Note: Descriptions are shown in the official language in which they were submitted.
i287635
-- 1 --
DESCRIPTION
PROCESS FOR PRODUCING AZIRIDINES
ITEC~NICAL FIELDI
This invention relates to a process for produc-
ing an aziridine compound, which comprises intramol-
ecularly dehydrating an alkanolamine represented by the
following general formula ~I) in the vapor phase in the
presence of a catalyst, and recovering the resulting
aziridine of general formula ~II) from the reaction
product containing it in the presence of an amine
compound.
CH -CH-R CH -CH-R
, 2 , _~ \2/ + H20
H
~I) ~II)
In the above reaction scheme, R represents
hydrogen, or a methyl or ethyl group, X is OH or NH2, and
Y i8 NH2 when X is OH, and OH when X is NH2.
tBACXGRoUND ARTI
The aziridine is a cyclic compound having a
3-membered ring with a large distortion. It has both
ring-opening reactivity and th0 reactivity of an amine,
and is useful as an intermediate for various compounds.
In particular, ethylenimine has already gained widespread
acceptance in the industry as a material for agricultural
chemicals and pharmaceuticals and for amine-type polymers
which are useful as textile treating agents.
A generally well-known method of producing an
aziridine compound is typically a method of producing
ethylenimine which comprises treating monoethanolamine
sulfate in the liguid phase with a concentrated alkali
solution, which has already been industrially practiced.
This method, however, has many defects in industrial
practice. For example, because of the need for using
~ .
;..
.
.
, ' ' -.
~2~7635
-- 2 --
large amounts of sulfuric acid and an alkali as sub-
sidiary materials, it has low productivity. Moreover,
inorganic salts of low utilitarian value are formed as
by-products.
In an attempt to remove the defects of
aziridine production by such a liquid-phase method,
various methods have been reported recently for the
direct production of an aziridine compound by intra-
molecular dehydration reaction of an alkanolamine in the
vapor phase in the presence of a catalyst without using
subsidiary materials (U. S. Patents Nos. 4301036,
4289656, 4337175 and 4477591, and European Laid-Open
Patent Publications Nos. 227461, 228898 and 230776).
The~e prioe attempts, however, were directed mainly to
cataly5t8 or the intramolecular dehydration reaction in
the vapor phase, and failed to propose an industrial
process for obtaining the desired aziridine compound from
the gaseous reaction product mixture.
In the production of an aziridine compound from
an alkalnolamine by the vapor-phase method, the reaction
- product gas contains by-products such as a carbonyl
compound of the following general formula ~III)
CH3-C-R ~III)
O
wherein R is as defined in formula (I) above,
25 which corre8pond8 to the starting alkanolamine, and
various amines. If the starting alkanolamine is mono-
ethanolamine, monoisopropanolamine or 2-amino-1-butanol,
the corresponding carbonyl compound of formula (III) is
acetaldehyde, acetone or methyl ethyl ketone, respec-
30 tively. For example, U. S. Patents Nos. 4,337,175 and4,477,591 describe that in the production of ethylenimine
from monoethanolamine, acetaldehyde is formed as a main
by-product and monoethylamine and pyrazines are also
:
~ ~ .
, ~', ' '
128763S
-- 3 --
formed as by-products. European Laid-Open Patent
Publications No. 227,451, 228,898 and 230,776 al~o
describe that the main by-products are the carbonyl
compounds of formula (III) and various amines.
In the industrial production of organic com-
pounds by a vapor-phase method, it is the general
practice to recover the desired product from the vapor-
phase reaction product and purify it in order to obtain
the final product. The industrial production of
aziridine compounds from alkanolamines by the vapor-phase
method, however, has the following problems. First of
all, the aziridine compounds of formula (II) generally
have a low boiling point and a very high vapor pressure.
Moeeover, they are very reactive, and liable to undergo
polymerization and other reactions. Furthermore, among
the various by-products formed in the vapor-phase method
as mentioned above, the carbonyl compounds tend to react
with the desired aziridine compounds and form adducts.
Hence, the yield of the desired aziridine compounds is
reduced. Theæe problems should therefore be taken into
consideration in producing aziridine compounds by the
vapor-phase method.
IDISCLOSURE O~ INV~NTIONI
It is an object of this invention to provide a
process for producing an aziridine compound from an
alkanolamine in the vapor-phase, in which the aziridine
compound i8 efficiently recovered from the vapor-phase
reaction product.
The present inventors made extensive investi-
gations in order to achieve this object, and have foundthat it is very effective to carry out a recovery step
and/or a distillation step in the presence of an amine
compound.
Thus, according to this invention, there is
provided a process for producing an aziridine compound
represented by the following general formula
,~
,
lZ87635
-- 4 --
CH2-/ H-R (II)
\N
H
wherein R represents hydrogen, or a methyl or
ethyl group,
which comprises intramolecularly dehydrating an alkanol-
amine represented by the following formula
CH2-CH-R (I)
X Y
wherein R i8 as defined, X is OH or N~2, and Y
i8 NH2 when X i8 OH, and OH when X is NH2,
in the presence of a catalyst in the vapor phase in a
reaction 8tep (A) to form a reaction product containing
the aziridine compound, and subjecting the reaction
product to any one of the following procedures (1) to
(3),
~l) send.ing the reaction product to a recovery
step (B) and recovering the aziridine compound in the
presence of an amine compound,
(2) sending the reaction product to a distil-
lation step (C) and distilling the aziridine compound in
the presence of an amine compound, and
(3) sending the reaction product to the re-
covery step (8), recovering the aziridine compound in the
presence of an amine compound, sending the recovered
aziridine compound to the distillation step ~C) and
distilling it in the presence of an amine compound.
The reaction step ~A) of intramolecularly
dehydrating the alkanolamine of formula ~I) in the
vapor-phase in the presence of a catalyst is described in
the above-cited patent documents, and methods similar to
those described there may also be used in this invention.
, . . . .
" ' ' ' ~' .' .
''''' .,'' ' ,
. .
- ,
128763S
For example, the alkanolamine is passed through a cata-
lyst layer. If desired, the alkanolamine is diluted with
an inert gas such as nitrogen to a suitable con~entra-
tion, or in order to inhibit side reactions, ammonia,
steam, hydrogen, etc. may be added to the starting gas.
Alternatively, all inert gas may be replaced by ammonia.
The operating pressure is atmospheric pressure or a
reduced or elevated pressure. The reaction temperature
is usually in the range of 300 to 500 C. The space
velocity of the starting gas varies depending upon the
type and concentration of the starting gas, the type of
the catalyst, etc., but is generally within the range of
50 to 5,000 hr 1. The catalyst may be those described in
the above-cited patent documents. The silicon- or phos-
phorus-containing catalysts shown in European Laid-Open
Patent Publications Nos. 227l461, 228,898 and 230,776 are
preferred.
The characteristic feature of the present
invention i8 that an aziridine compound is produced from
the reaction product formed in the reaction step (A) by a
~ treatment according to the above procedure ~1), (2) or
~3). The essential feature of the present invention is
that in the production of the aziridine compound from the
reaction product formed in the reaction step ~A), the
recovery step ~B) and/or the distillation step ~C) is
carried out in the presence of an amine compound. The
amine compound inhibits polymerization of the aziridine
compound in the recovery step ~B) and/or the distillation
gtep ~C), and enables it to exist stably. A problem
arises in that a by-product carbonyl compound contained
in the reaction product may react with the aziridine
compound to form an adduct in the recovery step ~B)
and/or the distillation step ~C). However when the amine
compound exists, the by-product carbonyl compound reacts
with the amine compound to form a stable adduct before it
reacts with the aziridine compound. As a result, the
amine compound produces the following effects.
-: - -
-
~7t~35
-- 6
(1) The decrease of the yield of the aziridinecompound can be prevented.
(2) The decrease of the quality of the
aziridine compound owing to the inclusion of the by-
product carbonyl compound can be prevented.
(3) The decrease of the stability of the
aziridine compound can be prevented.
Various amine compounds can be used as the
aforesaid amine compound in this invention. Primary
amine compounds are particularly effective because they
immediately react with the by-product carbonyl compound
in the reaction product to form stable Schiff bases.
Secondary amine compounds are also effective because they
react with the carbonyl compound to form stable adducts.
Specific examples of the amine compound include primary
alkanolamines having 2 to 6 carbon atoms such as mono-
ethanolamine and monoisopropanolamine; primary aliphatic
amines having 2 to 8 carbon atoms such as ethylamine and
ethylenediamine; primary aromatic amines having not more
than 8 carbon atoms such as aniline; and secondary
alkanolamines having not more than 8 carbon atoms such as
diethanolamine and diisopropanolamine. Preferably, the
amine compound is the same as the starting alkanolamine
because it can be recovered with the unreacted starting
material and recycled, and the process can be relatively
simplified. Use of other amine compounds requires a
recovery and re-use of the amine compound which has to be
carried out independently of the recovery and re-use of
the unreacted starting material. This complicates the
process, and the amine compound is likely to be included
in the product as an impurity.
The procedure (1) of sending the reaction
product obtained in the reaction step (A) to the re-
covery step ~B) to recover the aziridine compound of
formula (II) in the presence of the amine compound will
be described. If the unreacted alkanolamine in the
lZ87t;35
reaction product obtained in the reaction step (A) ex-
hibits a sufficient effect as the amine compound, the
recovery step (B) may, if desired, be carried out by
simply cooling the reaction product to condense and
recover it. On the other hand, if the amount of the
unreacted alkanolamine alone is insufficient to exhibit
the effect of the amine compound, the reaction product
obtained in the reaction step (A) is sent to recovery
equipment where the aziridine compound of formula ~II) is
recovered in the presence of a sufficient amount of the
amine compound. In the latter case, it is convenient to
use an absorption tower and feed the amine compound as an
absoebing liquid, as is generally practiced.
When the amount of the unreacted alkanolamine
i~ insufficient but the recovering step is carried out
using water as the absorbing liquid without adding an
additional supply of the amine compound, the aziridine
compound undergoes polymerization. If an aqueous
solution of sodium hydroxide is used as the absorbing
liquid to prevent polymerization, the by-product carbonyl
compound will react with the aziridine compound to form
an adduct, and the yield of the aziridine compound will
be decreased.
The amount of the amine compound to be present
in the recovery step varies depending upon various
factors such as the composition of the reaction product,
the type of the ami~e compound, the temperature and the
structure of the recovering device. Preferably, it is at
least 0.1 mole per unit time per mole of the aziridine
compound in the reaction product, and at least 1 mole per
mole of the carbonyl compound. If it is too small, the
stability of the aziridine compound is low, or the
carbonyl compound does not sufficiently change to an
adduct with the amine and the yield of the aziridine
compound is decreased. An aqueous solution of the amine
compound may be used as the absorbing liquid. If the
1287635
viscosity of the amine compound is relatively high, the
efficiency of contact of the amine compound with the
reaction product can be increased by dissolving the amine
compound in water and using the resulting solution of a
low viscosity as the absorbing li~uid. Volatilization of
an amine compound having a relatively low boiling point
may be inhibited by ~sing it as an aqueous solution. Too
much water in the absorbing liquid, however, induces the
reverse reaction of the adduct of the carbonyl compound
and the amine compound, and the carbonyl compound cannot
be removed smoothly. Accordingly, when the amine com-
pound is fed as an aqueous solution, the concentration of
the amine compound in the absorbing liquid to be fed is
preferably adjusted to at least 30 mole%, especially at
least 80 mole%, although it may vary depending upon the
water content of the reaction product. The temperature
at which the aziridine compound is absorbed by the ab-
sorbing liquid is suitably within the range of 0 to 80 C
although it may vary depending upon the type of the
absorbing liquid. The operating pressure may be atmos-
pheric pressure or a reduced or elevated pressure. The
absorption may be conveniently carried out by using an
absorption tower. The absorption tower may be of a
packed type, a tray type, a multitubular type, a spray
type or a wetted wall, or of a combination of such types.
Now, the procedure ~2) or ~3) of sending the
reaction product obtained in the reaction step ~A) to the
distillation step ~C) where the aziridine compound of
formula (II) i8 distilled in the presence of a sufficient
amount of the amine compound will be described.
The liquid containing the aziridine compound
obtained in the presence of the amine compound in the
recovery step ~B) is then sent to the distillation step
~C) to distill the aziridine compound in the presence of
the amine compound IProcedure ~3)1. Alternatively, the
liquid containing the aziridine compound obtained from
- . , .
. .
lZ87635
the reaction product of the reaction step (A) without
subjecting it to the recovery step (B) of the invention
may be very effectively distilled in the presence of the
amine compound according to this invention tProcedure
(2)]. If the aziridine compound-containing liquid con-
tains the amine compound such as the unreacted starting
alkanolamine in an amount sufficient to produce the
desired effect, no fresh supply of the amine compound may
be required. If not, a fresh supply of the amine com-
pound is added to the distillation system, and then theaziridine compound is distilled. Preferably, the amine
compound is added by mixing it with the aziridine com-
pound-containing liquid and feeding the mixture into a
distillation tower. Alternatively, the amine compound
may be separately fed into the distillation tower.
Particularly, when water is present in the aziridine
compound-containing liquid fed to the distillation tower,
the amine compound is preferably added from above the
material feeding tray of the distillation tower. This is
effective for preventing formation of an adduct between
the desired aziridine compound with the by-product
carbonyl compound which is liberated by reverse reaction
from the adduct of it with the amine compound owing to
the presence of water near the material feeding tray and
rises as a low-boiling component through the distillation
tower. Because of the above manner of adding the amine
compound, the amine compound is present in a sufficient
amount above the material feeding tray and the amount
of water there is decreased as a result of descending
through the distillation tower as a high-boiling
impurity. Hence, even when the by-product carbonyl
compound is liberated, it is immediately converted back
into an adduct with the amine compound/ and the distil-
lation step can be carried out without any substantial
1088 of the aziridine compound~
The amount of the amine compound to be present
1~
-- 10 --
in the distillation tower may be properly selected de-
pending upon the types and proportions of the compounds
present in the distillation system, the amount of the
carbonyl compound, etc. Preferably, it is at least 0.05
mole per mole of the aziridine compound fed into the
distillation system, at least 1 mole, especially at least
5 moles, per mole of the carbonyl compound, and at least
1 mole per mole of water. If the amount of the amine
compound is too small relative to the aziridine compound,
its ability to inhibit polymerization of the aziridine
compound is insufficient. If, on the other hand, the
amount of the amine compound i8 too small relative to
the carbonyl compound or water, the carbonyl compound is
not fully converted to an adduct with the amine compound.
As a result, theee is a possibility that the yield of the
aziridine compound will be decreased, the carbonyl com-
pound will be included in the aziridine compound distil-
late, or the stability of the resulting aziridine com-
pound will be reduced.
When the operation of distilling the aziridine
compound is performed repeatedly-several times, the
; distillation step ~C) of this invention can be applied to
any of such distillation operations.
The distillation may be carriéd out batchwise
or continuously by any ordinary method properly chosen in
a distillation tower of any type such as a packed tower
or a plate tower. The operating pressure may be atmos-
pheric, reduced or elevated pressure. The operating
temperature may be properly determined depending upon the
type of the aziridine compound, the stability of the
adduct of the carbonyl compound and the amine compound,
etc. Preferably, the temperature of the top of the
distillation tower is in the range of 10 to 100 C. If
the temperature is too high, the aziridine compound might
be converted to another compound. If the temperature is
too low, the cost of condensation in a heat-exchanger
lZ~376;35
increases and the process becomes industrially disadvan-
tageous. The above distillation can give the aziridine
compound of a high purity in a high yield.
The process of this invention can be more
conveni~ntly carried out if in the reaction step (A),
the alkanolamine of formula (I) is intramolecularly
dehydrated in the vapor phase by passing it through the
catalyst layer without substantially diluting it, pre-
ferably under a reduced pressure of 10 to 500 mmHg. When
the alkanolamine is diluted with a diluting gas such as
nitrogen and then reacted in the reaction step, the
discharged gas at the time of recovering the reaction
product in the recovery step still contains the desired
product and the unreacted material, and the yield of the
desired product may possibly be decreased. Furthermore,
equipment is required for circulating the discharged gas
or treating it to make it nontoxic. In contrast, if the
starting alkanolamine i8 not substantially diluted prior
to the reaction, the decrease of the yield of the desired
aziridine compound can be prevented in the recovery step,
and the large-scale discharged gas treating equipment can
be omitted or simplified. Moreover, in this case, the
reaction product may be directly introduced to the dis-
tllation step, and the recovery step can be omitted.
The distillation operation can be carried out without a
problem because no non-condensable gas exist. In the
latter case, the reaction product is preferably passed
through a cooler to cool it to a temperature suitable for
the operation conditions of the distillation tower and
then introduced into the distillation tower. In the
distillation tower, it is separated into a fraction
composed mainly of the aziridine compound and a distil-
lation residue composed of the unreacted alkanolamine,
water and other high-boiling impurities. As required,
the fraction containing the aziridine compound as a main
component obtained from the top of the distillation tower
1287635
- 12 -
may be purified by further introducing it into a rectifi-
cation column to obtain the aziridine compound of a
higher purity. In the meantime, a mixture composed of
the unreacted starting alkanolamine, the water formed and
other high-boiling impurities is withdrawn from the
bottom of the distillation tower. As required, the
alkanolamine may be recovered from the mixture for reuse.
Needless to say, in order to evaporate the starting
alkanolamine smoothly or increase the activity of the
catalyst, it is permissible to add such a small amount of
nitrogen, ammonia, etc. as will not impair the aforesaid
object of the invention in the reaction step.
IBRIEF DESCRIPTION OF DRAWINGS]
Figures 1 and 2 are flowsheets showing pre-
ferred embodiments of the present invention. Theinvention will now be described with reference to these
drawings.
lBEST ~OD~ FOR CARRYING OUT T~E INVENTIONl
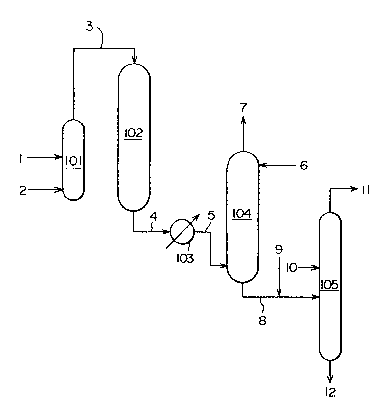
Figure 1 illustrates a process for producing an
aziridine compound comprising a reaction step, a recovery
step and a distillation step.
The star~ing alkanolamine is fed into an evapo-
rator 101 through a line 1, and gasified by heating. An
inert gas such as nitrogen, helium or argon is fed into
the evaporator 101 through a line 2 optionally together
with ammonia, steam or hydrogen for inhibiting side
reactions, and the concentration of the alkanolamine is
adjusted. The starting gaseous mixture is then in-
troduced into a vapor-phase dehydration reactor 102
filled with a catalyst via a line 3. The reactor 102 may
be of a general fixed bed, fluidized bed or moving bed
type. The reaction product which has left the reactor
102 comprises the aziridine compound, the unreacted
alkanolamine, water, the carbonyl compound and amine
compounds excepting the inert gas used as a diluent for
the starting material. The reaction product is sent to a
1~763S
- 13 -
cooler 103 via a line 4 and cooled to a suitable tem-
perature. After cooling, it is introduced into the lower
portion of an absorption tower 104 via a line 5. A
suitable packing is packed into the inside of the ab-
sorption tower 104, and from a line 6, an absorbingliquid composed of a liquid amine compound or its aqueous
solution is fed from the upper part of the absorption
tower 104 and brought into contact with the reaction
product on the packing, and thereby absorbs the reaction
product. The unabsorbed gaseous composition is dis-
charged from the top of the absorption tower 104 via a
line 7. The liquid which has absorbed the aziridine
compound i8 withdrawn from the bottom of the tower via a
line 8 and sent as a distillation feed material to a
di~tillation tower 105 where it is distilled. This
device is consteucted such that if desired, the amine
compound can be added to the starting material through a
line 9, and the amine compound can also be introduced
into the distillation tower through a line 10 provided
above the material feeding tray. The carbonyl compound
contained in the liquid which has absorbed the aziridine
compound reacts with the amine compound to form a high-
boiling adduct. The desired aziridine compound is ob-
tained a~ a distillate from the top of the distillation
tower 105 via a line 11. High-boiling compounds in-
cluding water, the high-boiling adduct of the carbonyl
compound and the amine compound and the added amine
compound are withdrawn from the bottom of the tower
through a line 12, and as required, the alkanolamine or
the amine compound is recovered from the high-boiling
compounds. If desired, the aziridine compound obtained
from the line 11 is sent to a rectification tower and
distilled again in the presence of the amine compound to
obtain the aziridine compound of a higher purity.
Figure 2 illustrates an embodiment in which the
alkanolamine is passed through a catalyst layer without
1287635
being substantially diluted and intramolecularly de-
hydrated in the vapor phase in the reaction step, and the
resulting reaction product is sent to the distillation
step.
The starting alkanolamine is fed to an evapo-
rator 201 via a line 21, and gasified by heating under
reduced pressure. The gasified alkanolamine is in-
troduced into a vapor-phase dehydrating reactor 202 via a
line 22. The reaction product which has left the reactor
202 comprises an aziridine compound, the unreacted
alkanolamine, water, the carbonyl compound and amine
compounds. The reaction product is sent to a cooler 203
via a line 23 and cooled to a suitable temperature. It
is then fed into a distillation tower 204 via a line 24
and distilled. This device is constructed such that as
required, the amine compound can be added to the staeting
material through a line 25, or the amine compound can be
introduced into the distillation tower through a line 26
provided above the material feeding tray. The carbonyl
compound contained in the reaction product reacts with
- the amine compound to form a high-boiling adduct. As a
result of distillation, the desired aziridine compound is
obtained as a distillate via a line 27. High-boiling
compounds containing water, the high-boiling adduct of
the carbonyl compound and the amine compound, the un-
reacted alkanolamine and the added amine compound are
withdrawn from the bottom of the distillation tower via a
line 28, and as required, the alkanolamine or the amine
compound is recovered from the high-boiling compounds.
If desired, the aziridine compound obtained through the
line 27 may be sent to a rectification tower, and dis-
tilled in the presence of the amine compound to obtain
the aziridine compound having a higher purity.
The following examples illustrate the present
invention more specifically. Analysis of the composition
of the reaction product was carried out by gas chromato-
lZ87635
graphy with respect to the amine compound, the aziridinecompound, the ~arbonyl compound and the amine-carbonyl
compound adduct, and by using â Karl-Fischer water con-
tent meter with respect to water. The recovery ratio (%)
of the aziridine recovered in the recovery step is
defined as follows:-
the aziridine
compound recovered
Recovery (moles/hour)
ratio (%) the aziridine x 100
compounds formed
(moles/hour)
EXAMPLE 1
This example illustrates the process of this
invention in accordance with procedure (1) using the
apparatus of Figure 1.
~paration of a catalYst
A catalyst was prepared in accordance with
Example 13 of European Laid-Open Patent Publication No.
227,461.
Silicon dioxide ~300 g), 788.7 g of barium
hydroxide octahydrate, 10.0 g of sodium hydroxide and 6.2
g of zirconium oxide were suspended in 3 liters of water.
With sufficient stirring, the suspension was heated and
concentrated to give a white clay-like material. It was
molded into solid cylindrical pellets having a diameter
of about 5 mm and a length of about 5 mm. The pellets
were dried, and then calcined at 600 C for 2 hours to
give a catalyst having the following composition
1.0 a0.5Na0.05zro.ol (by atomic ratio, excepting
oxygen).
Dehydration reaction step (A)
Two hundred milliliters of the catalyst was
filled in a stainless steel reaction tube having an
inside diameter of 25 mm and set up in reactor 102, and
heated to 390 C by a heat medium. A starting gaseous
mixture composed of monoethanolamine and nitrogen in a
~2~7635
volume ratio of 10:90 was passed through the reaction
tube at a space velocity of 1500 hr 1 to react the
monoetheanolamine. Analysis showed that the reaction
product contained 4.9% by volume of ethylenimine, 0.3% by
volume of acetaldehyde, 3.6% by volume of monoethanol-
amine, 5.0% by volume of steam, 85.3% by volume of
nitrogen and small amounts of ammonia and a dimerized
product of monoethnanolamine.
Recovery step (B)
The reaction product discharged from the re-
action tube was cooled to 110 C in cooler 103 and
introduced into the lower part of absorption tower 104
consisting of a stainless steel tube having an inside
diameter of 25 mm and a length of 1000 mm. The inside of
the absorption tower 104 contained packings having a
diameter of 3 mm (Dickson packings) packed to a layer
height of 700 mm. Monoethanolamine as a absorbing
liquid kept at 40 C was fed into the absorption tower at
a flow rate of 980 g/hour from its top via line 6, and
brought into contact with the reaction product. The
- absorbing liquid which captured ethylenimine was with-
drawn from the bottom of the absorption tower. The
recovery ratio of ethylenimine was determined by
analyzing the withdrawn liquid. It was thus ascertained
that 99.6~ of ethylenimine formed in the reaction step
was absorbed by monoethanolamine. Acetaldehyde fomred
as a by-product in the reaction step did not exist in the
above liquid, and the formation of N-ethylidene-l-
hydroxyethylamine ~the reaction product of acetaldehyde
and the monoethanolamine usd as the absorbing liquid) in
an amount corresponding to the by-product acetaldehyde
was determined.
EXAMPLES 2-6
These examples illustrate the process in
accordance with procedure ~1) using the apparatus of
Figure 1.
,;
12~7635
Example 1 was repeated except that the type,
temperature and amount fed of the absorbing liquid were
changed. The recovering conditions and the results are
shown in Table 1.
EXAMPLE 7
This example illustrates the process in
accordance with procedure (1) using the apparatus of
F igu re 1.
Preparation of a catalyst
A catalyst was preapred in accordance with
Example 3 of U. S. Patent 4,477,591.
Niobium pentoxide (20 g) was dissolved in 200
ml of warm water with stirring. Aqueous ammonia was
added to adjust the pH of the solution to 7Ø The
precipitate formed was sepaeated by filtration, washed
with water, and dissolved in 320 ml of a 10% by weight
aqueous solution of oxalic acid. Furthermore, 0.8 g of
barium hydroxide octahydrate was added. Silicone carbide
(240 cc) having a particle diameter of 5 mm was immersed
in the solution, and the solution was evaporated to
dryness, and calcined at 500 C in an air current for 3
hours to give a supported catalyst containing 307% by
weight of niobium pentoxide and 0.5% by weight of barium
oxide (Nbl.2BaO.12.6
Dehydration reaction step (A)
The dehydration reaction step of Example 1 was
repeated except that the above catalyst was used and the
reaction temperature was changed to 420 C. The reaction
product was found to contain 2.1% by volume of ethylen-
imine, 0.7% by volume of acetaldehyde, 6.5% by volume of
monoethanolamine, 2.5% by volume of steam, 87.3% by
volume of nitrogen and small amounts of ammonia, ethyl-
amine and a dimerized product of monoethanolamine.
Recovery step (B)
The reaction product was submitted to the
recovery step in the same way as in Example 1. The
S
- 18 -
recovering conditions and the results are shown in Table
1.
EXAMPLE 8
This example illustrates the process in accord-
ance with procedure (1) using the apparatus of Figure 1.
Preparation of a catalyst
A catalyst was prepared in accordance with
Example 25 of European Laid-Open Patent Publication No.
230,776.
10 Aluminum nitrate nonahydrate t900 g) was dis-
solved in 2.4 liters of water, and a solution of 357.6 9
of triammonium phosphate in 2.4 liters of water was added
with stirring. The precipitate formed was separated by
filtration and washed with water. Barium oxide (73.6 g)
and 100 ml of water were added, and the mixture was well
kneaded. The resultinq clay-like material was molded
into solid cylindrical pellets having an outside diameter
of about 5 mm and a length of about 9 mm, dried and then
calcined at 1000 C for 2 hours to give a catalyst having
the composition AllPlBa0 2 (atomic ratio excepting
- oxygen).
DehYdration reaction step ~Al
The same dehydration reaction step as in
Example 1 was repeated except that the resulting catalyst
was used, the reaction temperature was changed to 420 C
and the starting gaseous mixture was changed to a mixture
of monoisopropanolamine and nitrogen in a volume ratio of
20:80. The reaction product was found to contain 7.9% by
volume of 2-methylethylenimine, 1.2% by volume of
acetone, 8.0% by volume of monoisopropanolamine, 8.0% by
volume of steam, 72.6% by volume of nitrogen and small
amounts of ammonia and a dimerized product of monoiso-
propanolamine.
Recovery step ~B)
The reaction product was submitted to the
recovery step and 2-methylethylenimine was recovered in
~Z876~
-- 19 --
the same way as in Example 1 except that monoisopropanol-
amine at 20 C was used as the absorbing liquid, and fed
at a rate of 2200 g/hour. The recovering conditions and
the results are shown in Table 1.
EXAMPLE 9
This example illustrates the process in accord-
ance with procedure (1) using the apparatus of Figure 1.
Preparation of a catalyst
Cesium carbonate (114.0 g), 92.4 g of di-
ammonium phosphate, 17.4 g of magnesium hydroxide, 26.6 g
of thallium nitrate and 255.0 g of aluminum oxide were
added to 2 liters of water, and concentrated by heating
to give a white clay-like material. This material was
molded into solid cylindrical pellets having an outside
diameter of about 5 mm and a length of about 5 mm. The
pellets were dried, and calcined at 600 C in an air
current for 2 hours to give a catalyst having the com-
MgO.2CS0.7Po.7Tlo.lAls.0 (atomic ratio exceptingoxygen).
Dehydration reaction step (A)
The same dehydration reaction step as in
Example 1 wa~ repeated except that the above catalyst was
used, the reaction temperature was changed to 400 C, and
the starting gaseous mixture was changed to a gaseous
mixture consisting of 2-amino-1-butanol and nitrogen in a
volume ratio of 20:80. The reaction product was found to
contain 7.3% by volume of 2-ethylethylenimine, 1.4% by
volume of methyl ethyl ketone, 9.1% by volume of 2-
amino-l-butanol, 7.5~ by volume of steam, 73.0~ by volume
of nitrogen and small amounts of ammonia and a dimerized
product of 2-amino-1-butanol.
Recover steD (B)
Y
The reaction product was subjected to the same
recovery step as in Example 1 to recover 2-ethylethylen-
imine. The recovering conditions and the results areshown in Table 1.
1287635
-- 20 --
~ 8 B ~ ~o B ~D B B B B
~ 3 ~J~ 'O ~ 'a ~ ~ ~ ~ ~ ~
1 .8 ~1 ~ J~ ~ J ~ ~ J~ J-
v ~, 8 v ~ 8 _ ~ ~ ~ ~ ~
~, æ _ _
O L~ ~ O~ ~ ~ ~D ~ ~n ~ ~D
o~3~ ~ ~ ~ ~ a~ ~ ~ a~ a
. _ _
o oo o~ o o o a~ o ~
o~ ~ a~ a~ _~ ~ a~ ~ a~
__ _ _
~ ~ ~ o ~ u~ u~ u~ ~ O ~r
,1 .~ ~ 3 - _ _
~1 ~ ~
- -
~ ~g ~g .3g s g
- ~ ,~ ~r
~! ~ o ~ o !~: -' !~: -'
.s~ 3g ~g sg s~g
3~ J~ eO~ e~
s,~
~ ~ ~ ~ ~a~
I I _
h~ ~ ~ ~1 ~ u~ ~D ~ ~ ~n
~ l _ _ _
.
~Z~7~35
-- 21 --
EXAMPLE 1 0
This example illustrates the process in accord-
ance with procedure ~3) using the apparatus of Eigure 1.
Preparation of a catalYst
A catalyst was prepared in accordance with
Example 7 of European Laid-Open Patent No. 228,~98.
Calcium nitrate tetrahydrate (590.5 9) was
dissolved in 1 liter of water, and while the solution was
heated at 80 C with stirring, 1 liter of an aqueous
solution containing 537.0 g of disodium hydrogen phos-
phate (dodecahydrate) was added. The mixture was aged
for 1 hour while its pH was maintained basic by adding
aqueous ammonia. The mixture was then cooled, and the
precipitate was separated by filtration and washed with
water to obtain a white solid. The solid was molded into
solid cylindrical pellets having an outside diameter of
about 5 mm and a length of about 5 mm, dried and then
calcined at 500 C in an air current for 2 hours to give
a catalyst having the composition CaO gNa0 lPo 5 ~atomic
ratio excepting oxygen).
~ehydration reaction step (A)
The same dehydration reaction step as in
Example 1 was carried out except that 200 ml of the
resulting catalyst was used, the reaction temperature was
changed to 400 C, and the starting gaseous mixture was
changed to a mixture composed of monoethanolamine and
nitrogen in a volume ratio of 20:80. The reaction
product gas was analyzed by gas chromatography, and it
was determined that acetaldehyde formed in addition to
ethylenimine as a main product.
RecoverY step (B)
Subsequently, the reaction product was sub-
jected to the same recovery step as in Example 1 to
recover ethylenimine. Analysis showed that the liquid
widhdrawn from the bottom of absorption tower 104 did not
contain the acetaldehyde formed as a by-product in the
~28~6~
reaction step (A), and the formation of N-ethylidene-l-
hydroxyethylamine (the reaction product of acetaldehyde
with the monoethanolamine used as the absorbing liquid)
in an amount corresponding to the by-product acetaldehyde
formed.
Distillation step ~C)
The liquid withdrawn from the bottom of the
absorption tower in the recovery step was fed into a site
0.6 m above the packed layer of a stainless steel distil-
lation tower having an inside diameter of 50 mm and alayer length of 2.4 m and packed with Dickson packings ~6
mm), and continuously distilled at a reflux ratio of 4
under a tower top pressure of 200 mmHg. An ethylenimine
reaction having a tower top tempeeature of 25 C was
obtained through line 11. The composition of the liquid
fed into the distillation tower and the results obtained
are shown in Table 2.
EXAMPLE 11
This example illustrates the process in accord-
ance with peocedure ~3) using the apparatus of Figure 1.
Example 10 was repeated except that ethylene-
diamine was used as the absorbing liquid in the recovery
step ~B) instead of the monoethanolamine, and the distil-
lation device in the distillation step was one-fifth of
the device used in Example 10 in scale. The composition
of the liquid fed into the distillation tower and the
results are shown in Table 2.
EXAMPLE 12
This example illustrates the process in accord-
; 30 ance with procedure ~3).
The reaction product gas discharged from thereaction step ~A) of Example 10 was cooled to room tem-
perature with cooling water, and then cooled to -30 C
using a cooling medium to condense the solution con-
taining ethyleni~ine. Monoethanolamine was added to thecondensed liquid, and the resulting solution was
~2876~5
- 23 -
subjected to distillation in the same way as in Example
10. The composition of the solution fed to the distil-
lation tower, and the results are shown in Table 2.
EXAMPLE 13
This example illustrates the process in accord-
ance with procedure (3).
Monoethanolamine was added to the ethyleneimine
fraction obtained by distillation in Example 10, and the
resulting solution was distilled by the same operation as
in Example 1. The composition of the solution fed into
the distillation tower and the results are shown in Table
2.
EXAMPLE 14
This example illustrates the process in accord-
ance with procedure (3) using the apparatus of Figure 1.
Preparation of a catalyst
A catalyst was prepared in accordance withExample 1~ of European Laid-Open Patent Publication No.
227,461.
Silicon dioxide (300 g), 132.8 g of strontium
hydroxide octahydrate, 2.4 g of lithium hydroxide and
12.8 g of aluminum oxide were suspended in 1 liter of
water. The suspension was concentrated by heating while
the solution was fully stirred. The concentrated product
was molded into solid cylindrical pellets having an
outside diameter of about 5 mm and a length of about 5
mm, dried and calcined at 600 C for 2 hours to give a
catalyst having the composition SilSrO lLio 02Alo 05
(atomic ratio excepting oxygen).
Deb~dration reaction step (A)
The same dehydration reaction step as in
Example 10 was carried out except that the resulting
catalyst was used, the apparatus was reduced in scale to
one-fifth of that used in Example 10, the heating tem-
perature in the heat medium was changed to 400 C, and
isopropanolamine was used instead of monolethanolamine.
lZ8763~
- 24 -
A gas-chromatographic analysis of the reaction product
gas led to the determination of the presence of acetone
in addition to 2-methylethylenimine as a main reaction
product.
Recovery step ~B)
2-Methylethylenimine, etc. in the reaction
product gas discharged from the dehydration reaction step
(A) were recovered in the same way as in Example 10
except that a recovery device of a smaller scale was used
and isopropanolamine was used instead of monoethanol-
amine.
Analysis showed that the liquid withdrawn from
the bottom of the absorption tower 104 did not contain
acetone formed as a by-product in the reaction step, and
1-isopropylideneamino-2-propanol as the reaction product
of acetone with the isopropanolamine used as the absorb-
ing liquid was formed.
Distillation step (C)
The liquid withdrawn from the bottom of the
absorption tower in the recovery step (B) was distilled
~ in the same way as in ~xample 11 except that a distil-
lation device of a smaller scale was used. The com-
position of the liquid fed into the distillation tower
and the results are shown in Table 2.
lZ87635
U'~
~ ~ ~ ~ c~
-., ~--~.. ..- ,
~1 ~ ~ ~
~11
-
__--o ~ ,~, ~ ~
128~63S
~ ,
`~_
1287635
-- 27 --
EXAMPLE 1 5
This example illustrates the process in accord-
ance with procedure ~2) using the apparatus of Figure 2.
Dehydrating reaction step (A)
One liter of the catalyst prepared by the
method of Example 8 was filled in a stainless steel
reaction tube having an inside diameter of 25 mm and set
up in reactor 202, and heated to 420 C with a heat
medium. Monoethanolamine was fed into evaporator 201,
and gasified monoethanolamine was passed through the
reaction tube at a space velocity of 1000 hr 1 under a
tube outlet pressure of 400 mmHg and continuously re-
acted. ~he reaction product was found to contain 67.6%
by volume of monoethanolamine, 12.7% by volume of
ethylenimine, 15.6% by volume of water, 1.35% by volume
of acetaldehyde and small amunts of ammonia and a
dimerized product.
Distillation step IC)
The reaction product obtained in the reaction
step (A~ was cooled to 100 C in cooler 203, introduced
into distillation tower 204 consisting of a stainless
steel having an inside diameter of 50 mm and a height of
2000 mm at a site about 1/3 of the total length of the
di8tillation tower from its top, and distilled at a
reflux ratio of 4. The stainless steel tube contained
Mcmahon packings having a diameter of 6.35 mm to a
height of 400 mm in the concentrating portion and a
height of 1200 mm in the recovery portion.
From the top of the distillation tower,
ethylenimine having a purity of 98.1% by weight was
obtained through line 27 at a rate of 291 9 per hour.
Water was almost the sole impurity. Acetaldehyde reacted
with the unreacted monoethanolamine to form an adduct
which was withdrawn from the bottom of the tower via line
28.
12~6~3S
-- 28 --
EXAMPLE 1 6
This example illustrates the process in accord-
ance with procedure ~2) using the apparatus of Figure 2.
Dehydration reaction step ~A)
The same dehydration reaction step as in
Example 15 was carried out except that monoisopropanol- -
amine was used instead of monoethanolamine, and gasified
monoisopropanolamine was passed through the reaction tube
at a space velocity of 200 hr 1 under a tube outlet
pressure of 60 mmHg. The reaction product was found to
contain 9.9~ by volume of monoisopropanolamine, 36.0% by
volume of 2-methyl-ethylenimine, 40.1% by weight of
water, 5.0% by volume of acetone, and small amounts of
ammonia and a dimerized product.
Distillation step ~C)
The reaction product obtained in the above
reaction step (A) was introduced into the distillation
tower in the same way as in Example 15. Monoiso-
propanolamine was fed into the distillation tower at a
rate of 447 g/hour from an amine adding opening formed
~ above the reaction product feed opening of the distil-
lation tower. The reaction product was thus distilled at
a reflux ratio of 8 under a pressure of 60 mmHg in the
same way as in Example lS.
From the top of the distillation tower, 2-
methyl-ethylenimine having a purity of 97.8% by weight at
a flow rate of 335 9 per hour. 98.1% of the 2-methyl-
ethyleneimine formed by the above reaction was recovered.
Water is almost the only impurity. Acetone reacted with
monoisopropanolamine to form an adduct, which was then
withdrawn from the bottom of the tower through line 28.
EXAMPLE 17
This example illustrates the process in accord-
ance with procedure (3) using the apparatus of Figure 2.
3s Dehydration reaction step (A)
The same dehydration reaction step as in
, ,. , ,~, ~
1287635
Example 15 was repeated except that the reaction pressure
was changed from 500 mmHg to 100 mmHg and the space
velocity was changed from 100 hr 1 to 400 hr 1. Analysis
showed the reaction product to contain 42.4% by volume of
monoethanolamine, 24.7% by volume of ethylenimine, 27.1%
by volume of water, 1.8% by volume of acetaldehyde and
small amounts of ammonia and a dimerized product of
monoethanolamine.
Recovery step (B)
The reaction product discharged from the de-
hydration reaction step was $irst cooled to eoom tem-
perature with cooling water and further to -10 C with a
cooling medium, and thus condensed and recovered to give
a solution containing ethylenimine. Analysis showed that
this ~olution did not contain acetaldehyde formed as a
by-product in the reaction step, and the formation of
N-ethylidene-l-hydroxyethylamine (the reaction product
between acetaldehyde and the monoethanolamine used as the
starting material) in an amount corresponding to the
by-product acetaldehyde was determined.
Distillation step ~C)
The solution obtained in the recovery step
was fed into a distillation tower and distilled in the
same way as in the distillation step in Example 15.
From the top of the tower, ethylenimine having a purity
of 98.3% was obtained at a rate of 267 g/hr in a yield
of 99.0% based on the ethylenimine formed in the re-
action step. The composition of the feed solution in
the distillation tower and the results are shown in Table
3o 2.
, . . .
, , ,. , - - ,. . . ...