Note: Descriptions are shown in the official language in which they were submitted.
h
MULTII~YEPc COATED CORROSION R~3SISTANT METAL PIPE
BACKGROUND OF THE INVEMTION
1. Field of the Invention:
This invention relates to a multilayer coated cor-
rosion resistant metal pipe, or a metal pipe having an outer
surface coated with a plurality of layers o corrosion resis-
tant materials. It i5 ~icularly concerned with a metal pipe
having a relatively small inside diameter not exceeding 20 mm.
BRIEF DESCRIPTION OF THE DRAWINGS
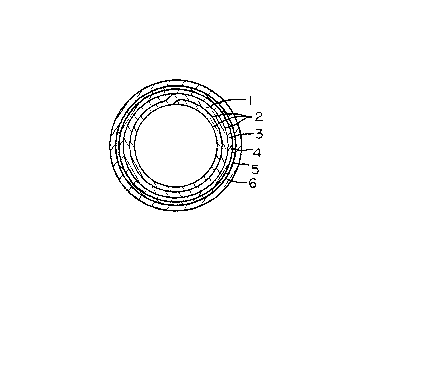
FIGURE 1 is a cross sectional view of a coated pipe
embodying this invention;
FIGURE 2 is a cross sectional view of a coated pipe
according to another embodiment of this invention; and
FIGURE 3 is a cross sectional view of a known coated pipe.
2. Descri~tion of the Prior Art:
1~ Metal pipes often have their outer surfaces covered
with protective coating. These pipes are used for making, for
example, pipelines for conveying a brake fluid and fuel in a
motor vehicle. These pipelines are locat~d under the body of
the vehicle. As they are used in such a bad environment, the
pipes are required to have high degrees of corrosion resis-
tance, scratch resistance, impact strength and mechanical wear
resistance. Spiked tires have come to be often used in the
cold season during which ice is likely to form on the road
surfacs. These tires, however, damage the road surface and
raise dust when it is not covered with ice. Rock salt is
sprinkled onto the road surface to prevent its freezing. A
yearly increase in the use of rock salt, however, is making
the problem of pipe corrosion more serious.
-- 1 --
,.. ..
2~S~
The pipes are also liable to damage or wear by stones or
mud spattered by the rotating wheels of the vehicle. It
is, therefore, necessary that the pipes be so coated as to
resist both chemical corrosion and mechanical damage or
wear.
Reference is made to FIGURE 3 showing ~y way of
example a known pipe of the type to which this invention
pertains. It comprises a double-rolled steel pipe 11 made
by rolling a steel strip or hoop twice and brazing its lon-
gitudinal edges by means of a copper plating layer 12, or
a seam welded steel pipe. The pipe 11 has an outer surface
coated with an electroplated zinc film 13. The film 13 has
an outer surface coated with a relatively thick special
chromate film 14 having an olive color. The chromate Silm
14 has an outer surface coated with a fluorinated resin film
16. Pipes of this kind are disclosed in, for example,
Japanese Patent Publications Nos. 60434jl982 and 23271/19~6.
The fluorinated resin film is formed by impregnat-
ing the chromate film with a dispersion of polyvinyl fluo-
ride immediately after the formation of the chromate film
when it ls still in the state of a gel, and drying them
under heat, so that~the fluorinated resin film may form an
intimate bond with the chromate film. When the chromate
film is formed by treating the pipe with a solution, it
requires large amounts of a chromium compound and an organic
acid, such as formic acid, used as a reducin~ a~ent. It
31.2~ L5~
is necessary to supply the treating solution with the
chromium compound, etc. frequently and yet renew it at
regular intervals of time in order to maintain a constant
film forming capacity. The waste solution, however, con-
tains a large amount of chro~ium having a valence of 6,
which is a toxic substance, and its disposal, therefore,
costs a great deal. Although the chromate film as formed
is highly resistant to corrosion, the heat to which it is
exposed during the formation of the resin film deprives it
of water and thereb~ makes it brittle. The plastic defor-
mation, such as bending or double flaring, of the pipe forms
in the chromate film fine cracks which lower its rustproof-
ing property.
SU~MARY O~? THE INVENTI ON
Under these circumstances, it is an object of this
invention to provide a multi~ayer coated corrosion resistant
metal pipe which can overcome the drawbacks of the prior art
as hereinabove pointed out and is highly resistant to chemical
corrosion, mechanical damage and wear.
This object is attained by a coated pipe which com-
prises a metal pipe having an outer surface coated with a
zinc film, a chromate film, an intermediate layer consisting
substantially of an epoxy resin, and a polyvinyl fluoride
film formed one upon another ln the order mentioned.
The coated pipe of this invention is particularly
characterized in that the intermediate layer consistincJ
-- 3 --
~2~
of an epoxy resin is pxesent between the chromate film
and the polyvinyl fluoride fllm.
Other features and advantages of this invention
will be apparent from the following description and the
accompanying drawings.
DETAILED DESCRIPTION OF THE INV~;NTION
A multilayer coated pipe embodying this invention
is sho~n in FIGURE 1. It comprises a metal pipe 1. More
specifically, it is a double-rolled steel pipe formed by
la rolling a steel hoop twice and brazing its longitudinal
edges and other mutually contacting surfaces by means of a
copper plating layer 2. The pipe has a relatively small
inside diameter not exceeding 20 mm. A copper plating
layer 2 is also provided on the outer surface of the pipe
1 ana has a thickness of, say, three microns.
The metal pipe 1 may alternatively be a seam welded
steel pipe, or a seamless steel pipe made by drawing and
having an appropriate diameter. Moreover, the pipe 1 does
not necessarily need to ~e of steel, but may also be of
-- 4 --
A
~LZ9;~
other material, such as aluminum or copper. A modified
form of pipe is shown by way of example in ~IGURE 2.
~ he outer surface of the metal pipe I is coated
wi-th a zinc film 3. ~he zinc film 3 can be formed by a
known electroplating method employing an acidic electrolyte
containing sulfuric acid or an alkaline electrolyte contain-
ing zinc cyanide. It has a thickness of ~ to 35 microns~
A zinc film having a thickness below five microns wou]d
fail to provide satisfactory corrosion resistance, while
a zinc film having a thickness over 35 microns would peel
off the pipe when it is bent.
A chromate film 4 covers the zinc film 3. It is
usually a film or chromate having a yellow color and is
formed by using an ordinary solution containing chromic
acid at a low concentration immediately after the formation
of the zinc film 3. The film 4 may alternatively of
chromate havlng an olive color if the pipe is of the type
which will not be sub3ected to plastic deformation.
An intermediate layer 5 consisting substantially
of an epoxy resin covers the chromate film 4, the epoxy
resin of which being one member selected from bisphenol-
A type epoxy resin, bisphenol-F type epoxy resin, bisphenol-
AD type epoxy resin, phenol novolac based epoxy resin, cresol
novolac based epo~y resin, brominated bisphenol-A -type epoxy
resin and/or polyglycol epoxy resin and having a viscosity of
10 to 60 seconds (E`ord cup #4) at a temperature of 25 ~ 5C,
-- 5 --
~9;~
being capable of baking at a temperature OI 2000 to 700C
for 10 to 25 seconds and having a hardness of formed film
of B to 4H (pencil). ~he intermediate layer 5 is formed by
dipping the chromate film 4 in the above-mentioned epoxy
resin or spraying the epoxy resin onto t;he chromate film
4, and is dried under heat.
~he intermediate layer 5 has a thickness of 1 to 20 microns.
~Z~54
~ layer having a thickness below one micron would fail to
adhere to the chromate film 4, while a layer having a thick-
ness over 20 microns would crack, or be separated from a
polyvinyl fluoride film which will hereinafter be described
in detail. The intermediate layer 5 having an appropriate
thickness forms a strong bond with both the underlying
chromate film 4 and the overlying resin film.
The polyvinyl fluoride film 6 covers the intermedi-
ate layer 5. It is formed by employing a solution prepared
by dispersing polyvinyl fluoride in a high-boiling solvent,
such as dimethyl or diethylene phthalate, until no solid
fluoride can be found. The intermediate layer is dipped
in the solution, or the solution is sprayed onto the inter-
mediate layer, and the solution covering the layer lS dried
under heat at a temperature up to, say, 350C. The poly-
vinyl fluoride film 6 has a thickness of 5 to 50 microns.
A polyvinyl fluoride film having a thickness below five
microns would fail to provide satisfactory corrosion resist-
ance, while a film having a thickness over 50 microns would
not be more effective than any film having a thickness of
50 microns or below, but would only be more costly.
The films or layers 3 to 6 shown in FIGURE 2 are
identical to their counterparts in FIGURE 1 which have
hereinabove been described. No further description of
the coated pipe shown in FIGURE 2 is, therefore, made.
The intermediate layer bonds -the chromate film and
~2~2~S4
the polyvinyl fluoride film so strongly that neither of
these films may crack or peel away when the pipe is bent
or otherwise deformed. The layer also adds to the corro-
sion resistance of the pipe. The polyvinyl fluoride film
S is a very good protective film because of its excellency
in mechanical strength, and heat, weather and chemical
resistances. The coated pipe of this invention is, there-
fore, very suitable for use in a highly corrosive and abra-
sive environment, such as under the bottom of a motor vehicle.
This invention is also valuable as it facilitates the control
of the solution which is used to form the chromate film.
The invention will now be described more specifically
with reference to a number of examples.
EXAMP~E 1
Five coated pipes were prepared in accordance with
the following procedure:
(1~ Metal pipe:
Each pipe was a double-rolled steel pipe formed
from a steel hoop coated with a copper layer having a thick-
ness of three microns, and having an outside diameter of
4.76 mm and a wall thickness of 0.7 mm;
(2) Formation of a zinc film:
An electroplated zinc film having a thickness of
25 microns was formed on the outer surface of the steel pipe
by employing an acidic electrolyte consisting mainly of
zinc sulfate and containing an organic additive, and apply-
~2~ 5~
ing an electric current at a density of 60 A/dm2 at a
temperature of 55C to 60C;
(3) Formation of a chromate film~
A yellow chromate film was formed on the zinc film
by employing a chromating solution containing chromium
having a valence of 6 at as low a concentration as 1 g per
liter;
(4) Formation of an intermediate layer:
The steel pipe was, then, dipped in a paint which
had been prepared by dissolving an epoxy resin and a pigment
in a solvent, and the paint on the pipe was heated at a
temperature of 300C for 60 seconds, whereby an intermediate
epoxy resin layer having a thickness of five microns was
formed on the chromate film; and
(5) Formation of a polyvinyl fluoride film:
Then, the pipe was dipped in a solution which had
been prepared by dispersing polyvinyl fluoride in diethyl
phthalate, and the soIution on the pipe was dried by heat-
ing at a temperature of 350C for 60 seconds, whereby a
polyvinyl~fluoride film having a thickness of 15 microns
was formed on the intermediate layer.
Each coated pipe was tested for corrosion resistance.
A test specimen having a length of 300 mm was prepared from
each coated pipe. A plurality of cuts spaced apart from
one another by a distance of 10 mm were formed by a knife
in the coating on each speclmen, so that -they might reach
~9~
the steel pipe. A salt solution spray test was conducted
on the specimen in accordance with the method as specified
by JIS Z2371. The salt solution caused a strlpe to appear
along each cut as a result of the corrosion and discolora-
tion of the chromate or zinc film. Time was counted until
the stripes which had appeared along every two adjoining
cuts and gradually spread eventually joined each other. The
results are shown in a table below.
EXAMPLE 2
Five coated pipes were prepared in accordance with
the following procedure:
(1) Metal pipe:
Each pipe was a seam welded steel pipe formed from
the same steel hoop as that used in EXAMP~E 1, and having
an outside diameter of 6.35 mm and a wall thickness of 0.7
mm;
(2) Formation of a zinc film:
The procedure of EXAMPLE 1 was repeated for forming
an electroplated zinc film having a thickness of 25 microns
on the outer surface of the steel pipe;
(3) Formation of a chromate film:
A yellow chromate film was formed on the æinc film
by employing a chromating solution containing chromium hav-
ing a valence of 6 at a concentration of 5 g per liter;
(4) Formation of an intermediate layer:
The same paint as that used in EXAMPLE 1 was sprayed
_ g _
onto the chromate film, and was heated at a temperature
of 320C for 60 seconds, whereby an intermediate epoxy resin
layer having a thickness of five microns was formed on the
chromate film; and
(5) Formation of a polyvinyl fluoride film:
The same dispersion of polyvinyl fluoride as that
used in EXAMPLE 1 was sprayed onto the intermediate layer,
and was dried by heating at a temperature of 380C for 65
seconds, whereby a polyvinyl fluoride film having a thick-
ness of 15 microns was formed on the intermediate layer and
a coated pipe having a cross section as shown in FIGURE 2
was obtained.
The procedure of EXAMPLE 1 was repeated for conduct-
ing a corrosion resistance test on each coated pipe. The
results are shown in the table below.
COMPARATIVE EX~PLE
Five coated pipes were prepared in accordance with
the following procedure:
(1) Metal pipe:
. 20 Each pipe was a double-rolled steel pipe duplicat-
ing each pipe that had been used in EXAMPLE 1;
(2) Zinc film-
The procedure of EXAMPLE 1 was repeated for forming
an electroplated zinc film having a thickness of 25 microns
on the outer surface of the steel pipe;
. (3) Chromate film:
, - 10 -
~2~
The steep pipe was dipped for 20 seconds in a
chromating solution containing chromium having a valence
of 6 at a concentration of 13 g per liter and further
containing formic acid and acetic acid, whereby a chromate
film having an olive color was formed on the zinc film; and
(4) Polyvinyl fluoride film:
The pipe which had been coated so far was washed
with water and air was jetted against it to remove water
from it. Then, the procedure of EXAMPLE 1 was repeated
for forming a polyvinyl fluoride film on the chromate film.
The procedure of EXAMPLE l was repeated for conduct-
ing a corrosion resistance test on each coated pipe. The
results are shown in the table below.
TABLE
Time elapsed before merger of stripes
along two adjoining knife cuts (hours)
Specimen COMPARATIVE
No. EXAMPLE l EXAMPLE 2 EXAMPLE
1 1104 1080 504
2~ 2 1032 1104 600
3 1176 1224 57~
4 1008 1080 480
1104 1008 504
As is obvious from the table, the coated pipes
embodying this invention were by far superior to the known
coated pipe in corrosion resistance.