Note: Descriptions are shown in the official language in which they were submitted.
~ 38~9~;
The present invention relates to novel, specific
7-aminoazoloLl~5-a]pyrimidines~ and to fungicides containing
them.
It is known that 7-aminoazoloC1,5-a~pyrimidines,
in particular 7-amino-6-phenyl-5-methyl-1,2,4-triazolo~1,5-
a~pyrimidine, possess pharmacological properties (U.S.
Patent 2,553,500).
It is also known that 7-aminoazolo Cl,5-
a~pyrimidines, in particular 7-amino-6-(4-tert-butoxybut-1-
yl)-2,5-di-methylpyrazoloC1,5-a~pyraimidine, can be used as
a fungicidal active ingredient (European Patent 141,317).
However, their fungicidal action is not adequate.
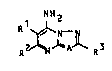
It has now been found that novel, specific 7-
aminoazolo-[1,5-a3pyrimidines of the formula:
12~2
where Rl is aryloxyalkoxyalkyl, alkoxyalkoxyalkyl, alkoxyal-
koxyalkoxyalkyl or dialkylaminoalkyl, in which the aryl
moiety is unsubstituted or monosubstituted or polysubsti-
tuted by straight-chain or branched alkyl, aryl, alkoxy,
aryloxy, halogen, arylalkyl, arylalkoxy, dialkylamino or
alkylarylamino, R2 and R3 are each hydrogen or alkyl and A
is =N-, =CH-, =CBr- or =CCl-, are superior to
the known compounds in their fungicidal action, in
particular in the case of Oomycetes.
More specifically, R is phenyl- or naphthyloxy-
C2-C6-alkoxy-C2-C6-alkyl where the alkoxy and alkyl group
have a straight-chain or are branched and the phenyl or
naphthyl group can be monosubstittued or polysubstituted by
." ~
l3809~;
- la -
straight-chain or branched C1-C10-alkyl, C1-C10-alkoxy,
aryl, aryloxy, fluorine, chlorine, bromine, aryl-Cl-C4-
alkyl, aryl-Cl-C4-alkoxy, di-Cl-Clo alkylamino or Cl-C10-
alkylarylamino; aryl being phenyl or 1- or 2-naphthyl. R
may furthermore be C1-C10-alkoxy-C2-C6 alkoxy-C2-C6-alkyl,
C1-ClO-alkoxy-c2-c6-alkoxy-c2-c6-al~oxy-c2-c6-alkyl~ where
the alkoxy and alkyl group once again have a straight-chain
or are branched, or di-C1-C10-alkyl-amino-C2-C6-alkyl.
R and R3 are each hydrogen or C1-C4-alkyl, methyl
being preferred.
/
/
o~
-- 2 --
7-aminoazoloC1,5-a]pyrimidines of the formula I are
obtained, for example, by a method in ~hich an approp-
riately substituted B-ketoester of the formula II
1 ~ OR
~ I 1,
uhere R is lower alkyl, is reacted with an appropriate
aminoazole of the formula III
R3
H2~ III,
to give a condensate of the formula V
R ~ R3
and this is halogenated at the hydroxyl group an'd reacted
~ith ammonia (process A).
The preparation of the B-ketoesters tII) can be
carried out as described in Organic Synthesis Coll., vol. 1,
page 248, or in German Laid-Open Application DOS3,227,388
The reaction (condensation) ~ith the aminoazoles' (III) can
be carried out in the presence or absence of solvents.
Suitable solv'ents are, in particular, alcohols, such as
ethanol, propanols, butanols, glycols or glycol monoethers,
. .
? ~ ~ O~,
0.~. 0050/37984
diethylene glycols and their monoethers, amides, such as
dimethylformamide, diethylformamide, dibutylformamide or
N,N-dimethylacetamide, lower alkanoic acids, such as formic
acid, acetic acid or propionic acid, and mixtures of these
solvents with water. The reaction temperature is in
general from 50 to 300C, preferably from 50 to 150C,
when a solvent is employed.
The condensates are generally obtained in pure
form and are washed tfor example with the same solvent or
10 with water) and then dried, after which they are halo-
genated with, for example, a phosphorus halide at the
reflux temperature, preferably at from 50 to 150C in excess
phosphorus oxytrichloride. A stoichiometric amount or an
excess of a base, eg. N,N-dimethylaniline, may be added.
The excess pho~phorus oxytrichloride is evaporated, after
which the mixture is treated w;th icewater, with or without
the addition of a water-immiscible solvent, and, if neces-
sary, the base ;s removed by extract;on with hydrochloric
acid.
The chLorination product finally obtained is gener-
ally very pure and is therefore most advantageously reacted
Jirectly with ammonia to give the novel 7-aminoazolol1,5-
a]pyrimidines. This is preferably carried out using am-
monia in an excess of from 1 to 10 moles per mole of the
pyrimidine, under superatmospheric pressure (up to 100 bar)
above about 100C and, if necessary, in a solvent.
The novel 7-aminoazolo~1,5-a~pyrimidines are gen-
erally crystalline compounds obtained directly in very pure
form.
The 7-aminoazolo~1,5-a]pyrimidines (I) may also
be prepared by a method in which ar, appropriately sub-
stituted ~-acylnitrile of the formula
R1-CH-CN
C=O IV,
R2
~ ~8~309~
O.Z. ~050/37984
is reacted with an aminoazole of the formula (III) (process
~), this process too being carried out in the presence or
absence of a solvent. The solvents and the process con-
ditions are substantially similar to those recommended
for process A. Process B gives the novel 7-aminoazolo~
S-a~pyr;midines directly; they are isolated as crystalline,
generally very pure compounds, if necessary after evap-
oration of the solvent or dilution with water. When !ower
alkanoic acids (fatty acids) are used as solvents, it is
advantageous to neutralize residual acid, if necessary
after partial evaporation of the excess.
Some of the substituted ~-acylnitriles (VI) required
for the preparation of the 7-amino-azolo~1,5-a]pyrimidines
are known, individual unknown nitriles of this type may
be prepared by a known method from nitriles possessing
hydrogen and carboxylic esters using strong bases, eg.
alkali metal hydrides, alkali metal amides or metalalkyl-
enes (J. Amer. Chem. Soc. 73, (1951), page 3766).
Preparat;on example
Process A
7-amino-5-methyl-6-r2-(2-methoxy-1-ethoxy)-prop-1-yl]-
1,2,4-triazoloC1,5-a]pyrimidine (corresponds to Example
no. 97 in the Table)
a) 7-hydroxy-5-methyl-6-r2-(2-methoxy-1-ethoxy)-prop-1-yl]-
Z5 1,2,4-triazolo~1,5-a~pyrimidine
43.6 9 of 86 percent strength (corresponding to 37.5 9
of 100 percent pure material, 161 millimoles) of methyl
2-r2-(2-methoxy-1-ethoxy)-prop-1-yl]-acetoacetate are
reacted with 16.8 9 (200 millimoles) of 3-amino-1~-1,2,4-
triazole in 300 ml of propionic acid for 24 hours at
60~ under a protective gas. The mixturé is cooled,
stirred into icewater and then neutralized with 2 N
NaOH, and any precipitate is filtered off. The aqueous
phase is extracted four times with methylene chloride,
and the extracts are dried and evaporated down. The
resulting oil is triturated with diethyl ether and
~ ~8~309~i
-- 5
O.Z. OOS0/37984
the crystal~ ~hich separate out are filtered off under
suction and dried. Yield: 17.5 9 (41% of theory);
mp. 127 - 128C. The infrared spectrum shows that the
substance is predominantly in the form of the 7-oxo-4H
tautomer.
b) 7-Chloro-5-methyl-6-t2-(2-methoxy-1-ethoxy)-prop-1-yl3-
1,2,4-triazolot1,5-a]pyrimidine
16.0 9 (56.2 millimoles) of the intermediate obtained as
described in method a) are boiled for 20 hours in
300 ml of phosphorus oxytrichloride. Excess phosphorus
oxytrichloride is then distilled off. The residue is
treated first ~ith ~ater and then ~ith aqueous sodium
bicarbonate solution. Extraction is carried out
several times ~ith methylene chloride and the extract
is extracted several times ~ith water. Drying and
evaporat;ng down the extract gives 12~5 9 (78%, based
on the intermediate) of an oil, which is used for the
next stage c) ~ithout further purificat;on.
c) Active ingredient, corresponding to Example 97 in the
Table
460 millimoles of gaseous ammonia are allo~ed to act on
a solution of 12.0 9 (42.1 millimoles) of the chlorine
compound from b) in 200 ml of dry 1,4-dioxane in an
autoclave under an initial pressure of 100 bar at 130C
for 60 hours. The autoclave is cooled and let do~n,
after which the mixture is taken up in water and ex-
tracted several times with methylene chloride. The
extract is dried, the solvent is distilled off and the
residue is triturated ~ith n-pentane to give S.0 9
(45X, based on the chlorine compound) of a crystalline
material of melting point 143-144C~
Preparation example
Process P
7-amino-S-methyl-6-{3-t2-(2,4,6-trichlorophenoxy)-1-ethoxy~-
prop-1-yl}-1,2,4-triazolot1~5-a]pyrimidine (Example no.
8 in the Table)
809~
-- 6 --
0.~. 0050/37984
a) 2-acetyl-5-C2-(2,4,6-trichlorophenoxy)-1-ethoxy]-
valeronitrile
245 9 (760 millimoles) of 5-l2-(2,4,6-trichlorophenoxy)-
1-ethoxy]-valeronitrile are dissolved in 1 l of dried
tetrahydrofuran and the solution is cooled to -68C
under a protective gas. 572 ml of a 1.5 molar solution
of n-butyllithium in n-hexane (corresponding to 858
millimoles of n-butyllithium) are added dropwise in the
course of 3 hours, and the mixture is stirred ~or a
1û further 3 hours at -60C. 74.0 ml (66.7 9; 758 milli-
moles) of dry ethyl acetate, disso(ved in 200 ml of
dry tetrahydrofuran, are then slowly added. The
mixture is left for a further 3 hours at -60C and
allowed to reach room temperature overnight. Excess
butyllithium is destroyed by carefully adding water
and the pH is brought to four by adding 2 N hydrochloric
acid. Thereafter, the organic phase is separated off,
washed with water, dried and evaporated down. The
residue which remains comprises 267 9 ~crude yield
73Z) of a yellow oil, which can be used directly for
reaction b).
b) Active ingredient, corresponding to Example 8 in the
Table
The total amount (732 mmoles) of the -acetylnitrile
prepared as described above and 61.5 9 (731 mmoles)
of 3-amino-1~-1,2,4-triazole in 1~0 l of propionic acid
are kept at the boil for 24 hours, after which the
mixture is allowed to cool and is filtered, and the
filtrate is evaporated down. The residue is taken up
in methylene chlor;de, and the solution is washed
several times with water until the aqueous phase is
neutral, and is then dried and evaporated down. 166 9
of (53%, based on the nitrile) of a crystalline mate-
rial of melt;ng point 193-194C result.
309~
-- 7
0.~. 0050/37984
Preparation example
Process B
7-amino-5-methyl-6-{2-~N-(3,5,5-trimethylhex-1-yl)-N-methyl-
-
amino]-1-ethyl}-1,2,4-triazolo[1,5-a]pyrimidine (Example
no. 125)
a) 2-acetyl-4-lN-(3,5,5-trimethylhex-1-yl)-N-~ethylamino]-
butyronitrile
As described above, 31.3 9 (139.5 mmoles) of 4-[N-(3,
5,5-trimethylhex-1-yl)-N-methylamino]-butyronitrile
1û in 300 ml of dry tetrahydrofuran are first reacted
with 103 ml of 1.5 M n-butyllithium solution (154
mmoles) and the product is then reacted with 13.7 ml
(12.4 9; 141 mmoles) of dry ethyl acetate in 50 ml of
tetrahydrofuran at -68C. In working up the mixture,
the pH is brought to 6 with 2 N hydrochloric acid.
The solvent is evaporated off to give 33.0 9 (crude
yield 88%) of an oil, which is used directly for the
subsequent product.
b) Active ;ngredient, corresponding to Example 125
Z0 The total amount (124 mmoles) of the resulting nitrile
is reacted with 10.4 9 (1Z4 mmoles) of 3-amino-lH-1,
2,4-triazole in 300 ml of boiling propionic acid for
18 hours. The solvent is removed, the residue is tri-
turated with n-pentane, the mixture is filtered under
suction, the residue is taken up in methylene chloride
and the solution is filtered over a short column of
silica gel, with the addition of 5 percent by volume
of methanol. The eluate is e~tracted by shaking with
aqueous sodium carbonate solution, dried and evaporated
down. 13.0 9 (32X, based on the nitrile) of a solid of
melting point of 109-110C remain.
The active ingredients characterized more e~actly
(melting point, state of aggregation, etc.) in the tables
below are prepared by the stated processes (A or B).
Those compounds which are not characterized can readily
be obtained by appropriately changing the starting
materials and adapting the methods of preparation; because
S09~
-- 8
O.Z. 0050/37984
of their structural similarity, they are expected to have
a si~ilar action.
- J ~3809~
- 9 - O.Z. 0050/37984
Tabls 1 a NH2
05 ~R)n (CH2)2 ~ N
No. (R)n -X- M.p. (C)
1 H -(CH2)2-
2 H -cH(cH3)cH2-
3 H -(CH2)3- 157-158
4 H -(CH2)4-
lS 5 H . -(CH2)5-
6 2,4,6-C13 -(CH2)2-
7 2,4,6-C13 -CH(CH3)CH2-
8 2,4,6-C13 -(CH2)3-
9 2,4,6-C13 -(CH2)4-
2010 2,4,6-C13 -(CH2)5-
11 2-Cl -(CH2)2-
12 2-C1 -cH(cH3)cH
13 2-Cl -(CH2)3-
14 2-Cl -(CH2)4-
2515 2-Cl -(CH2)5-
16 4-C1 -(CH2)2-
17 4-Cl CH(CH3)CH2
18 4-C1 -(CH2)3-
19 4-C1 -(CH2)4-
3020 4-Cl -(CH2)5-
21 3-Cl -(CH2)2-
22 3-Cl -CH(CH3)CH2-
23 3-Cl -(CH2)3- 135-137
24 3-C1 -(CH2)4-
3S25 3-C1 -(C~2)5-
26 2-Br -(CH2)2-
27 2-Br -cH(cH3)cH2-
28 2-Br -(CH2)3- 161-163
29 2-Br -(CH2)4-
~030 2-Br -(CH2)5-
31 4-Br -(CH2)2-
32 4-Br -cH(cH3)
33 4-9r -(CH2)3-
U9~
- 10 - O.Z. 0050/37984
No. (R)n -X- M.p. (C)
-
34 4-Br -(CH2)4-
05 35 4-Br -(CH2)5-
36 2-CH3 -(CH2)2-
37 2-CH3 -CH(CH3)CH2-
38 2-CH3 -(CH2)3- 164-166
39 2-CH3 -(CH2)4-
10 40 2-CH3 -(CH2)5- 220 (decomposition)
41 3-CH3 -(CH2)2-
42 3-CH3 -CH(CH3)CH2-
43 3-CH3 -(CH2)3- 147-149
44 3-CH3 -(CH2)4-
lS 45 3-CH3 -(CH2)5-
46 4-CH3 -(CH2)2-
47 4-CH3 -CH(CH3)CH2-
48 4-CH3 -(CH2)3- 155-158
49 4-CH3 -(CH2)4-
20 50 4-CH3 -(CH2)5-
51 2,4,6-(CH3)3 -(CH2)2-
52 2,4,6-(CH3)3 -cH(cH3)cH2-
53 2,4,6-(CH3)3 -(CH2)3- 190-191
54 2,4,6-(CH3)3 -(CH2)4-
2S 55 2,4,6-(CH3)3 -(CH2)5- 157-160
56 tert.-C4Hg-CH2~C(cH3)2 -(CH2)2-
57 tert.-C4Hg-CH2~C(cH3)2 -cH(cH3)cH
58 tert.-C4Hg-CH2~C(cH3)2 -(CH2)3-
59 tert.-C4Hg-CH2~C(cH3)2 -(CH2)4-
30 60 tert.-C4Hg-CH2~C(cH3)2 -(CH2)5- 149-151
61 4-C1-2~CH3 -(CH2)3- 144-145
62 2-(i-C3H7) -(CH2)3-
63 2-(sec-C4Hg) -(CH2)3- 118-120
64 2-(sec-C4Hg) -(CH2)5- 154-156
~5 65 4 C6H5 -(CH2)3- 176-179
66 4-C6H5 -(CH2)5- 172-174
67 4 H5C2 -(CH2)2- 162-163
68 4 H5C2 -CH(CH3)CH2- 158-160
69 4 H5C2 -(CH2)3-
~0 70 4-H5C20 -(CH2)4-
71 4 H5C2 -(CH2)5-
72 4-H5C60 -(CH2)2-
73 4-H5C60 -CH(CH3)CH2-
809~i
- 11 - O.Z. 0050/37984
No. (R)n -X- M.p. (C)
74 4-H5C60 -(CH2)3- 156-15805 75 4-H5C60 -(CH2)4-
76 4-H5C60 -(CH2)5-
77 2-(n-C4Hg)O -(CH2)3- 133-135
78 2-(n-C4Hg)O -(CH2)4-
79 2-(n-C4Hg)O -(CH2)5-
3-(n-C4Hg)O -(CH2)3-
81 3-(n-C4Hg)O -(CH2)4-
82 3-(n-C4Hg)O -(CH2)5-
83 4-(n-C4Hg)O -(CH2)3-
84 4-(n-C4Hg)O -(CH2)4-
lS 85 4-(n-C4Hg)O -(CH2)5-
86 2-(H5C6-CH2)0 -(CH2)3-
87 2 (H5C6-cH2) -(CH2)5-
88 3-(H5C6-cH2)0 -(CH2)3-
89 3-(H5C6-cH2)0 -~CH2)5-
20 90 4-(H5C6-CH2)0 -(CH2)3-
91 4-(H5C6-CH2)0 -(CH2)5-
92 3-(H5C2)N -(CH2)3-
93 3-(H5C2)N -(CH2)5-
2S
Table 1 b
NH
~ -(CH2~2--x ~ - N
CR n H3 N ~ CH3
No. (R)n -X- M.p. (C)
94 t-C4Hg-CH2-C(cH3)2~ -(CH2)3- 60
t-C4Hg-CH2-C(cH3)2~ -(CH2)5- (o~l)
38~
- 12 - O.Z. 0050t37984
Table 2
NH2
2 ) 2 ~N
No. R -X- M.p. (C)
96 CH3 -(CH2)2-
97 CH3 -CH(CH3)CH2- 142-144
98 CH3 -(CH2)3-
99 CH3 -(CH2)4-
15100 CH3 -(CH2)5_
101 n-C4H9 -(CH2)2-
102 n-C4Hg -cH(cH3)cH
103 n-C4H9 -(CH2)3-
1~4 n-C4Hg -(CH2)4-
20105 n-C4Hg -(CH2)5-
106 2-ethylhexyl -(CH2)2-
107 2-ethylhexyl -CH(Ms)CH2_
108 2-ethylhexyl -(CH2)3-
109 2-ethylhexyl -(CH2)4-
25110 2-ethylhexyl -(CH2)5-
111 3,5,5-trimethylhexyl -(CH2)2-
112 3,5,5-tr~methylhexyl -CH(CH3)CH2-
113 3,5,5-trimethylhexyl -(CH2)3-
114 3,5,5-trimethylhexyl -(CH2)4-
30115 3,5,5-trimethylhexyl -(CH2)5-
116 n-HgC4-0~(CH2)3 -(CH2)2-
117 n-HgC4-0-(CH2)3 -cH(cH3)cH2- resin
118 n-H9C4~0~(CH2)3 -(CH2)3-
119 n-HgC4-0~(CH2)3 -(CH2)4
35120 n-HgC4-0~(CH2)3~ -(CH2)5-
121 n-HgC4-0~(CH2)2 -cH(cH3)cH2-
122 CH20(CH2)2- -CH(CH3)CH2-
H5C2-CH-n-c4H9
123 (CH2)20(CH2)2- -cH(cH3)cH
H3C-CH-CH2-t-C4Hg
8~309~
- 13 - O.Z. 0050~37984
Table 3
R NH2
OS 3
No. R5 R4 M.p. (C)
124 n-C6H13- n-C6H13- 139-140
125 3,5,5-trimethylhexyl- CH3- 109-110
lS Table 4
,C l NH2
C l ~ 0 - ( C H 2 ) 2 - - ~ C H 2 ) 3~N- r
C l R2 N AJ~ R3
No. R2 R3 A M.p. (C)
126 H H N
127 CH3 CH3 N
128 CH3 CH3 CH
129 CH3 CH3 C-Or
o9l~
- 14 - O.Z. 0050/37984
The novel active ingredients have a strong fungitoxic
action on phytopathogenic fungi, especially from the
Phycomycetes class. The novel compounds are therefore
sl~itable for combatting Phytophthora infestans in tomatoes
05 and potatoes, Phytophthora parasitica in strawberries,
Phytophthora cactorum in apples, Pseudoperonospora
cubensis in cucumbers, Pseudoperonospora humuli in hops,
Peronospora destructor in onions, Peronospora sparsa in
roses, Peronospora tabacina in tobacco, Plasmopara viticola
in grapes, Plasmopara halstedii in sunflowers, Sclerospora
macrospora in Indian corn, Bremia lactucae in lettuce,
Mucor mucedo in fruit, Rhizopus nigricans in beets,Erysiphe
graminis in cereals, Uncinula necator in grapes,
Podosphaera leucotricha in apples, Sphaerotheca fuliginea
in roses, and Erysiphe cichoriacearum in cucumbers.
The active ingredients are well tolerated by plants.
Some of the active ingredients have curative properties,
i.e., the agents may also be applied after the plants have
been infected by the pathogen, and success is still
ensured.
The fungicidal agents contain from 0.1 to 95, and
preferably from 0.5 to 90, wt.% of active ingredient. The
application rates depend on the type of effect desired, and
range from 0.1 to 5 kg/ha.
The active ingredients may also be mixed and applied
together with other active ingredients, e.g., herbicides,
insecticides, growth regulators and other fungicides, or
with fertilizers. When they are mixed with other
fungicides, the spectrum of fungicidal action is often
increased, i.e., the fungicidal action of the combination
is greater than the sum of the actions of the individual
components.
Examples of fungicides which may be combined with the
novel compounds are:
sulfur
dithiocarbamates and derivatives thereof, such as
ferric dimethyldithiocarbamate
- 15 - O.Z. 0050/37984
zinc dimethyldithiocarbamate
zinc ethylenebisthiocarbamate
manganese ethylenebisdithiocarbamate
rnanganese zinc ethylenediaminebisdithiocarbamate
05 l:etramethylthiuram disulfides
ammonia complex of zinc N,N'-ethylenebisdithiocarbamate
ammonia complex of zinc N,N-propylenebisdithiocarbamate
zinc N,N'-propylenebisdithiocarbamate and
N,N-polypropylenebis(thiocarbamyl) disulfide
nitro derivatives, such as
dinitro (l-methylheptyl)-phenyl crotonate
2-sec-butyl-4,6-dinitrophenyl-3,3-dimethylacrylate
2-sec-butyl-4,6-dinitrophenyl isopropylcarbonate and
diisopropyl 5-nitroisophthalate
heterocyclic substances, such as
2-heptadecylimidazol-2-yl acetate
2,4-dichloro-6-(o-chloroanilino)-s-triazine
0,0-diethyl phthalimidophosphonothionate
5-amino-1-~bis-(dimethylamino)-phosphinyl]-3-phenyl-1,2,4-
-triazole
2,3-dicyano-1,4-dithiaanthraquinone
2-thio-1,3-dithio- r 4,5-b]-quinoxaline
methyl l-(butylcarbamoyl)-2-benzimidazole carbamate
2-methoxycarbonylaminobenzimidazole
2-[furyl-~2)]-benzimidazole
2-[thiazolyl-(4)]-benzimidazole
N-(1,1,2,2-tetrachloroethylthio)-tetrahydrophthalimide
N-trichloromethylphthalimide
N-dichlorofluoromethylthio-N+,N+-dimethyl-N-phenyl-
-sulfuric acid diamide
5-ethoxy-3-trichloromethyl-1,2,3-thiadiazole
2-thiocyanomethylthiobenzthiazole
1,4-dichloro-2,5-dimethoxybenzole
4-(2-chlorophenylhydrazono)-3-methyl-5-isoxazolone
2~thiopyridine l-oxide
8-hydroxyquinoline and its copper salt
~ ~S~09~;
- 16 - O.Z. 0050/37984
2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiin
2,3-dihydro-5-carboxanilido-6-methyl-1,4-oxathiin
4,4-dioxide
2-methyl-5,6-dihydro-4-H-pyran-3-carboxanilide
05 2-methylfuran-3-carboxanilide
2,5-dimethylfuran-3-carboxanilide
2,4,5-trimethylfuran-3-carboxanilide
2,5-dimethyl-N-cyclohexylfuran-3-carboxamide
N-cyclohexyl-N-methoxy-2,5-dimethyl-furan-3-carboxamide
2-methylbenzanilide
2-iodobenzanilide
N-formyl-N-morpholine-2,2,2-trichloroethylacetal
piperazine-1,4-diylbis-(1-(2,2,2-trichloroethyl)-formamide
1-(3,4-dichloroanilino)-1-formylamino-2,2,2-trichlorethane
2,6-dimethyl-N-tridecyl-morpholine and its salts
2,6-dimethyl-N-cyclododecyl-morpholine and its salts
N-[3-(p-tert.-butylphenyl)-2-methylpropyl]-cis-2,6-di-
methylmorpholine
N-[3-(p-tert.-butylphenyl)-2-methylpropyl]-piperidine
1-[2-(2,4-dichlorophenyl)-4-ethyl-1,3-dioxolan-2-yl-ethyl]-
-l-H-1,2,4-triazole
1-[2-(2,4-dichlorophenyl)-4-n-propyl-1,3-dioxolan-2-yl-
-ethyl]-l-H-1,2,4-triazole
N-(n-propyl)-N-(2,4,6-trichlorophenoxyethyl)-N+-imidazolyl-
urea1-(4-chlorophenoxy)-3,3-dimethyl-1-(lH-1,2,4-triazol-1-yl)-
-butan-2-one
1-(4-chlorophenoxy)-3,3-dimethyl-1-(lH-1,2,4-triazol-1-yl)-
butan-2-ol
alpha-(2-chlorophenyl)-alpha-(4-chlorophenyl)-5-pyrimidine-
methanol
5-butyl-2-dimethylamino-4-hydroxy-6-methylpyrimidine
bis-(p-chlorophenyl)-3-pyridinemethanol
1,2-bis-(3-ethoxycarbonyl-2-thioureido)-benzene
1,2-bis-(3-methoxycarbonyl-2-thioureido)-benzene
2-cyano-N-(ethylaminocarbonyl)-2-(methoximino)-acetamide
309~;
- 17 - O.Z. 0050/37984
and various fungicides, such as
dodecylguanidine acetate
3-[2-(3,5-dimethyl-2-oxycyclohexyl)-2-hydroxyethyl]-glutar-
amide
05 hexachlorobenzene
DL-methyl-N-(2,6-dimethylphenyl)-N-fur-2-yl alanate
methyl DL-N-(2,6-dimethylphenyl)-N-(2'-methoxyacetyl)-
-alanate
N-(2,6-dimethylphenyl)-N-chloroacetyl-DL-2-aminobutyro-
19 lactone5-methyl-5-vinyl-3-(3,5-dichlorophenyl)-2,4-dioxo-1,3-oxa-
zolidine
3-(3,5-dichlorophenyl)-5-methyl-5-methoxymethyl-1,3-oxa-
zolidine-2,4-dione
3-(3,5-dichlorophenyl)-1-isopropylcarbamoylhydantoin
N-(3,5-dichlorophenyl)-1,2-dimethyl-cyclopropane-1,2-di-
carboximide
The novel active ingre~ients may be applied or
instance in the form of directly sprayable solutions,
powders, suspensions (including high-percentage aqueous,
oily or other suspensions), dispersions, emulsions, oil
dispersions, pastes, dusts, broadcasting agents, or
granules by spraying, atomizing, dusting, broadcasting or
watering. The forms of application depend entirely on the
purpose for which the agents are being used, but they must
ensure as fine a distribution of the novel active ingre-
dients as possible.
For the preparation of solutions, emulsions, pastes
and oil dispersions to be used direct or after emulsific-
ation in water, mineral oil fractions of medium to highboiling point, such as kerosene or diesel oil, further
coal-tar oils, and oils of vegetable or animal origin,
aliphatic, cyclic and aromatic hydrocarbons such as
benzene, toluene, xylene, paraffin, tetrahydronaphthalene,
alkylated naphthalenes and their derivatives such as
. methanol, ethanol, propanol, butanol, chloroform, carbon
tetrachloride, cyclohexanoll cyclohexanone, chlorobenzene,
09~
- 18 - O.Z. 0050/37~84
isophorone, etc., and strongly polar solvents such as
dimethylformamide, dimethyl sulfoxide, N-methylpyrrolidone,
water, etc. are suitable.
Aqueou3 formulations may be prepared from emulsion con-
05 centrates, pastes, oil dispersions or wettable powders byadding water. To prepare emulsions, pastes and oil dis-
persions the ingredients as such or dissolved in an oil or
solvent may be homogenized in water by means of wetting or
dispersing agents, adherents or emulsifiers. Concentrates
which are suitable for dilution with water may be prepared
from active ingredient, wetting agent, adherent, emulsify-
ing or dispersing agent and possibly solvent or oil.
Examples of surfactants are: alkali metal, alkaline
earth metal and ammonium salts of ligninsulfonic acid,
lS naphthalenesulfonic acids, phenolsulfonic acids, alkylaryl
sulfonates, alkyl sulfates, and alkyl sulfonates, alkali
metal and alkaline earth metal salts of dibutylnaphthalene-
sulfonic acid, lauryl ether sulfate, fatty alcohol sul-
fates, alkali metal and alkaline earth metal salts of fatty
acids, salts of sulfated hexadecanols, heptadecanols, and
octadecanols, salts of sulfated fatty alcohol glycol
ethers, condensation products of sulfonated naphthalene and
naphthalene derivatives with formaldehyde, condensation
products of naphthalene or naphthalenesulfonic acids with
phenol and formaldehyde, polyoxyethylene octylphenol
ethers, ethoxylated isooctylphenol, ethoxylated octylphenol
and ethoxylated nonylphenol, alkylphenol polyglycol ethers,
tributylphenyl polyglycol ethers, alkylaryl polyether
alcohols, isotridecyl alcohol, fatty alcohol ethylene oxide
condensates, ethoxylated castor oil, polyoxyethylene alkyl
ethers, ethoxylated polyoxypropylene, lauryl alcohol poly-
glycol ether acetal, sorbitol esters, lignin, sulfite waste
liquors and methyl cellulose.
Powders, dusts and broadcasting agents may be prepared
by mixing or grinding the active ingredients with a solid
carrier.
~.~88~9~
- 19 - O.Z. 0050/37984
Granules, e.g., coated, impregnated or homogeneous gra-
nules, may be prepared by bonding the active ingredients to
solid carriers. Examples of solid carriers are mineral
earths such as silicic acid, silica gels, silicates, talc,
05 kaolin, attapulgus clay, limestone, lime, chalk, bole,
loess, clay, dolomite, diatomaceous earth, calcium sulfate,
magnesium sulfate, magnPsium oxide, ground plastics,
fertilizers such as ammonium sulfate, ammonium phosphate,
ammonium nitrate, and ureas, and vegetable products such as
grain flours, bark meal, wood meal, and nutshell meal,
cellulosic powders, etc.
Examples of formulations are given below.
I. 90 parts by weight of the compound of Example 3 is
mixed with 100 parts by weight of N-methyl-alpha-pyrroli-
done. A mixture is obtained which is suitable for applica-
tion in the form of very fine drops.
II. 20 parts by weight of the compound of Example 8
is dissolved in a mixture consisting of 80 parts by weight
of xylene, 10 parts by weight of the adduct of 8 to
10 moles of ethylene oxide and 1 mole of oleic acid-N-mono-
ethanolamide, 5 parts by weight of the calcium salt of
dodecylbenzenesulfonic acid, and 5 parts by weight of the
adduct of 40 moles of ethylene oxide and 1 mole of castor
oil. By pouring the solution into water and uniformly
distributing it therein, an aqueous dispersion is obtained.
III. 20 parts by weight of the compound of Example 23
is dissolved in a mixture consisting of 30 parts by weight
of cyclohexanone, 30 parts by weight of isobutanol, and
20 parts by weight of the adduct of 40 moles of ethylene
oxide and 1 mole of castor oil. By pouring the solution
into water and finely distributing it therein, an aqueous
dispersion is obtained.
IV. 20 parts by weight of the compound of Example 38
is dissolved in a mixture consisting of 25 parts by weight
of cyclohexanol, 65 parts by weight of a mineral oil
fraction having a boiling point between 210 and 280C, and
809~:i
- 20 - O.Z. 0050/37984
lO parts by weight of the adduct of 40 moles of ethylene
oxide and 1 mole of castor oil. By pouring the solution
into water and uniformly distributing it therein, an
aqueous dispersion is obtained.
05 V. 20 parts by weight of the compound of Example 43
is well mixed with 3 parts by weight of the sodium salt of
diisobutylnaphthalene-alpha-sulfonic acid, 17 parts by
weight of the sodium salt of a lignin-sulfonic acid
obtained from a sulfite waste liquor, and 60 parts by
weight of powdered silica gel, and triturated in a hammer
mill. By uniformly distributing the mixture in water, a
spray liquor is obtained.
VI. 5 parts by weight of the compound of Example 94
is intimately mixed with 95 parts by weight of particulate
kaolin. A dust is obtained containing 5~, by weight of the
active ingredient.
VII. 30 parts by weight of the compound of
Example 117 is intimately mixed with a mixture consisting
of 92 parts by weight of powdered silica gel and 8 parts by
weight of paraffin oil which has been sprayed onto the
surface of this silica gel. A formulation of the active
ingredient is obtained having good adherence.
VIII. 40 parts by weight of the compound of
Example 124 is intimately mixed with 30 parts of the sodium
salt of a phenolsulfonic acid-urea-formaldehyde condensate,
2 parts of silica gel and 48 parts of water to give a
stable aqueous dispersion.
IX. 20 parts of the compound of Example 23 is
intimately mixed with 2 parts of the calcium salt of
dodecylbenzenesulfonic acid, 8 parts of a fatty alcohol
polyglycol ether, 2 parts of the sodium salt of a phenol-
sulfonic acid-urea-formaldehyde condensate and 68 parts of
a paraffinic mineral oil. A stable oily dispersion is
obtained.
The following experiments demonstrate the biological
action of the novel compounds. The prior art compounds
7-amino-6-phenyl-5-methyl-[1,2,4]-triazole-~1,5-a]-pyrimi-
~ 2~ 9~,
- 21 - O.Z. 0050/37g84
dine (A) (U.S. 2,553,500) and 7-amino-6-(4-tert-butoxy)-
--5-methyl-2-methylpyrazolo-[1,5-a]-pyrimidine ~B)
(EP 141,317) were used for comparison purposes.
~xperiment 1
05 Action on Plasmopara viticola
Leaves of potted vines of the Muller-Thurgau variety
were sprayed with aqueous suspensions containing (dry
basis) 80% of active ingredient and 20~, of emulsifier. To
assess the duraction of action, the plants were set up,
after the sprayed-on layer had dried, for 10 days in the
greenhouse. Then the leaves were infected with a zoospore
suspension of Plasmopara viticola. The plants were first
placed for 16 hours in a water-vapor saturated chamber at
24C and then in a greenhouse for 8 days at from 20 to
30C. To accelerate and intensify the sporangiophore
discharge, the plants were then again placed in the moist
chamber for 16 hours. The extent of fungus attack was then
assessed on the undersides of the leaves.
The results of the experiment show that for instance
compounds nos. 3, 8, 23, 38, 43, 94, 117 and 124, applied
as 0.05~ liquors, have a better fungicidal action (e.g.,
97%) then comparative compounds A and B (e.g., 60~.).
Experiment 2
Action on PhYtoPhthora infestans in tomatoes
Leaves of potted tomatoes of the "Gro~e Fleischtomate"
variety were sprayed with aqueous liquors containing (dry
basis) 80~ of active ingredient and 20~, of emulsifier.
After the sprayed-on layer had dried, the leaves were
infected with a zoospore suspension of Phytophthora
infestans. The plants were then placed for 5 days in a
water vapor-saturated chamber kept at 16 to 18C. After
this period, the disease had spread on the untreated
control plants to such an ex~ent that the fungicidal action
of the compounds was able to be assessed.
The results of this experiment show that compounds 8,
63 and 124, applied for instance as 0.025~ liquors, have a
better fungicial action ~e.g., 97%) than prior art active
ingredient B (0~).