniline, and
~5
::'
- ,
, ~ ~
:
:
~2~37277
-- 5 --
their alkylene oxide addition products; compounds having
at least two amino groups, such as diaminodiphenyl-
methane, methylene-bis(orthochloroaniline) (hereinafter
referred to as "MOCA"), phenylene diamine, tolylene
5 diamine, ethylene diamine and piperazine; and compounds
having at least two amino and hydroxyl groups, such as
monoethanol amine, diethanol amine, aminoethylethanol
amine and compounds formed by adding an alkylene oxide
to parts of amino groups of the above-mentioned compounds
having at least two amino groups. The hydroxyl value
and/or the amine value of each of these compounds is
larger than 120 mg KOH/g.
The polyurethane resin used for formation of the
main coating layer in the present invention may further
comprise a catalyst, an extender, a platicizer, and a
hygroscopic compound according to need.
As the catalyst, there can be mentioned organo-
metallic compounds such as dibutyltin dilaurate,
stannous octoate, dibutyltin diacetate, lead octylate
and lead naphthenate, and amine compounds such as
triethylamine and triethylenediamine.
As the extender, there can be mentioned inorganic
powders such as calcium carbonate, silicates, mica
powder and glass flake.
As the plasticizer, there can be mentioned coal
tar, process oil, a liquid petroleum resin, dibutyl
phthalate, dioctyl phthalate and chlorinated paraffin.
As the hygroscopic compound, there can be mentioned
powdery silica gel and zeolite having a variety of fine
pores capable of absorbing water therein, anhydrous
calcium chloride and gypsum absorbing water as water of
crystallization, and calcium oxide capable of absorbing
water by reaction with water.
In the polyurethane resin to be used for formation
of the coating in the present invention, the mixing
ratio of the polyol (a) and the optional polyol (c) is
such that the amount of the polyol (a) is 100 to 50
~J ~rc J 3~Z77
parts by weight and the amount of the polyols (c) is 0
to 50 parts by weight, with the proviso that the sum of
the amounts of the polyols (a~ and (c) is 100 parts by
weight. If the amount of the polyol (a) is smaller than
50 parts by weight, the water absorption of the resin
is undesirably increased and hence, the insulation
resistance is reduced. Where a polyester polyol is used
as the polyol (c), if the amount of the polyol (a) is
smaller than 50 parts by weight, deterioration of the
resin by hydrolysis is caused.
The compound (d) may be used in an amount of 0 to
300 parts by weight per 100 parts by weight of the sum
of the polyols (a) and (c). It is preferred that the
average hydroxyl value of the polyols (a) and (c)
and the compound (d) or the sum of the average hydroxyl
value and the average amine value be 100 to 300 mg
KOH/g. Generally, a reduction of the average hydroxyl
value results in a reduction of the mechanical strength.
On the other hand, the cold resistance is improved by a
reduction of the average hydroxyl value, and conversely,
as the hydroxyl value is increased, the mechanical
strength is improved but the cold resistance is degraded.
If the hydroxyl value is further increased, the resin
becomes brittle.
The compound (b) should be used in an amount such
that the molar ratio NCO/(OH + NH2) of the isocyanate
groups of the compound (b) to the sum of the hydroxyl
and amino groups of the polyols ~a) and (c) and the
compound (d) is in the range of from 0.85 to 1.5. If
this ratio is lower than 0.85, curing is insufficient
and the resin becomes viscous. On the other hand, if
this ratio is higher than 1.5, the free isocyanate group
reacts with water in the air and blistering readily
occurs in the coating layer.
It is preferred that the amounts of the catalyst,
extender, plasticizer and hygroscopic compound, to be
used according to need, be 0 to 10 parts by weight, 0 to
,
~ ~37~77
500 parts by weight, 0 to 100 parts by weight and 0 to
30 parts by weight, respectively.
Sometimes the steel article having a heavy-duty
anticorrosive coating is cut by a gas burner for
alteration of the size, etc. at the piping step or the
step of assembling a structure. During this cutting
operation, cutting is often rendered difficult because
the coating layer composed of a polyurethane resin
catches alight. In this case, if a flame retardant such
as aluminum hydroxide, antimonyl oxide or chlorinated
paraffin is added to the polyurethane resin, the cutting
operation by a gas burner is facilitated.
Where the coated steel article is used outdoors for
a long time, whitening of the surface of the coating
composed of the polyurethane resin, that is, so-called
"chalking", occurs. Chalking is a phenomenon which
occurs only in a very top surface portion of the coating
and has no substantial influence on the anticorrosive
function of the coating. Where, in view of the ap-
pearance characteristics, it is necessary to preventchalking, this can be done by forming a coating of an
acrylic urethane resin on the coating composed of the
polyurethane resin. Preferably a product formed by
reacting an acrylic polymer with a urethane prepolymer
having a terminal isocyanate group is used as the
acrylic urethane resin.
As the steel article used in the present invention,
there can be mentioned, for example, a steel pipe, a
steel pipe sheet pile, a steel sheet pile, an H beam and
a steel plate. r~Ore specifically, there can be mentioned
steel pipes for underground piping, submarine piping and
ground piping, and piling steel pipes, steel pipe sheet
piles, steel sheet piles and H beams for marine con-
struction, sea and river shore protection construction
and other exposed constructions such as bridges. To
improve the adhesion between the coating layer composed
of the polyurethane resin and the steel material, it is
:
'
~ XJ~7277
-- 8
preferred that, after cleaning the surface of the steel
material by blast working or the like, (l) an epoxy
primer layer be formed, (2) a chemical conversion
treatment of the chromic acid type be carried out or (3)
a chromic acid type chemical conversion treatment be
carried out and an epoxy primer layer be then formed
thereon~ As the chromic acid type chemical conversion
treatment agent, there may be used an aqueous solution
of a composite oxide of hexavalent chromium oxide and
trivalent chromium oxide obtained by partially reducing
hexavalent chromium oxide, and a solution formed by
adding to the above aqueous solution glycerol or
polyvinyl alcohol as a reduction-promoting substance or
silica as a substance capable of improving the adhesion
to the coating.
As the epoxy type primer lacquer, there may be used
a composition comprising a bisphenol A type epoxy resin
as a main component and, incorporated therein, a modified
amine curing agent and an inorganic pigment according to
need.
To obtain a steel article having a heavy-duty
anticorrosive coating according to the present invention,
the surface of the steel article is cleaned by blasting
or the like and a coating composed of a polyurethane
resin is then formed thereon. Before formation of the
coating layer of the polyurethane resin, and according
to need, an epoxy type primer layer is formed on the
surface of the steel article or a chromic acid type
chemical conversion treatment is carried out, or after
the chromic acid type chemical conversion treatment, an
epoxy type primer layer is formed.
For formation of the polyurethane resin coating,
the following methods may be adopted.
(l) A method in which a first liquid formed by
uniformly mixing the polyols (a) and (c) and the compound
(d), optionally with predetermined amounts of the
catalyst, extender and plasticizer, and the second
~ ~3'~Z77
g
liquid of the compound ~b) are separately stored, and at
the time of application, the first and second liquids
are mixed together and coated on the steel article by
using a two-liquid mixing type spray coater.
~2) A method in which a first liquid (prepolymer)
formed by reacting parts of the polyols (a) and ~c) and
the compound ~d) with all of the compound ~b) and a
second liquid formed by uniformly mixing together the
remaining parts of the polyols (a) and (c) and the
compound ~d), optionally with the catalyst, extender and
plasticizer, are separately stored, and at the time of
application, the first and second liquids are mixed
together and coated on the steel article by a trowel.
(3) A method in which a liquid composition formed
by mixing together the respective components is cast on
the steel article from the upper portion thereof by
using a multiple-component mixing foaming machine and
uniformly coated by a trowel or the like.
In these methods, the curing rate may be controlled
according to the amount of the catalyst. It is preferred
that a relatively thick coating be carried out so that
the thickness of the coating layer is at least 1 mm. If
a desired thickness cannot be obtained by performing the
coating operation only once, the coating operation may
be carried out repeatedly.
If spray coating is carried out under specific
conditions, for example, under high humidity conditions,
water in the air is absorbed and reacts with the
isocyanate to form foam in the coating, which results in
a reduction of the strength, water absorption and
insulating property of the coating. In this case, the
formation of foam in the coating can be prevented if
coating is carried out after incorporating a hygroscopic
compound into the polyurethane resin.
As apparent from the foregoing description, the
coated steel article of the present invention has a
heavy-duty anticorrosive coating of a polyurethane
~ ~r37277
-- 10 --
resin, which has a low water-absorption, a high electrical
insulation property, excellent mechanical characteristics
and a long-period stability. Accordingly, the coated
steel article of the present invention has an excellent
corrosion resistance under the ground, in the sea and
for other exposed conditions.
In the polyurethan~ resin of the present invention,
the contents of hydrophilic atomic groups such as ether
linkages and ester linkage are much lower than in
conventional polyurethane resins, and therefore, the
water absorption of the polyurethane resin is small.
Moreover, the proportion of non-polar hydrocarbon groups
in the molecule is large and hence, the electrical
insulation property is high. Even if the polyurethane
coating is exposed to sea water or fresh water, reduction
of the electrical insulation property is very small.
Accordingly, the coating layer of the present invention
retains an excellent corrosion resistance for a long
time. The molecular structure of the polyurethane resin
of the present invention comprises very soft portions
composed of long-chain hydrocarbon groups and rich in
the softness, which are known as "soft segments", and
portions composed of aromatic rings and highly
crosslinked atomic groups and having a high rigidity,
which are known as "hard segments". Accordingly, the
polyurethane resin of the present invention is charac-
terized in that it has a combination of a high mechanical
strength and a high softness. Therefore, the coating of
the present invention has a high durability against
shock or bending during transportation or working.
The polyurethane resin of the present invention is
distinguishable over conventional polyurethane resins in
that since the content of the ester linkage readily
undergoing hydrolysis or the ether linkage readily
undergoing ultraviolet ray deterioration or oxidative
deterioration in the molecule is very low, the coating
layer of the polyurethane resin layer of the present
~ i~r37~'77
-- 11 --
invention has an excellent resistance to ultraviolet ray
deterioration, reslstance to oxidative de-terioration,
water resistance and chemical resistance. Therefore,
the polyurethane resin of the present inventlon is
suitable for forming an anticoxrosive coating for
piping work or construction which will be in use for a
long time and in which intermediate maintenance is
difficult.
The present invention will now be described in
detail by the following examples.
All of "parts" in the examples are by weight.
Example l
The outer surface of a steel pipe for water service,
which had a thickness of 12 mm, an outer diameter
of 1600 mm and a length of 12 m, was cleaned by grit
blasting (G-70), and an epoxy type primer was then
spray-coated on the cleaned outer surface to form a
primer layer having a thickness of 30 ~m (after curing).
A polyurethane coating material comprising the first and
second components shown in Table l was coated on the
primer layer by a two-components mixing type spray
coating machine to form a coating of a polyurethane
resin having a thickness of 2.5 mm (after curing).
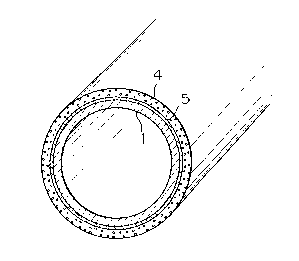
Fig. 1 is a perspective view of the thus-obtained coated
steel pipe having a heavy-duty anticorrosive coating.
In Fig. 1, reference numerals 1, 4 and 5 represent the
steel pipe, the coating composed of the polyurethane
resin and the epoxy type primer layer, respectivelyO
The heavy-duty anticorrosive coating of this coated
steel pipe was aged for 7 days after the coating
operation to sufficiently advance the curing of the
coating layer. Test pieces were cut out and subjected
to various performance tests. The results obtained are
shown in Table 2.
7277
- 12 -
Table 1
_ _ _ _ _
Coating Material
Components KindsAmounts (parts)
First Component
Polyol (a) Polybutadiene R-45HTl)100
Polyol (c)
Compound (d) Polyhardener PA-4002)65
Catalyst Dibutyltin dilaurate0.8
Extender Mica powder 15
Plasticizer Process oil 45
Hygroscopic compound Anhydrous gypsum 4
Second Component
Compound (b) Crude MDI 80
. .
Note
l) Product of ARCO Co. having a hydroxyl value of
46.5 mg KOH/g
2) Product of Daiichi Kogyo Seiyaku Co. having a
hydroxyl value of 420 mg KOH/g
12~3'7277
-- 13 --
o '~ ~ '~
~ .~ r)l ~ ~
0
o ~ X ~ ~,
~ ~ ~ ~ ~ ~
C~
H 4~ ~ o
o
z ~ z~ ~ ~
a ~ a)
.~ ~ o~o ~ ~ I
~ ~ o ~ ~
~1
u~ ~ h
a~ ~ ~ ~0
~ ~ o a~ a~
<~J ~ ~ ~ o ~ u~
r ~ ~ l~ ~n u~
o ~ ~ a c) ~9 a) u~ r~
a a ~ , ~ ~ ~ ~ 3
~ ~ $ '~
~ 0~
~ ~ ~$ ~'
~ ~ 3 ~ ~ a)
1~ ~ h ,-1 O
Q ~ o ~ o ~ ~ ~
'8 ~u~ ~0 ;~ h :~
~ ~ o
" - :
,- . '
' ~ ' ,
'7Z77
- 14 -
Example 2
The outer surface of a steel pipe sheet pile having
a thickness of 15 mm, an outer diameter of 800 mm, a
length of 16 m and a shape as shown in Fig. 2 was cleaned
by a grit blast treatment (G-70) and subjected to a
chromic acid type chemical conversion treatment to form
a film of a chromic acid compound having a total chromium
deposition amount of 500 mg/m~. A polyurethane coating
material comprising first and second components shown in
Table 3 was coated on the chromic acid compound film by
a two-components mixing type spray coating machine to
form a coating of a polyurethane resin having a thickness
of 2.5 mm after curing. Fig. 2 is a perspective view
showing the thus-obtained steel pipe sheet pile having
the heavy-duty anticorrosive coating. In Fig. 2,
reference numerals 2, 4 and 6 represent the steel pipe
sheet pile, the coating layer of the polyurethane resin
and the film composed of the chromic acid compound,
respectively.
The heavy-duty anticorrosive coating of the coated
steel pipe sheet pile was aged for 7 days to sufficiently
advance curing of the coating, and test pieces were cut
out and subjected to various performance tests. The
results obtained are shown in Table 4.
;:
.
'7X~7
- 15 -
Table 3
Coating Material
Components Kinds Amounts (parts)
First Component
Polyol (a) Polybutadiene R-45HT lOO
Polyol (c)
Compound (d) Polyhardener PA-400 70
Catalyst Dibutyltin dilaurate 0.6
Extender Talc 15
Plasticizer Process oil 40
Hygroscopic compound Synthetic zeolite 4
Second Component
Compound (b) Crude MDI 85
,
" '
7Z77
-- 16 --
~ (~ g ~
~ u~ r~ ~~ ,1 ~ U~
~ X O~
~ ~ o o~ ~ o
~o ~$
~3 ~
0 Z 0 Z U
e, ~ e
:~ ~
~r
~1 ~
~ ~ ~
~ o
~ ~) O ,~ U ~ ~
a ~ fi-~ ~ ~ ~ ~
~ ~ a a a 5~ ~
U~ 1 Q ~ U O a?
a) a) ~ '
~ ts u
~ P
.~
: ''
7277
- 17 -
Example 3
One surface of a steel sheet pile (FSP III type)
haviny a shape shown in Fig. 3 was cleaned by a grit
blasting (G-70) and subjected to a chromic acid type
chemical conversion treatment to form a film of a
chromic acid compound having a total chromium deposition
amount of 500 mg/m2. Then, an epoxy resin primer was
coated on the chromic acid compound film to form a
primer layer having a thickness of 30 ~m after curing.
A polyurethane coatlng material comprising first and
second components shown in Table 5 was coated on the
primer layer by using a two-component mixing type spray
coating machine to form a coating of a polyurethane
resin having a thickness of 2.5 mm after curing. The
obtained coated steel sheet pile having the heavy-duty
anticorrosive coating is shown in the perspective view
of Fig. 3. In Fig. 3, reference numerals 3, 4, 5 and 6
represent the steel sheet pile, the coating composed of
the polyurethane resin, the epoxy resin primer layer and
the film composed of the chromic acid compound,
respectively.
The heavy-duty anticorrosive coating of the coated
steel sheet pile was aged for 7 days to sufficiently
advance the curing of the coating. The test pieces were
cut out and subjected to various performance tests. The
results obtained are shown in Table 6.
~ ~f37Z7~7
- 18 -
Table 5
Coating Material
Components _ Kinds Amounts (parts)
First Component
Polyol ~a) Polybutadiene R-45HT lO0
Polyol (c) - -
Compound (d) Polyhardener PA-400 75
Catalyst Dibutyltin dilaurate0.6
Extender
Plasticizer Chlorinated paraffin 40
Hygroscopic compound Synthetic zeolite 4
Flame retardant Aluminum hydroxide 55
Second Component
Compound (b) Crude MDI 9O
.
!
~f~Z77
- 19 ~
u7 ~ ~J h~ ~0 -/
a~ ~ ~ ,1 o h
p:j ~ ~ U~ ~ X 1
o O
O ~ ~ ~ $ ~ h
~ o ~ $
Zo ~ ~ Zo Zo
â
~D~ ~ .~
~, $
d~
a) o
r~
1 1 o ~ ~ a~ ~ c)~D U7
i~ i a~
~ a
.~
~ O ~ ~
U~
a) "~ ~ ~ ~ hu~ ,l
au ~ u ~ o
h E; O ~ ~ ~ a)~; ~1
~ v
f3~7277
- 20 -
From the foregoing performance test results of the
steel articles having a heavy-duty anticorrosive coating,
obtained in the examples, it is seen that each of the
heavy-duty anticorrosive coatings according to the
present invention had a high impact resistance and a
good bendability. Furthermore, even if the coating was
immersed in tap water or aqueous sodium chloride, water
absorption of the coating layer was saturated below 1%
and increase of the water absorpiton was not observed
afterward. In the dew cycle weathermeter test, no
reduction of the tensile elongation was observed in any
of the coated steel articles having a heavy-duty
anticorrosive coating even after the lapse of 8000
hours, and it was found that each of the coated steel
articles according to the present invention had a good
weatherability. Moreover, the coated steel article
having a heavy-duty anticorrosive coating according to
the present invention had a much higher electrical
insulation resistance than that of conventional
polyurethane resin-coated steel articles. Furthermore,
from the results of the tap water and aqueous sodium
chloride immersion tests, it was found that the heavy-
duty anticorrosive coating of the coated steel material
of the present invention could maintain a good adhesion
for a long time in either tap water or aqueous sodium
chloride.
As seen from the results obtained in the examples,
it will be readily understood that the steel article
having a heavy-duty anticorrosive coating according to
the present invention has excellent impact resistance,
bendability, water absorption, weatherability, electrical
insulation resistance and adhesion, and the coated steel
article shows an excellent long-period durability in sea
water or fresh water or under the ground or in other
- 35 exposed conditions.