Note: Descriptions are shown in the official language in which they were submitted.
~8~;~37
A process for removing gaseous sulfur compounds, such as sulfur
dioxide, from the flue gases of a furnace
The present invention relates to a process for removing gaseous
sulfur compounds, such as sulfur dioxide, from the flue gases of
a furnace which burns sulfur-containing fuel, coal or oil.
It is previously known to decrease the sulfur dioxide content of
the flue gases of a furnace by feeding calcium oxide, calcium
carbonate or some other alkaline compound into the combustion
chamber of the furnace. In a fluidized-bed furnace with a circul-
ating bed it is possible by means of a calcium addition to de-
crease the sulfur dioxide content of the flue gases by as much as
90 ~ when the furnace is operating within the temperature range
which is optimal for the chemical reactions, i.e. 800-1000 C.
The sulfur dioxide thus absorbed leaves the furnace in the form
of gypsum, along with the fly ash.
In other furnaces, in which it is necessary to use temperatures
higher than those mentioned above and in which the retention of
the additive is short due to the nature of the combustion, it is
expected that the decrease in the sulfur dioxide content of the
flue gases stays substantially lower, about 50 % or less, and
therefore this process has not been applied on an industrial
scale to such furnaces.
On the other hand, it is known that the sulfur dioxide content of
flue gases can be decreased by various absorption processes out-
side the furnace. One such process, known per se, is the so-
called spray or semidry process, in which the flue gas leaving
the furnace is led into a separate reactor, into which an aqueous
slurry of calcium hydroxide is sprayed in the form of small droplets through
specific nozzles. The reactor is typically a rather large vessel, in which
the ve]ocity of the flue gases is allowed to decrease and the aqueous slurry
is sprayed downwards from above, from the upper part of the vessel. The
temperature of the reactor is at this time about 50-80C, and the control
of the spraying of the aqueous slurry of calcium hydroxide is very important,
since drops which are too large will remain as liquid on the bottom of the
reactor. The thickness of the aqueous slurry of calcium hydroxide should be
maintained at such level that the heat content in the flue gases would
evaporate the water entering the reactor, so that adsorption product can be
recovered in the form of dry powder. By this process it is possible to
remove as much as 90 % of the sulfur dioxide. The disadvantages of the
process include the tendency of the nozzle to become clogged, an extra
preparing and batching apparatus for the aqueous slurry of calcium hydroxide,
which raises the investment costs, and problems of controlling the drop size
during the spraying.
The goal of the present invention is to provide a process for remov-
ing gaseous sulfur compounds, such as sulfur dioxide, from the flue gases of
a furnace, a process by which the gaseous sulfur compounds can be converted
to solid sulfur compounds which can easily be separated from the gases and
thereby effectively removed from the flue gases of the furnace in a simple
and economical manner.
According to the present invention there is provided a process for
removing a gaseous sulfur compound, from a furnace flue gas, which comprises
a) feeding a pulverous alkali metal oxide or alkaline earth metal or a
compound which converts to an oxide under the conditions in the furnace, into
a furnace in addition to the sulfur-containing flue gas to be burned and an
'7
-- 3 --
oxygen-containing gas, or feeding said oxide into the sulfur containing flue
gas which leaves the furnace,
b) separately spraying water or steam into the furnace or into the flue gas
to convert the oxide to a hydroxide which reacts with sulfur dioxide and
finally
c) separating a solid which contains alkali metal or alkaline earth metal
sulfate, obtained as a reaction product, from the gas.
Preferably the pulverous compound fed into the furnace is calcium
carbonate, calcium-magnesium carbonate or a corresponding oxide thereof.
In the process according to the present invention, a material which
reacts with gaseous sulfur compounds, and particularly with sulfur dioxide,
and water are fed into the process separately, whereby the problems of pre-
paring, handling and feeding in a slurry are avoided, in the following
manner:
a) a pulverous alkali metal oxide and/or alkaline earth metal oxide and/or a
respective compound which can be converted to oxide in the furnace, such as
carbonate, is fed into the furnace in addition to the sulfur-containing mater-
ial to be burned and an oxygen-containing gas, or the said oxide powder is
fed into the sulfur dioxide bearing flue gases which emerge from the furnace,
b) water and/or steam is fed separately into the furnace and/or into the flue
gases in order to convert the oxide to a hydroxide which reacts with the
sulfur dioxide, and finally
c) solid particles which contain the sulfate and possibly sulfite of the
alkali metal or the alkaline earth metal are separated from the gases.
The basic idea of the invention is thus that the calcium and
magnesium oxides which are inactive from the viewpoint of the removal of sulfur
dioxide are activated only in situ in the flue gases by means of water and/or
steam, whereby they are converted to the respective hydroxides and react with
~ 3,~ 3 ~
sulfur di.oxide, forming a solid sulfate/slllfite mixture which can thereafter
be removed effect;vely from the flue gases by physical separation methods.
A pulverous oxide and/or carbonate is fed into the combustion
chamber of the furnace i.n a~cordance with the sulfur content of the fuel in
such a way that the amount of alkali and/or alkaline earth metals is at least
the amount equivalent to the sulfur in the molar proportion according to the
reaction formula, but preferably higher than the amount required for the
reaction. By feeding the oxide and/or carbonate in the form of powder separa-
tely into the combustion chamber, or the oxide directly into the flue gas ducts,
it need not be fed in the form of a slurry through nozzles, whereby nozzle
clogging and the use of extra preparation and batching devices for the aqueous
slurry are
.
~ ~39337
omitted. On the contrary, the feeding of water and steam through
nozzles is uncomplicated and easy.
The feeding of water or steam into the flue gases is in practice
carried out at a temperature of 50-800 C, preferebly within the
temperature range 90-200 C. If it is desired to recover the
absorption product substantialLy in the form of dry powder, water
is used in only such an amount that the thermal energy and the
heat of reaction of the flue gases suffice to vaporize it, or a
small amount of energy introduced from outside the system is used
as an addition to the heat of reaction.
The invention is described below in greater detail with reference
to the accompanying drawing, which depicts diagrammatically an
apparatus suitable for carrying out the process according to the
present invention.
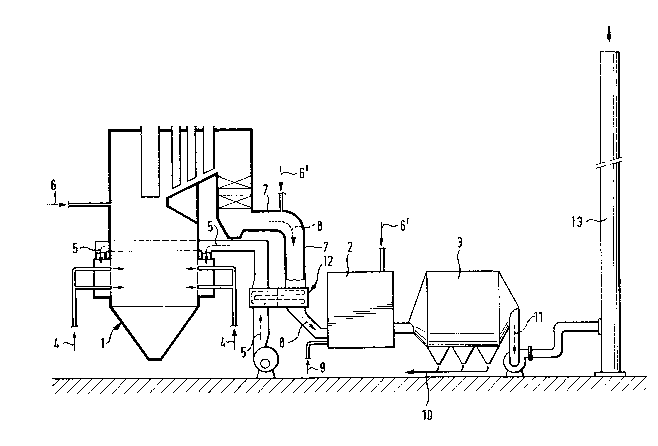
In the drawing, the furnace in general is indicated by reference
numeral 1. A sulfur-containing fuel for combusting 4, usually
preheated, an oxygen-bearing gas 5, and calcium and/or magnesium
oxide 6' and/or carbonate 6, preferebly in excess in proportion
to the amount of the sulfur dioxide gas forming in the combustion
chamber, are fed into the combustion chamber of the furnace 1. By
the expression "in excess" is meant in this context that the
amount of calcium, magnesium, or calcium and magnesium present in
the calcium and/or magnesium oxide and/or carbonate is greater
than would in theory, according to the reaction formula, be
required to react with all of the sulfur fed into the combustion
chamber.
The carbonate fed into the furnace breaks down in the furnace
into oxide and carbon dioxide. The oxide, for its part, can react
with the sulfur dioxide, forming first sulfite and thereafter,
upon oxidation, sulfate. Owing to the short retention time in the
. , .
~139~37
furnace, only part of the oxide has time to react with the sulfur
dioxide at a temperature sufficiently high for the reaction, and
for this reason calcium oxide bearing and/or magnesium oxide
bearing flue gases 8 which contain combustion residue and also
unabsorbed sulfur dioxide leave the combustion chamber of the
furnace through the flue gas conduit 7. Additionally or alterna-
tively, pulverous oxide 6' can be fed directly into the flue gas
conduit 7 or into a subsequent reactor 2.
In practice the temperature of the flue gases 8 is so low that
the reaction between the calcium and/or magnesium oxide and sulfur
dioxide is relatively weak, and the oxides can under these condi-
tions be regarded as relatively inactive in terms of sulfur re-
moval. However, the flue gases 8 can be used in the heat exchanger
12 to heat the air 5 fed into the furnace 1.
The calcium and/or magnesium oxide bearing and sulfur dioxide
containing flue gases 8 which emerge from the combustion chamber
of the furnace 1 are thereafter directed into a reactor, which is
generally indicated by reference numeral 2. In order to activate
the calcium and/or magnesium, water 9 or steam is sprayed into
the flue gases in the reactor 2, and this water or steam reacts
with the calcium and/or magnesium oxide, thereby forming the res-
pective hydroxide. The hydroxide for its part reacts with the re-
maining sulfur dioxide in the flue gases 8, thereby forming the
respective sulfite, which, in the presence of oxygen, at least in
part further oxidizes to the respective sulfate.
The amount of water 9 fed into the reactor 2 is adjusted to so
low a value that the heat of the flue gases 8 suffices to evapo-
rate the water fed into the reactor 2. Thereupon the dry, fly ash-
like reaction product can be removed, in the same manner as the
other fly ash, in a conventional fly ash separator 3, from which
the flue gases 11 are directed further into the flue 13 and the
, ' '.
37
separated fly ash 12 and reaction product are possibly directed
to a further treatment.
The order in which the water or steam and the pulverous oxide are
added is in no way critical. Thus, for example, the water or
steam can be fed into the furnace and the pulverous oxide only at
a point subsequent to the furnace, either into the flue gas con-
duit or into the subsequent reactor. The additional advantages of
the process according to the present invention include the fact
that the process can be applied to a furnace provided with any
type of burner. The size of the furnace is not a restricting
factor, and it is not necessary to circulate the calcium and/or
magnesium oxide in the combustion chamber, whereby the expensive
circulating-bed alternative with the complicated recirculating
devices and at the same time the excessive dust which is a dis-
advantage of the recirculating-bed alternative due to its prin-
ciple of operation, as well as the separation of the dust, are
avoided. Compared with the prior known spray process, the spray-
ing of water or steam into the reactor 2 is, furthermore, con-
siderably less complicated and easier to implement than when
using slurry which clogs the nozzles and is difficult to mix. It
is an additional advantage that the carbonate can be economically
burned in the combustion chamber of the furnace itself.
The invention is described below in greater detail with the aid
of examples.
Example l
Coal having a sulfur content of 1.4 ~ is fed at a rate of 70 t/h
into a pulverized-coal furnace having a thermal output of 600 MW,
the furnace being operated at full capacity. An excess of combus-
tion air is fed in , so that the oxygen content in the flue gases
is 4 ~. Calcium, which may be for example calcium carbonate,
dolomite or calcium oxide, is fed into the furnace. For example,
~1.?~ 37
calcium carbonate having a calcium carbonate content of 90 ~ is
fed into the furnace at a certain varying proportion to the
sulfur amount entering the furnace in the fuel. The theoretical
equivalent amount is about 3.4 t/h calcium carbonate.
The calcium carbonate decomposes
(1) CaCO ~ CaO + CO
3 2
in the furnace at a high temperature to calcium oxide and carbon
dioxide, which leave the furnace along with the flue gases. Part
of the calcium oxide in the furnace reacts with the oxides of
sulfur present in the flue gases, thereby forming calcium sulfate
or calsium sulfite.
(2) Cao + SO + 1/2 O ---> CaSO
2 2 4
or
CaO + SO ---> CaSO
CaS03 + 1/2 O ---> CaSO
Water and/or steam is sprayed into the flue gases either in the
furnace or in the flue gas conduit, or in a separate reactor sub-
sequent to the flue gas duct.
In terms of energy economy it is most economical to increase
the moisture content of the flue gases by spraying water into
them in a separate reactor, at a point after all heat recovery
surfaces.
The increased moisture content of the flue gases enables a highly
reactive calcium hydroxide to form in the furnace from the un-
reacted calcium oxide,
12~ 7
~3) Cao ~ H2O ---> Ca(OH)
Ca(OH) ~ SO ---~ CaSO + H O
2 2 3 2
which rapidly reacts with the oxides of sulfur present in the
flue gases. The higher the moisture content of the flue gases
upon their leaving, the more effectively the sulfur dioxide is
removed from t'ne flue gases. In terms of energy economy it is,
however, advantageous to proceed in such a manner that the heat
released in the chemical reactions suffices to evaporate the
water amount added. If it is desired to increase the final tempe-
rature of the flue gases, this is done either by using external
heat or by means of a warm flue gas flow.
It is essential that the compound arriving in the reaction zone,
derived from calcium carbonate or dolomite, is in the form of
oxide.
The results are presented in the table below, which shows in per-
cent how much sulfur dioxide was removed from the flue gases when
varying amounts of calcium carbonate were fed into the furnace in
accordance with the present invention, the amounts of the calcium
carbonate being reported as molar ratios of the calcium content
of the pulverous calcium carbonate to the sulfur content of the
fuel fed into the furnace. The temperatures of the flue gases
were measured at a point immediately prior to the feeding point
of the water or steam, except at 800 C, at which the water or
steam was fed directly into the furnace.
.
3~
Table 1
B)
Ca/S Flue gas Flue gas SO
temperature temperature reduction
S w
o A) o
0.48 800 C 108 C 42 %
0.52 50C 65C 56 ~
o o
1.52 202 C 74 C 77 ~
o o
1.56 90 C 68 C 82 %
2.20 200 C 72C 87 %
2.22 120C 62C 96 %
o o
2.3 110 C 68 C 93 %
o o
2.5 90 C 66 C 97 %
o o
4.1 800 C 110 C 72 %
o o
4.0 120 C 68 C 98 %
A) water or steam fed into the furnace
B) at a point immediately prior to the feeding point of water
Example 2
Dolomite which contained 45 % calcium carbonate (CaCO ), 45 %
magnesium carbonate (MgCO ) and 10 % impurities was fed into a
pulverized-coal furnace according to Example 1, using the same
operating values. On the basis of equivalence, the amount of
dolomite required in proportion to the sulfur amount fed in is
about 6.8 tn/h.
The calcium and magnesium carbonates contained in the dolomite
break down in the furnace into calcium oxide, magnesium oxide and
carbon dioxide, which leave the furnace along with the flue gases.
Part of the oxides in the furnace reacts with the oxides of
sulfur present in the flue gases, thereby forming sulfate or
sulfite.
Water and/or steam is sprayed into the flue gases either in the
furnace or in the flue gas duct, or in a separate reactor situated
39;~37
at a point subsequent to the flue conduit, whereupon the oxides
which have not reacted in the furnace can, due to the increased
moisture, ~orm hydroxide. The hydroxide for its part reacts with
the oxides of sulfur present in the flue gases, thereby forming a
pulverous reaction product.
When dolomite is used, the highly reactive calcium hydroxide
reacts before the reacting of the slower magnesium hydroxide,
which, when the calcium amount is sufficient, passes through the
reactor almost unreacted. By designing the process so as to be
carried out only on the basis of the calcium present in the dolo-
mite, the equivalent amount presented above is arrived at. When
the molar ratio of calcium to sulfur is at least 1, the results
of the process are substantially in compliance with the corres-
ponding values in Table 1.
Example 3
Calcium oxide which contains impurities 10 % is fed into a
furnace according to Example 1, using the corresponding operating
values. In terms of the reaction, the theoretical equivalent
amount of calcium oxide in proportion to the sulfur amount enter-
ing the furnace in the fuel is about 1.9 tn/h.
Part of the calcium oxide reacts in the furnace with the oxides
of sulfur present in the flue gases, thereby forming calcium
sulfate or sulfite.
Water and/or steam is spra~ed into the flue gases either in the
furnace or in the flue gas duct, or in a separate reactor located
at a point after the flue gas conduit.
Due to the increase in moisture, the calcium oxide forms highly
reactive calcium hydroxide, which reacts rapidly with the oxides
of sulfur still present in the flue gases. The higher the moisture
content in the flue gases upon leaving, the more effectively the
sulfur dioxide is removed from the flue gases. In terms of energy
economy it is, however, advantageous to operate in such a way that
the heat released in the chemical reaction will suffice to
evaporate the water amount added.
When the calcium fed in, in calcium oxide, is calculated in a
molar ratio to the sulfur, the results are in accordance with
those shown in Table 1 of Example 1.