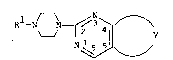Note: Descriptions are shown in the official language in which they were submitted.
3,,2~84q~9
-- 1 --
SPECIFICATION
2-PIPERAZINOPYRIMIDINE DERIVATIVES AND
PROCESS FOR THEIR PRODUCTION
Technical Field
This invention relates to novel 2-piperazinopy-
rimidine derivatives and a process for their production.
Background Art
Compounds having a structure formed by the conden-
sation of a nitrogen- or carbonyl containing ring and a
pyrimidine ring, for example, compounds having a pyridopy-
rimidine structure are known as mentioned in, for example, -
Chemical Abstracts, Vol. 90, 94893 (1979) and Yol. 97,
182350 (1982). However, these known compounds are greatly
different in structure from the 2-piperazinopyrimidine
derivatives of the present invention and their usefulness in
agricultural applications are neither explained nor suggested
in the above publications.
The present inventors conducted an extensive study
on 2-pipera2inopyrimidine derivatives. ~s a result, the
inventors have succeeded in the synthesis of the novel 2-
piperazinopyrimidine derivatives of the present invention
greatly different in structure from the known compounds
mentioned above and further have found that these novel
compounds have an excellent herbicidal activity~ and have
completed the present invention.
Disclosure of the Invention
The present invention provides 2-piperazinopy-
rimidine derivatives represented by the following generalformula [Il.
Rl-N ~ - ~ 3 ~ ~ y ... [I]
~ / .
wheeein R is a hydrogen atom or an aralkyl group and Y
is one of the groups represneted by the following general
,
formulas [II] to [VII]
atcH2 ~ C-b ... LII]
NR2
a~CH2 ~ C b ... ~III]
R3 O
t.
a-N~CH2 ~ C~CH2 ~ b ... ~IV]
o R4
atCH2 ~ C N b ... [V~
R5 o
~.
atCH2 ~ N - C-b ... [VI]
R6 R7 o
..
a-N~CH2 ~ N - C-b ... [VII]
(wherein a and b are positions to be bound to positions 4
and 5 of the pyrimidine ring of the formula [I], respec-
tively; Ql and Q2 each are an integer of 2 to 4; Q3 is 2 or0; Q4 is 0 or 1, provided that Q4 is 0 when Q3 is 2 and Q4
is 1 when Q3 is 0; Q5 is 2 or 3; Q6 is 1 or 2; Q7 is 2 or 3;
R is a hydroxyl group or a toluenesulfonyloxy group; and
R3, R4, RS, R6 and R7 each are a hydrogen atom or a lower
alkyl group).
As the arlkyl group of Rl of the general formula
[Il, there can be mentioned a benzyl group, a diphenylmethyl
group, a triphenylmethyl group, etc. O~ these, a benzyl
group is particularly preferred. Y of the general formula
[I~ is one of the groups represented by the general formulas
[II] to [VII].
In the general formulas ¦II] and [III], Ql and Q2
each are an integer of 2 to 4 and preferably 2 or 3O In the
geneal formula [IV], Q3 is 0 or 2 and Q4 is 0 or 1, pro-
vided that Q4 is 1 when Q3 is 0 and Q4 is 0 when Q3 is 2.
.
~ 2~34~
As the lower alkyl group of R3, there can be mentioned, forexample, alkyl groups of 1 to 4 carbon atoms such as methyl
group, ethyl group, propyl group, isopropyl group, n-butyl
group, isobutyl group, sec-butyl group, tert-butyl group and
the like. Of these, an ethyl group is particularly pre-
ferred.
In the general formula [V], Q5 is 2 or 3 and
preferably 3. R4 can be a hydrogen atom or one of the lower
alkyl groups mentioned with respect to R3 and particularly
preferably is a hydrogen atom or an ethyl group.
In the general formula [VI], Q6 is 1 or 2. R5 is
a hydrogen atom or one of the lower alkyl groups mentioned
with respect to R3 and particularly preferably is a hydrogen
atom or an ethyl group.
In the general formula [VII], ~7 is 2 or 3 and
preferably 2. R6 and R7 each are a hydrogen atom or a lower
alkyl group. As the lower alkyl group, there can be men-
tioned those listed with respect to R3 and an ethyl group is
preferable.
The compounds of the present invention can take a
free form as well as a salt form such as an acid addition
salt or the like. Therefore, in the present invention, the
2-piperazinopyrimidine derivatives of the general formula
[I] include their salts. These salts as well can be used as
a herbicide. As the acid addition salts, there can be
mentioned, for example, salts with a mineral acid such as
hydrochloric acid, sulfuric acid, phosphoric acid or the
like or with an organic acid such as acetic acid, maleic
acid, citric acid or the like.
Examples of the present invention compounds are
shown below. In the following formulas, Et is ethyl and prl
is isopropyl.
~ ~`84~3
6 5 2
(C6H5 ) 2CHN~,~
o
HN/~1~
(C6HS) 3CN~?
C6HSCH2~N~
NOH
HN/~
NOH
6 5 2 ~ ~
N502--(~CH3
C6 H5 CH 2 N~
2~ 6 5
HN
NOS02-~CH3
, ~ ~
8~3
C 6 H 5 C Ei 2 N ,N ~N E t
6 5 2 --/ ~CH 3
H N N - ~
--/ N~ NEt
HN~ N-~ r
C6H5CH2N ~N-~pr i
/--\ N~
C6H5CH2N ~ 3 NH
O
HN ~N ~H
O
C6H5 CH 2N~
O
.
~2~842~
HN ~N-~ ~ NEt
HN ,~ CH3
Et
HN/~-~'3
o
CH
/ \ N N 3
H N
6 5 2 /
C6H5CH2N~
HN~
Et
HN N~
C~13
6 5 2 \
;9
~ o
Et
C 6 H5 CH 2 N ~N~ ,o
Et
H N~ \=
Et
H N ~N ~ t
CH3
HN~
C,H3
C6H5 CH2 N/~-N~ ~
o CH3
C6H5CH21 ~1~C,\N_CH3
5 2 ~ N~3~C ~N
prl
6 5 2 / N~=O
..~
~ ~3842~
,CH3
6 5 2 ~ H~3~C=
HN ,~ ~ N-Pr
o
pri
HN N~
~ ~C=O
CH3
HN~N~ \C=0
S [Production Process No. 1]
Of the presnet invention compounds of the general
formula ~I], those having the Y represented by the formula
[II] can be produced according to the following process.
For exampler a compound ~ wherein Ql f the formula ~ is
3 and Rl is a benzyl group, or a compound ~ wherein Ql f
the formula [II] is 3 and Rl is a hydrogen atom can be
produced as follows.
0 0 CH
+ 3 ~H-N/ 3 ~ CH-N~' 3
CH30 CH3 1 ~ :
(~) (~) (~)
(~ + C6 HS C H 2--N ~N `h ' 2 H 2 50 4 --3 C 6 Hs cH 2 , ~3
0 ~ H - N ,rJ ~
O
~,
:
38'~
The starting material is a cycloalkane-1,3-dione
or the like. Specifically, cyclopentane-1,3-dione or cyclo-
hexane-1,3-dione ~ is used. For example, 1 mole of the
compound ~ is mixed with about 2 mole of N,N-dimethylform-
amide dimethylacetal with ice-cooling and they are reacted
for several hours at normal temperature to obtain a compound
The compound ~ is reacted with a compound ~ for
1 to several hours in an alcohol medium such as methanol,
ethanolr propanol or the like in the presence of a caustic
alkali, with refluxing the alcohol medium, to obtain a
compound ~ The compound ~ is subjected to hydrogenolysis
to obtain a compound ~ Examples of the hydrogenolysis
catalyst include Pd-C, Raney-Ni, Pt-C, PdO, etc. Examples
of the reaction solvent include alcohols such as methanol,
ethanol, isopropanol and the like; carboxylic acids such as
formic acid, acetic acid, propionic acid and the like; and
mixed solvents such as ethanol-formic acid, ethanol-acetic
acid and the like. The reaction can be conducted, for
example, at 10 to 100C for 0.1 to 10 hours with feeding
hydrogen at normal pressure to 10 kg~cm2.
[Production Process No. 2]
Of the present invention compounds of the general
formula [I], those having the Y represented by the general
formula IIII~ can be produced by using, as the material, one
of the compounds obtained according to the above Production
Process No. 1, for example, the compound ~ or ~. When, for
example, the compound ~ is used, the production is conducted
as follows~
~ ~38~9
-- 10 --
C6H5CH2-N~JN~ + H;;~NOH ~ HCl
(~)
6 H5 CH 2 N ~3~'3~ ~ HCl
N-OH
(~) + ~H3-C6H4-S02Cl
----~C6H5CH2N N- ~
~ N O SO2-C6H4-CH3
The compound ~ can be obtained by subjecting to
reaction an about equimolar mixture consisting of the com-
pound ~ and hydroxylamine hydrochloride, for example, at 40
to 200C for 0.5 to 10 hours in a solvent such as an alcohol
(e.g~ methanol, ethanol), toluene, xylene, dimethylformamide
or the like. The compound ~ can be obtained by reacting
1 mole of the compound ~ with 1 to 2 moles of p-toluene-
sulfonyl chloride, for example, at 0 to 100C for 0.5 to 10
hours in a solvent such as water, acetone, an alcohol,
toluene or their mixture. The essentially same production
process as above can be applied to obtain compounds having
Ql and Q2 each of 2 or 4.
[Productin Process No. 3]
Of the present invention compounds of the general
formula lI], those having the Y represented by the formula
[IV~,:Q3 of 0 and Q4 of 1 and having, as R , a benzyl group
can be produced as follows.
.
.
f~8~f2~
C H CH ~\ ~3~C1 2 5NH2
~CH2COOEt
C2H5
6 5 2 ~ N~3/ =o
In the above case, a desired compound can be
obtained by heating the compound ~ and an alkylamine cor-
responding to R3 of the formula [IV] such as ethylamine, forexample, at 100 to 140C for several hours in an alcohol
medium such as isopropanol or the like. Compounds having
the same Y, ~3 and Q4 as above but having, as Rl, a hydrogen
atom can be obtained by subjecting a compound such as the
compound ~ to hydrogenolysis in the same manner as men-
tioned in [Production Process No. 1].
[Production Process No. 4]
Of the present invention compounds of the general
formula [I~, those having the Y represented by the formula
1IV~, Q3 of 2 and Q4 of 0 can be synthesized, for example,
as follows from a known compound ~ .
,C2H5 C2H5
CH -S ,3J + NH~ H --~ ~ ,,3 - J
~ o ~ o
.
In the above case, 1 mole of the compound ~ and,
for example, 2 to 3 moles of a compound ~ are reacted, for
:20example, at 100~ to 150C for 1 to several hours in a sol-
: vent such as dimethylsulfoxlde, N,N-dimethylformamide,
pyridine or the like. Compounds having the same Y, Q3 and
38429
- 12 -
Q4 as above ~ut having, as Rl, an aralkyl group can be
synthesized in the same manner as above by using a 1-
aralkylpiperaæine in place of the compound ~.
[Production Process No. 5]
Of the present invention compounds of the general
formula [I], those having the Y represented by the formula
[V] can be synthesized, for example, according to the fol-
lowing reaction which is the rearrangement reaction of the
compound ~ mentioned in [Production Process No. 2].
C C /~ ~3~
~ N-OsO2c6H4 CH3
C C
The compound ~ having, as Rl, an aralkyl group
can be obtained by heating the corresponding compound ~ at,
for example, 0 to 200C, preferably 30 to 140C for, for
example, 0.5 to 10 hours in a carboxylic acid te.9. formic
acid, acetic acid, propionic acid) or an alcohol (e.g.
ethanol). Compounds having, as R4, a lower alkyl group can
be obtained by reacting a compound corresponding to the
compound ~ with a lower alkyl bromide such as ethyl bromide
in tetrahydrofuran or an alcohol in the presence of NaH,
NaOEt, KOEt~ K2CO3, Na2CO3, NaOH or RO~. This reaction can
be conducted under conditions of, for example, 0 to 100C
and 0.5 to 10 hours.
Compounds of the general formula [I3 having a
hydrogen atom as Rl and the Y represented by the formula [V~
can be obtained by subjecting the compound ~ to hydrogeno-
lysis in the same manner as mentioned in [Production Process
No. 1]~
~ 2~34~3
¦Production Process No. 6]
Those compounds of the genral formula [I~ having
the Y represented by the formula lVI] and Q6 of 2 can be
produced according to the following reaction formula.
~ + C6H5CH2-N ~ ~ NH 2H2
O OMe
~ C6 H5 CH 2 -N .~N~ Et
The above reaction can be conducted by, for ex-
ample, hPating the compound ~ and the compound ~ for 1 to
several hours in an ethanol solvent in the presence of a
base such as NaOH, KOH or the likev with refluxing ethanol.
Incidentally, the compound ~ can be synthesized according
to a method mentioned in Reference Examples 1 to 5 which
appear later. Materials other than the compound ~ , used
Eor the production of compounds of the general formula [I]
having the Y represented by the formula [VI] and Q~ of 1 can
also be synthesized in the same manner. Compounds of the
present invention having a hydrogen atom as Rl and the Y
represented by the formula [VI] can be produced by subject-
ing a compound such as the compound ~ having an aralkyl
group as Rl to the same hydrogenolysis as mentioned in
[Production Process No. li. The present invention compound
can be produced also by hydrogenating the following
compound
C6H5CH2-N~-~N-Et
O
2S which is obtained according to Mitsui, laid-open Japanese
application No. 43190/1986~
,
84~g
- 14 - ,
[Production Process No. 7] f
Co~pounds of the general formula [I] having the Y
represented by the formula [VI] and 6 of 1 can be produced
also according to the following reaction formula.
OEt
~ OEt + C6H5CH2~N~_~1 \NH
6 5 2 ~ ~ ~ ~oEt
~ O
~ + NH3 ~ 6H5CH2-N~_~N ~N ~ NH
(~) O
(~ + EtNH2 ~ C6H5CH2-N/ \N_ ~ ~ N-Et
The compound ~ can be synthesized, for example,
according to Reference Example 6 which appears later. The
reaction between the compound ~ and the compound ~ can be
conducted, for example, at 0 to 100C for 0.5 to 10 hours
in a solvent such as water, methanol, ethanol, tetrahydro-
furan, dimethylformamide or the like. The compound ~ and
the compound ~ can be synthesized by reacting the compound
with ammonia and an amine having an alkyl group corres-
ponding to R5, respectively, that is, by reacting the mate-
rials~ for example, at 0 to 150C for 0.5 to 20~ hours in
a solvent such as water, an alcohol, tetrahydrofuran, di-
methylformamide, toluene, xylene or the like.
Compounds of the general formula [I] having ahydrogen atom as Rl and the Y represented by the formula
,,
'' '
:,, ` '
1VI] can be obtained by subjecting to hydrogenolysis a
compound corresponding to the compound ~ or ~ in the same
manner as mentioned in [Production Process No. 1].
lProduction Process No~ 8]
Compounds of the general formula [I] having the Y
represented by the formula [VII] can be obtained by, for
example, reacting (1) a pyrimidodiazepine derivative as
obtained in Reference Example 9 which appears later and (2)
a l-aralkylpiperazine such as l-benzylpiperazine or the
like.
This reaction can be conducted at 140 to 170C
for several tens of hours by using 1 mole of the former
compound and 4 to 5 moles of the latter compound~ Compounds
of the general formula [I] having a hydrogen atom as Rl and
the Y represented by the formula lVII] can be produced by
subjecting to hydrogenolysis corresponding compound having,
as Rl, an aralkyl group, particularly, a benzyl group in the
same manner as mentioned in [Production Process No. 1].
Best Mode for Carrying Out the Invention
The present invention compounds of the general
formula [I] can specifically be produced as follows. These
compounds can be utilized as a herbicidally active compound
as shown later by the data on some of the compounds.
Reference Example 1 E~hyl 3-ethylaminopropionate
50 g (0.50 ~ole) of ethyl acrylate was dissolved
in 500 ml of ethanol. To the resulting solution being
stirred in an ice bath was added dropwise a mixed solution
consisting of 36 g of a 70~ aqueous ethylamine solution
(containing 0.55 mole of ethylamine~ and 100 ml of ethanol,
in 3.5 hours. The reaction was continued for further 3
hours and then the solvent was distilled off. The resulting
residue was subjected to vacuum distillation to obtain 50.5
g of a desired product with an yield of 70~.
Colorless liquid
Boiling point: 65C/10 mmHg
Infrared absorption spectrum ~neat~ cm
3320 (broad), 1735
. :
. .
8~
- 16 -
H-NMR spectrum (CDCl3 solution, ~ ppm)
1.0-1.4 (6H, m), 2.4-3.0 (6H, m), 4.16
(2H, q, J=7.0Hz)
Reference Example 2 Ethyl N-ethoxycarbonylacetyl-3-ethyl-
amino~ropionate
To a mixture consisting of 45 g (0.30 mole) of
ethyl 3-ethylaminopropionate, 37.3 g (0.27 mole) of potas-
sium carbonate, 250 ml of toluene and 250 ml of water being
stirred in an ice bath was added dropwise 67.7 9 (0.45 mole)
of ethylmalonyl chloride in 0.5 hour. Stirring was con-
tinued for further 3 hours at room temperature and the
reaction mixture was subjected to phase separation. The
toluene layer obtained was washed with a 5% aqueous HCl
solution, a saturated aqueous sodium bicarbonat~ solution
and a saturated aqueous NaCl solution in this order and then
dried with anhydrous magnesium sulfate. The resulting
solution was subjected to vacuum distillation to remove
toluene, whereby 64.3 g of a desired product was obtained
with an yield of 83~.
Colorless liquid
Infrared absorption spectrum (neat, cm l)
1735, 1648
H-NMR spectrum (CDC13 solution, ~ ppm~
1.1-1.3 (6EI), 2.64 (2H), 3.2-3.8 (6H),
~5 4.0-4.2 (4H)
Reference Example 3 3-Carbethoxy-l-ethylpiperidine-
2,4-dione
-
5.8 9 of metallic sodium was added to 300 ml of
ethanol and they were reacted to obtain an ethanol solution
containing sodium ethoxide. To this solution was added 62.
g of ethyl N-ethoxycarbonylacetyl-3-ethylaminopropionate,
and they were refluxed for 4 hours and allowed to cool.
Ethanol was distilled off. Thereto were added ethyl aceta~e
and a dilute aqueous hydrochloric acid solution and thP
resulting mixture was shaken The organic layee was sep-
arated, water-washed, dried and concentrated to obtain
. ,
.
'
.. : .
~.~8~29
- 17 -
36.3 g of 3-carboethoxy-1-ethylpiperidine-2,4-dione as an
oily matter with an yield of 71%.
H-NMR spectrum (CDC13 solution, ~ ppm)
1.28 (6H, m), 2.66 (2H, m), 3.44 (4H, m),
4.32 (2H, m)
Reference Example 4 1-Ethyl~peridine-2,4-dione
300 ml of a 10% aqueous hydrochloric acid solution
was added to 36.0 9 of 3-carboethoxy-1-ethylpiperidine-2,4-
dione, and they were refluxed for 40 minutes and allowed to
cool. The resulting mlxture was subjected to extraction by
chloroform. The resulting chloroform layer was water-
washed, dried and concentrated to obtain 16.6 9 of l-ethyl-
piperidine-2,4 dione as a light yellow oily matter with an
yield of 70%.
H-NMR spectrum (CDC13 solution, ~ ppm)
1.20 ~3H, t, J=7Hz), 2.64 (2H, t, J=7Hz),
3~36 (2H, s), 3.54 (4H, m)
Reference Example 5 1-Ethyl-3-methoxymethylen~piperidine-
2i4-dione
11 g of methyl orthoformate and 20 ml of acetic
anhydride were added to 8.3 g of 1-ethylpiperidine-2,4-
dione, and they were refluxed for 7 hours and then allowed
to cool. Excessive methyl orthoformate and acetic anhydride
were removed by vacuum distillation. The resulting brown
reside was subjected to vacuum distillation (0.5 mmHg, bath
temperature of 200 to 250C) using a Kugelrohr apparatus,
whereby 2.7 g of 1-ethyl-3-methoxymethylenepiperidine-2,4-
dione was obtained as a crystal with an yield of 25~. This
crystal was recrystallized from a mixed solvent consisting of
ethyl acetate and hexane to obtain a needle-like crystal of
said compound.
H-NMR spectrum (CDC13 solution, ~ ppm)
1.20 (3H, m3, 2.64 (2H, t, J=7Hz), 3.50
(4H, m), 4.12 ~3H, s), 7086 ~lH, two s).
Example 1 2-(4-Benzyl~iperazino)-6-et~y~1-5-oxo-5,6,7,8-
tetrahydro~yrido[4,3-d~pyrimidine
.~
., , ,~.
,
.
'
.
,
34~
- 18 -
(In the general formula [I], Y is [IV~.)
1.56 g of 1-amidino-4-benzylpiperazine sulfate was
added to an ethanol solution containing 0.23 g of sodium
hydroxide. To the resulting suspension was added 1.07 g of
1-ethyl-3-methoxymethylenepiperidine-2,4-dione, and they
were refluxed for 2 hours. Ethanol was distilled off.
Then, water was added and extraction by chloroform was
conducted~ The chloroform layer was dried and concentrated
to obtain 1.4 g of 2-(4-benzylpiperazino)-6-ethyl-5-oxo-
5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine with an yield of
69~.
Melting point: 128-130C
H-NMR spectrum (CDC13 solution, ~ ppm)
1.20 (3H~ t, J=7Hz), 2.50 (4H, m), 2,94
(2H, t, J=8Hz), 3.55 (6H, m), 3.92 (4H, m),
7.32 (5H, m), 8.92 (lH, s)
Example 2 6-Ethyl-5-oxo-2-piperazino-5,6,7,8-tetrahydro-
pyrido[4,3-d]pyrimidine
(In the general formula [I], Y is [VI].)
In 20 ml of ethanol was dissolved 0.70 g of
2-(4-benzylpiperazino)-6-ethyl-5-oxo-5,6,7,8-tetrahydro-
pyrido[4,3-d] pyrimidine. Thereto was added 10% Pd-C, and
the resulting mixture was stirred at 60C for 4 hours in a
hydrogen atmosphere. After having been allowed to cool, the
mixture was filtered to remove the catalyst and the filtrate
was concentrated to obtain 0.50 g of 6-ethyl-5-oxo~2-
piperazino-5,6,7,8-tetrahydropyrido[4,3-dlpyrimidine as a
crystal with an yield of 96~ The crystal was purified by
immersion using ethyl acetate~
M~lting pointo 205~210C
~-NMR spectrum (CDC13 solution, ~ ppm)
1.20 (3H, t, J=7Hz~, 3.00 (6H, m), 3.60
(4H, m), 3.94 (4H, m), 8.84 (lH, s)
Reference Example 6 Ethyl-4-chloro-2-ethoxymethyleneaceto-
acetate
10 g (60.7 mM) of ethyl 4-chloroacetoacetate, 18 g
38~29
-- 19 --
(121 mM) of ethyl orthoformate and 25 g (245 mM) of acetic
anhydride were stirred at 110C for 3 hours. Then, exces-
sive ethyl orthoformate and acetic anhydride were distilled
off under vacuum and the residue was recrystallized from
hexane to obtain 12.1 g of a needle-like crystal with an
yield of 90%.
Melting point: 86.5C
H-NMR spectrum (CDC13 solution, 6 ppm)
1.35 (3H, t, J=7Hz), 1.44 (3H, t, J=7Hz),
4.30 (2H, q, J=7Hz), 4.33 (~H, q, J=7Hz),
4.55 (2H, s), 7.8B (lEI, s)
lnfrared absorption spectrum (KBr tablet, cm 1):
2900, 2830, 1686, 1~70, 1575, 1250, 1018
Reference Example 7 Ethyl-4-chloro-methyl-2-(4-benzyl-
piperazino)px-rimidine-5-carboxylate
A solution consisting of 15 ml of water and 1.5 g
of NaOH was added to a suspension consisting of 9.7 g (36.4
mM) of l-amidino-4-benzylpiperazine sulfate and 185 ml of
tetrahydrofuran, to conduct neutralization.
Thereto was added dropwise at 20C a solution
consisting of 200 ml of tetrahydro~uran and 8 g (36.4 mM) of
ethyl 4-chloro-2-ethoxymethyleneacetoacetate, after which
they were stirred at that temperature for 1 hour. Then, 300
ml of ether was added and water washing was conducted three
times. The organic layer was dried with anhydrous MgSO4.
The solvent was distilled off under vacuum to obtain 11.8 g
of a desired compound of light yellow color with an yield of
86.7%.
lH-NM~ spectrum (CDCl~ solution, 6 ppm)
1.37 (3H, t, J=7Hz), 2.51 (4H, t, J=6Hz),
3.75 (2H, s), 3.96 ~4H, t, J-6~z), 4.34
(2H, qg J=7Hz), 4.88 (2H, s), 7.32 (5H, s),
3.87 (lH, s)
Infrared absorption spectrum (neat, cm 1):
2873, 278~, 1706, 158~t 1526; 1445, 1350,
1250, 1090, I000~ 742, 696
': -
4~
- 20 -
Example 3 2-(4-Benzylpiperazino)-5-oxo-5~6-dihydro(7H)
~yrrolo[3,4-d]pyrimidine
(In the general formula [I], Y is IVI3.)
2.25 g t6 mM) of ethyl 4-chloromethyl-2-(4-
benzylpiperazino)pyrimidine-5-carboxylate was dissolved in
10 ml of ethanol. Thereto was added 10 ml of a 30% aqueous
NH4OH solution (containing 59 ml of NH40H) at 20C, and the
mixture was stirred for 12 hours. The reaction mixture was
poured into a 10% aqueous NaHCO3 solution, and extraction by
CHC13 was conducted. The solvent was distilled off from the
CHC13 layer and the resulting residue was recrystallized
from toluene.
Yield: 0.70 g (38%)
Melting point: 172C
lH-NMR spectrum ~DMSO-d6 solution, ~ ppm)
2.45 (4H, t, J=6Hz), 3.50 (2H, s), 3.83
(4H, t, J=6Hz), 4.20 (2H, s), 7.30 (5H, s),
8.20 (lH, br. s), 8.57 (lH, s)
lnfrared absorption spectrum (nujol, cm 1):
2~00~ 1715, 1674t 1607, 1562, 1218, 1145,
730, 720
Example 4 2-Piperazino-5-oxo-5,6-dihydro(7~) r- yrrolo-
..
[3,4-d]pyrimidine
(In the general formula II]r Y is [VI~.)
Hydrogen was bubbled through a mixture being
heated at 60C, consisting of 1.6 g (5.18 mM) of 2-
(4-benzylpiperazino)-5-oxo-5,6-dihydro(7H)pyrrolo[4,3-d~-
pyrimidine, 0.16 g of 10% Pd-C and 30 ml of AcOH. One hour
la~er, the solven~ was distilled of~. The residue was
suspended in a 10% aqueous NaHCO3 solution, and the in-
solubles were collected by filtration and dried to obtain
0.75 g of a desired compound as an oily substance with an
yield of 66%u
H-NMR spectrum (DMSO-d6 solution, ~ ppm)
2.80 (4H, br. s), 3.24 (lH, br. s), 3.78
(4H, br. s), 4.23 (2H, s), 8.18 ~lH, br. s),
8.58 tlH, s)
3429
- 21 -
Infrared absorption spectrum (KBr tablet, cm 1):
3210, 3100, 2920, 2880, 1700, 1612, 1260,
1150, 980
Example 5 6-Ethyl-2-(4-benzyl~iperazino)-s-oxo---5~6-di
hydro(7H)pyrrolo[3,4-d]pyrimidine
(In the general formula [I], Y is [V1].)
1.0 g (2.7 mM) of ethyl 4-chloromethyl-2-(4-
benzylpiperazino)pyrimidine-5-carboxylate was dissolved in
10 ml of ethanol. Thereto was added at 20C 5 g of a 70
aqueous ethylamine solution (containing 59 mM of ethyl-
amine). The mixture was stirred at 20C for 2 hours and at
80C for 0.5 hour.
After completion of the reaction, the reaction
mixture was poured into water and neutralized with a 10%
aqueous NaHCO3 solution. Then, extraction by ether was
conducted. The organic layer was dried, after which the
solvent was distilled off. The residue was recrystalliæed
from a mixed solvent consisting of toluene and hexane to
obtain 0.82 g of a desired compound with an yield of 91~.
Melting point: 159.2C
H-NMR spectrum (CDC13 solution, ~ ppm)
1.22 (3H, t, J-7Hz), 2.S0 (4H, t, J=6Hz),
3.55 (2H, s), 3.59 (2H, q, J=7Hz), 3.93
(4H, t, J-6Hz), 4.18 (2H, s), 7.32 (5H, s),
8.64 (lH, s)
Infrared absorption spectrum (nujol, cm 1):
2900, 1666, 1624, 1566, 1280, 1148, 1000,
975, 795, 733
By using methylamine or isopropylamine in place of
ethylamine, the following two compounds were obtained.
6-Methyl-2-(4-benzylpiperazlno)-5-ox---J 6-dihydro-
(7H)p~rrolo[3,4-d]~yrimidine
(In the general formula [I~, Y is [VI~.)
Yield: 94%
Melting point: 178-179C
"
.
.
.
~1.2~8~29
H-NMR spectrum (CDC13 solution, 6 ppm)
2.52 (4H, m), 3.15 (3H, s), 3.58 (2H, s),
3.96 (4H, m), 4.20 (2H, s), 7.36 (5H, m),
~.65 (lH, s)
Infrared absorption spectrum (CHC13 solution,
cm 1):
1685, 1618, 1522, 1350
6-Isopro~yl-2-(4-benzylpi~erazino~-5-oxo-5~6
di~y~ ~ rrolo~3,4-d]E~imidine
tIn the general formula lI], Y is [VI~.)
Yield: 39%
Melting point: 173~-174C
H-NMR spectrum (CDC13 solution, 6 ppm)
1.25 (6H, d~ J-7Hz), 2.52 (4H, m), 3.58
lS (2H, s~, 3.96 (4H, m), 4.16 (2H, s),
4.64 (lH, sept., J=7Hz), 7.36 (5H, m),
8.66 (lH, s)
Infrared absorption spectrum (CHC13 solution,
cm-l)
1680, 1618, 1530, 13~5
Example 6 6-Ethyl-2-piperazino-_-oxo-5,6-dihydro(7H)-
pyrrolo[3,4--d]pyrimidine
~In the general formula [I~, Y is ~VI].)
To 20 ml of AcO~I were added 1~5 g (4.45 mM~ of
6-ethyl-2-(4-benzylpiperazino3-5-oxo-5,6-dihydro(7H)-
pyrrolol3,4-d]pyrimidine and 0.15 g of 10% Pd-C. Hydrogen
was bubbled therethrough at 80C to conduct a reaction for
1 hour.
After completion of the reaction, Pd-C was remQved
by filtration and AcOH was distilled of~. The residue was
dissolved in chloroform and neutralized with a 10% aqueous
NaHC03 solution. The organic layer was dried with MgS04 and
the solvent was distilled off. The residue was purified
according to silica gel chromatography. The yield was 0.50
9 ~45~).
Melting point: 58.5C
~, :
.. . .
8a~2~
- 23 -
H-NMR spectrum (CDC13 solution, 6 ppm)
1.24 (3H, t~ J-7Hz), 2.12 (lH, br. s),
2.95 (4H, br. s), 3.62 (2H, q, J=7Hz), 3.92
(4H, br. s), 4.21 (2H, s), 8.66 (lH, s)
Infrared absorption spectrum (KBr tablet, cm 1):
34B0, 3300, 2900, 1650, 1618, 1520, 1438,
1235, 1160, 862
In the similar manner, the following two compounds
were obtained.
6-Methyl-2-piperazino-5-oxo-5,6-di ~ 7H)-
~yrrolo~3,4-d]p~rimidine
(In the general formula [I], Y is [VI].)
Yield: 100%
Melting point: 176-177C
lS lH-NMR spectrum (CDC13 solution, ~ ppm)
2.98 (4H, m), 3.18 (3H, s), 3.96 t4H, m),
4.24 (2H, s), 8.70 (lH, s)
Infrared absorption spectrum (CHC13 solution,
cm-l)
1685, 1618, 1522, 1350
6-Isopropyl-2-piperazino-5-oxo-5,6-dihydro(7H)
~yrrolo[3,4-d]pyrimidine
(In the general formula [I], Y is [VIl.
Yield: 96~
Melting point: 161-162C
H-NMR spectrum (CDC13 solution, ~ ppm)
1.28 (6~, d, J=7Hz), 2.96 (4H, m), 3.94
(4H, m), 4.18 (2H, s), 4.65 (lH, sept.,
J-7Hz3, 8.70 (lH, s)
Infrared absorption spectrum (CHC13 solution,
cm 1):
1680, 1618, 1520, 1345
Reference Example 8~ 2-Dime hylaminomethylenecyclohexane-
. ~ ~ 1, 3-dione
11.9 g (100 mM) of N.N-dimethylformamide dimethyl-
acetal was dropwise added to 5.6 g ~50 mM) of cyclohexane-
384~9
- 24 -
1,3-dione being stirred in an ice bath. The mixture was
subjected to a reaction at 25C for 7 hours. The low~
boiling substances in the reaction mixture were distilled
off. The residue was recrystallized from ethyl acetate -
hexane to obtain 7.7 g of a desired compound as a yellowcrystal with an yield of 92%.
Melting point: 106-107C
H-NMR spectrum (CDC13 solution, 6 ppm)
1.92 (2H, m), 2~47 (4H, m), 3.18 (3H, s),
3.40 (3H, s~, 8.05 (lH, s)
Example 7 2-(4-Benzylpiperazino)-S-oYo-5,6,7,B-tetrahydr
~inazoline
(In the general formula [I], Y is lII].)
To 60 ml of an ethanol suspension cGntaining 10.73
g (40 mM) of l-imidino-4-ben~ylpiperazine sulfate were added
80 ml of an ethanol solu~ion containing 1.6 g ~40 mM) of -
sodium hydroxide and then 6.69 g (40 mM) of 2-dimethylamino-
methylenecyclohexane-1,3-dione. The mixture was refluxed
for 4 hours and allowed to cool to room temperature. The
solvent was distilled off. To the residue was added 100 ml
of water, and extraction was conducted two times using 200
ml of ethyl acetate. The ethyl acetate layer was washed
with a a saturated aqueous NaCl solution and dried with
anhydrous magneisum sulfate. Ethyl acetate was distilled
off under reduced pressure. The residue was purified
according to silica gel chromatography [eluting solvent:
ethyl acetate (3)/hexane(7)1 to obtain 9.90 g of a desired
compound as a light yellow crystal with an yield of 77%.
Melting point: 96-97C
Infrared absorption spectrum (KBr tablet, cm~l):
1665, 1590, 1530, 1515
H-NMR spectrum (CDC13 solution, ~ ppm)
2.08 (2H, m), 2.5 (6H, m), 2.80 (2~, m),
3.54 (2H, sl, 3.96 (4H, m), 7.32 (5a~ s)~
8.83 (lH, s)
''
84~9
- 25 -
Example 8 2-Pi~razino-5-oxo-5,6,7,8-tetrahYdroquinazoline
(In the general formula [I], Y is lII].)
0.64 q (2 mM) of 2-(4~benzylpiperazino)-5-oxo-
5,6,7,8-tetrahydroquinazoline was dissolved in 30 ml of
ethanol and 10 ml of acetic acid. Thereto was added 64 mg
of 10% Pd-C, and hydrogenation was conducted at 50C for 1
hour under normal pressure. The reaction mixture was cooled
to room temp&rature. The catalyst was removed by filtration
and the filtrate was subjected to vacuum distillation. The
residue was recrystallized from ethyl acetate to obtain 0042
y of a desired compound as a light yellow crystal with an
- yield of ~0~.
Melting point: 161-162C
Infrared absorption spectrum ~KBr tablet, cm 1)
3400 (broad), 1670, 1600, 1530
H-NMR spectrum (CDC13 solution, ~ ppm)
2.10 (2H, m), 2.60 (2H, m), 2.84 (2H, m),
3.06 (48, m), 4.08 (4H, m), 8.85 (lH, s)
Example 9 2-(4-Benzyl~ie~azino)-5,6,7,8-tetrahydro-5-
hydroxyimino~uinazoline
(In the general formula [I], Y is [IIIl.)
To 30 ml of methanol were added 2.6 9 (8.07 mM) of
2-(4-benzylpiperazino)-5-oxo-5,6,7,8-tetrahydroquinazoline
and 0.67 g (9.64 mM) of hydroxylamine hydrochloride. The
25 mixture was stirred at 60C for 2 hours and cooled to 20C.
The resulting precipitate was collected by filtration,
whereby 2.85 g of a white solid was obtained with ~n yield
of 95%.
Melting point: Above 300C (decomposed)
l~-NMR spectrum (CDC13 solution, ~ ppm3
1.80 (2H, m), 2.40-2.90 (8H, m~, 3.58
(2H, s), 3.92 (4H, t, J=6Hz), 7.34 (5H, s),
8.91 (lH, s)
Infrared absorption spectrum (KBr tablet, cm 1)
3150, 2920, 2444, 1618, 1582, 1510, 1500,
1275, 1032, 955
.:
~.2~3842~
- 26 -
Example 10 2-(4-BenzYlPiperazino)-5-~6~7~8-tetr-ahydro-5
(p-toluenesulfonyl)aminoquinazoline
.. . . _ _
(In the general formula [I], Y is [III].)
2 g (5.35 mM) of 2-(4-benzylpiperazino)-5,6,7,8-
tetrahydro-5-hydroxyiminoquinazoline, 1.6 g (8.4 mM) of
p-toluenesulfonyl chloride and 0.7 g (11 mM) of KOH were
added to a mixed solvent consisting of 10 ml of water and
30 ml of acetone. The mixture was stirred at 200C for
4 hours.
After completion of the reaction, the reaction
mixture was poured into an aqueous NaCl solution. Extrac-
tion by ethyl acetate was conducted. The organic layer was
evaporated to dryness. The residue was purified according
to silica gel chromatography (etluting solvent: CHC13 -
ethanol) to obtain 1.5 g of a white solid with an yield of
59%.
Melting point: 180.6C
H-NMR spectrum
1.68-1.98 (2H, m), 2.44 (3H, s), 2.46-2.85
(8H, m), 3.57 (2H, s), 3.90 (4H, t, J=6Hz~,
7.26-7.40 (7H~ m), 7.86 (lH, s), 7.95
(lH, s), 8.69 (lH, s)
Infrared absorption spectrum (RBr tablet, cm 1)
2920, 1578, 1526, 1424, 1188, 1176, 1005,
992
Example 11 2-(4-BenzylPiPerazino)-6-oxo-6~7~8~9-tetrahydr
(5H)pyrimldo[5,4-d]aze~ine
(In the general formula [I~, Y is [V~
1.3 9 (4 mM) of 2-(4-benzylpiperazino)-5,6,7.8-
tetrahydro-5-(p~toluenesulfonyl)iminoquinazoline was dis-
solved in 30 ml of acetic acid. The solution was heated at
100C for 4 hours for reaction. After completion of the
reaction, acetic acid ~as distilled off. The residue was
dissolved in CHC13 and neutralized with a 10~ aqueous NaHCO3
solution. The solvent was distilled off and the residue was
recrystallized from toluene to obtain 0.70 g of a white
solid with an yield of 79%.
'
.
~1 2~ 34X9
- 27 -
Melting point: 210.7C
H-NMR spectrum (CDC13 solution, ~ ppm)
2.26-2.40 (4H, m), 2.49 (4H, t, J-6Hz),
2.83 (2H, t, J=7Hz), 3.55 (2H, s), 3.82
(4H, tr J=6Hz), 7.32 (5H, s), 7.83 (lH, s),
7.94 (lH, s)
Infrared absorption spectrum (RBr tablet, cm 1)
3150, ~900, 2830, 1660, 1595, 1346, 1010,
982
10 Example 12 5-Ethyl-2-(4-benzylpiperazino)-6-oxo~6,7,8,9-
tetrah~dro(5H)pyrimido[5,4-b]azepine
(In the general formula [I], Y is lV].)
0.65 g (1.84 mM) of 2-(4-benzylpiperaæino)-6-
oxo-6,7,8,9-tetrahydro(5H)pyrimido~5,4-b]azepine and 0.15 g
(3-75 mM) of 60% NaOH were added to 30 ml of tetrahydro-
furan. The mixture was stirred at 20C for 30 minutes.
Then, 3 g (27~8 mM) of ethyl bromide was added. The mixture
was subjected to a reactin at 60C for 5 hours. After
completion of the reaction, the solvent was distilled off
and the residue was purified according to silica gel
chromatography to obtain 0.65 g of a colorless oily compound
with an yield of 92~
H-NMR spectrum (CDC13 solution, ~ ppm)
1.15 (3H, t, J=7Hz), 2.16-2.38 (4H, m),
2.53 (4H, t, J-6Hz), 2.74 (2H, t~ J=5.5Hz),
2.58 (2H, s), 3.80 (2H, q, J=7Hz), 3.87
(4H, t, J=6Hz), 7.35 (5H, s~, 8~13 (lH, s)
Infrared absorption spectrum (KBr tablet, cm 1)
2920, 1657, 1589, 1444, 1250, 1000, 9800 Example 13
~5H)pyrimidol5,4-b~azepine
(In the general formula [I], Y is [V3.)
0.6 g (2.18 mM) of 5-ethyl-2-(4-benzylpiperazino)-
6-oxo-6,7,8,9-tetrahydro(5~)pyrimido[5,4-b3azepine and 0.06
g of 10% Pd-C were added to a mixed solvent consisting of
30 ml of acetic acid and 10 ml of ethanol. The mixture was
,, '~
~'
~.2~8429
- 28 -
subjected to a reaction at 100C for 4 hours with bubbling
hydrogen through the mixture. Then, Pd-C was removed by
filtration and the filtrate was evaporated to dryness to
obtain 0.5 g of a light yellow oily compound with an yield
of 100%.
lH-NMR spectrum (CDC13 solution, ~ ppm)
1.16 (3H, t, J=7Hz), 2~21-2.40 (4H, m),
2.75 (2H, t, J-5~5Hæ), 2.96 (4H, t, J=6Hz),
3.70-4.05 (6H, m)
Infrared absorption spectrum (neat, cm 1)
- 3460, 3300, 29~0, 165~, 1595, 1445, 1128,
984
In the similar manner, the following compound was
obtained as ~ollows.
2 Piperazino-6-oxo-6,7,8,9-tetrahydro(5H)pyrimido-
[5,4-b]azepine
(In the general formula ~I], Y is [V].)
0.1 g (0.3 mM) of 2-(4-benzylpiperazino)-6-oxo-
6,7,8,9-tetrahydro(5H)pyrimido[5,4-b]azepine and 0.01 g of
10~ Pd-C were added to 20 ml of ethanol. The mixture was
subjected to a reaction at 50C or 4 hours in a hydrogen
atmosphere. Then~ Pd-C was removed by filtration and the
filtrate was evaporated to dryness to obtain 0O074 g of a
white crystal quantitatively.
Melting point: 175-178C
H-NMR spectrum (CDC13 solution, ~ ppm)
2.2-2.5 (4H, m), 2.7-3.0 (2H, m), 2.94
(4H~ t, J=4.5Hz), 3.81 (4H, t, J=4.5Hz),
7.00 (lH, br. s), 7.98 (lH, s)
Infrared absorption spectrum (nujol, cm 1)
168~, 1600, 1505, 1350, 1235~
Example 14 2-(4-Benzylpiperazino)-5,6-dihydro-7-ethyl-
.
6-oxo(7H)pyrrolo~2,3-d]pyrimidine
.
tIn the general formula [I], Y is [IV].)
In a pressure vessel was placed a mixture
consisting of 1.41 g (3.76 mM) of ethyl 2-(4-benzyl-
~ ~38~2~3
- 29 -
piperazino)-4-chloropyrimidine-5-acetate, 5 ml of ethylamine
and 20 ml of isopropanol. The mixture was heated for 2
hours at 120C. Then, the solvent was removed under reduced
pressure. Water was added and extraction by chloroform was
conducted. The organic layer was dried with MgSO4 and
concentrated. The residue was purified according to silica
gel column chromatography to obtain 0.58 g of the captioned
compound with an yield of 46%.
Melting point: 110-113C
Infrared absorption spectrum (CHCH3 solution,
cm
1725, 1620, 1570
H-NMR spectrum (CDC13 solution, ~ ppm)
1.25 (3H, t, J=7.0Hz~, 2.49 (4~, t, J=5.2Hz),
3.37 (2H, s), 3.54 (2H, s~, 3.75 ~2H, q,
J=7.2Hz), 3.83 (4H, t, J=5.2Hz), 7.31 (5H, s),
7.89 (lH, s)
Using methylamine or isopropylamine in place of
ethylamine, the following two compounds were obtained.
2~ Benzylpiperazlno)-5,6 dihydro-7-isoprop~l-
6-oxo(7H)pyrrolo[2,3-d]pyrimidine
(In the general formula [Il, Y is [IV].)
Yield: 36~
Melting point: 125-127C
lH-NMR spectrum (CDC13 solution, 6 ppm)
1.48 (6H, d, J=7Hz), 2.50 (4H, m)~ 3.36
(2H, s), 3.56 (2H, s), 3.82 (4H, m),
4.62 (lH, sept., J=7Hz), 7.33 (5H, m),
7.89 (lH, s)
Infrared absorption spectrum (KBr tablet, cm 1)
1730, 1620, 1580, 1~40, 1220, 1100
2-(4-Ben~L~e~erazino)-5,6-dihydro-7-methyl-6-
oxo(7H)p~rrolo[2~3-d]pyrimidine
(In the general formula [I], Y is [IV].)
Yield: 72%
Melting point: 172-174C
42~
- 30 -
H-NMR spectrum (CDC13 solution, 6 ppm)
2.50 (4H, m), 3.18 (3H, m), 3.40 (2H, s),
3.54 (2H, s), 3.84 (4H, m), 7.34 (5H, m),
7.90 (lH, s)
Infrared absorption spectrum (KBr tablet, cm 1)
1735, 1630, 1575, 1520, 1480, 1340i 1245
Example 15 5~6-Dihydro-7-ethyl-6-oxo-2-~iperazino(7H)
pyrrolo[2 ! 3-d]eyrimidine
(In the general formula lI], Y is [IV].)
0.54 g (1.60 mM) of 2-(4-benzylpiperazino)-5,6-
dihydro-7-ethyl-6-oxo(7H)pyrrolo[2,3-d]pyrimidine was sub-
jected to hydrogenation at normal pressure in the presence
of 0.1 g of 10% Pd-C in 15 ml of an ethanol solvent con-
taining 0.12 ml of formic acid. Refluxing was conducted for
- 15 4.5 hours. The catalyst was removed by filtration and
ethanol was distilled off under reduced pressure. An
aqueous sodium carbonate solution was added and extraction
by chloroform was conducted. The organic layer was dried
with MgSO4 and concentrated to obtain 0.36 g of the cap-
tioned compound as an oily product with an yield of 91%.
H-NMR spectrum (CDC13 solution, ~ ppm)
1.27 (3H, t, J-7.0Hz), 2.02 (lH, br. s),
2.92 (4H, t, J=5.2Hz1, 3.40 (2H, s), 3.80
(6H, m), 7.91 (lH, s)
In the similar manner, the following two compounds
were obtained.
5,6-Dihydro-7-isopropyl-6-oxo ~ lno(?H)-
rrolol2, ~
(In the general formula [I], Y is [IV~.)
Yield: 83%
Melting point: 113-115C
H-NMR spectrum (CdC13 solution, 6 ppm)
1~52 (6H, d, J=7Hz), 2.08 (1~, brs),
2.93 (4H, mj, 3.40 (2H, s), 3.80 (4H, m),
4.65 (lH, sept.~ J=7Hz), 7.47 (lH, s),
7.93 (1~, s)
.
3.~1~8df~9
31 -
Infrared absorption spectrum (neat, cm 1)
3330, 1725, 1622, 1575, 1440, 1220, 1110
5,6-Dihydro 7-methyl-6-oxo-2-piperazino(7H)-
pyrrolo[2,3-d]pyrimidine
(In the general formula [I], Y is lIV]-)
Yield: 70%
Melting point: 145-147C
H-NMR spectrum (CDC13 solution, ~ ppm)
1.86 (lH, brs), 2.94 (4H, m), 3.21
(3H, s), 3.43 ~2H, s), 3.82 (4H, m), 7.93
(lH, s)
Infrared absorption spectrum (KBr tablet, cm 1)
3340, 1738, 1630, 1S80, 1450, 1105
Example 16 8-Ethyl-5--oxo-2-piperazino-5,6,7,8-tetrahydro-
pyrido[2,3-d ~ idine
(In the general formula [I], Y is [IV].)
A mixture consisting of 2. g (11.3 mM) of
8-ethyl-5-oxo-2-methylthio-5,6,7,8-tetrahydropyrido-
[2,3-d~pyrimidine (synthesized according to the process
described in Japanese Patent Laid-open Publication NoO
18600/1978), 2.93 g (34.0 mM) of anhydrous piperazine and 20
ml of dimethylsulfoxide was heated at 120C for 3.5 hours
and successively at 140C for 6.5 hours. Dimethylsulfoxide
was distilled o~f under reduced pressure for concentration.
Water was added to the residue and extraction by chloroform
was conducted. The organic layer was dried with MgSO4 and
concentrated to obtain 3~08 g of the captioned compound as
an oily product with an yield of 90%.
lH-NMR spectrum (CDC13 solutlon, ~ ppm)
1.20 (3H, t, J=7.2Hz), 1.77 (lH, s)l 2.61
(2H, dd, J=6.6, 6.7Hz), 2.90 (4H, m), 3.59
(4H, m), 3.88 ~4H, m), 8.59 (lH, s)
Reference Example 9 6,9-Dimethyl-2-metllylthio 5-oxo-
5,6,7,8-tetrahydro(9H)pyrimido-
[4,5-e]diazepine
." ' ' ' .
- 32 -
A solution consisting of 50 ml of ethanol and 5.0
g (21.5 mMJ of ethyl 4-chloro-2-methylthio-pyrimidine-5-
carboxylate was added dropwise in 50 minutes to a mixture
consisting of 4.58 ml (43.0 mM) of N,N'-dimethylethylene-
diamine, 150 ml of ethanol and 2.51 g of Na2C03. Refluxingwas conducted for 13 hours. Ethanol was distilled off.
Water was added to the residue and extraction by chloroform
was conducted. The organic layer was dried and concen-
trated. The residue was purified according to silica gel
column chromatography to obtain 3.55 g of the captioned
compound with an yield of 69%.
Melting point: 153-155C
H-NMR spectrum (CDC13 solution, ~ ppm)
2.52 (3H, s), 3.13 (3H, s), 3.23 (3H, s),
3.63 (4H, AB quart.), 8.72 (lH, s)
Example 17 6,9-Dimethyl-2-(4-henzylFnE~a~:lno)-5-oxo-
5,6,7,8-tetrahydro(9H)pyrim do[4,5-e]diaze~ine
-- .. _ ~ __ _ _ ~11
(In the general formula [I], Y is [VIIl.)
1.3 g (5.46 mM) of 6,9-dimethyl-2-methylthio-5-
oxo-5,6,7,8-tetrahydro(9H)pyrimido[4,5-e]diazepine and 4 9
(22.69 mM) of l-benzylpiperazin were stirred at 140 to
170C for 28 hours. After the mixture had been cooled,
ethyl acetate was added thereto and the insolubles were
removed by filtration. The filtrate was concentrated and
purified according to column chromatography to obtain 0.2 g
of an reddish brown oil with an yield of 10%.
H-NMR spectrum (CDC13 solution, ~ ppm)
2.47 (4H, m), 3.11 (3H, s), 3.12 (3H, s),
3.55 (6H~ m), 3 84 (4H, m), 7.32 (SH, m),
8.72 (lH, s)
Example 18 6,9-Dimethyl-2~e~perazino 5-oxo-5,6,7,8-
tetrahydro(9H)pyrimido[4,5 e]diaz~ine
(In the general formula [I~, Y is [VII].)
In 10 ml of ethanol was dissolved 0.2 g (0.55 mM)
of 6,9-dimethyl-2-(4-benzylpiperazino)-5-oxo-5,6,7,8-
tetrahydro(9H)pyrimido[4,5-e]diazepine. Thereto was added
~.~88429
- 33 -
20 mg of 10% Pd-C, and refluxing was conducted for 2 hours
at normal pressure to effect hydrogenation. After the
mixture had been cooled to room temperature, the catalyst
was removed by filtration and the filtrate was subjected to
vacuum distillation. The residue was purified according to
column chromatography to obtain 0.1 g of a desired compound
with an yield of 67~.
H-NMR spectrum (CDC13 solution, ~ ppm)
3.59 (6H, s), 3~27 (4H, m), 3.62 (4H, brs),
4.20 (4H, m), 8.70 (lH, s)
Exam~le of comp ~ as her _ ide
Next, an example of compounding of a herbicide
using the present invention compound will be explained.
The figures herein indicate % by weight.
Example of compounding (wettable powder)
Present invention compound 10%
Sodium salt of a higher
alcohol sulfate 3
Raolin 87~
The above mixture was homogeneously blended and
ground to use as a wettable powder.
Test_f r the herbicidal effect when ap~lied before
germination
A garden soil was packed in a ceramic pot having
an inside diameter of 9 crn. Amaranthus retroflexus L. and
Cyperus Iria L. were sowed therein. Successively, a dis-
persion consisting of 20 liters of water and 300 g of a
wettable powder containing a particular compound of the
present invention was sprayed on the entire surface of the
soil from above the pot using a small sprayer. (20 liters
and 300 g are the amounts per one are of the soil area to be
sprayed.) After this spraying, the pot was placed in a
- greenhouse for 21 days to examine the herbicidal effect of
the compound. The following evaluation criterion was used.
Herbicidal effect
5 Withered completely.
8~9
4 Herbicidal effect is high.
3 Herbicidal effect is medium.
2 Herbicidal effect is low.
1 Herbicidal effect is very slight.
0 No effect ~normal).
The test results are shown in the following table.
Table
Test compound Amaranthus Cyperus
retroflexus L. Lria L.
Compound of Example 1 3 3
Compound of Example ~ 3 3
Compound of Example 4 4 3
Compound of Example 6 3 3
Compound of Example 9 3 4
Compound of Example 11 3 3
Compound of Example 1~ 4 3
Industrial Applicability
The present invention compounds have an excellent
herbicidal activity and can be used as a herbicide for
paddies and gardens. These compounds are particularly
effective for paddy weeds such as Echinochloa crus galli
L~ Beauv. gar crusgalli, Cyperus difformis Lo~ Monochoria
vaginalis Presl, Scirpus juncoides Roxb. var. Hotarui Ohwi
and Alisma canaliculatum A~ Br. et Bouche, as well as for
garden weeds such as Panicum Crus-galli L. var. frumentaceum
Trin., Digitaria adscendens Henr., Polygonum nodosum Pers.,
Amaranthus retroflexus L., Cyperus Iria L. and Chenopodium
album L. var. cPntrorubrum Makino. ~
~ In using as a herbicide, the present invention
compounds can be diluted to an appropriate concentration as
they are or after having been mixed with a carrier, a sur-
factant, a dispersing agent, an auxiliary chemical, etc. to
prepare a wettable powder, an emulsion, a granular, a fine
granular or the like and then can be sprayed or directly
applied.