Note: Descriptions are shown in the official language in which they were submitted.
- 12~Z987
RAN 4430/18
The present invention is concerned with pyrimidine
aerivatives, a process for their manufacture and medicaments
containing said derivatives.
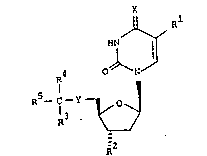
These pyrimidine derivatives have the general formula
X
.J~,
R4 N
¦
R5- C -Y
-
wherein R is halogen, Cl 4-alkyl or halo-(Cl ~-
-alkyl), R i8 hydrogen, hydroxy or acyloxy, R and
R4 each are hydrogen or Cl 4-alkyl, R5 is aryl or
aryloxy, X is 0 or NH and Y is -CO-CH2-,
-CH(OH)-CH2~, -CH2-CH2-, -S-, -SO- or -S02-,
and tautomers thereof.
As used therein, "Cl 4-alkylll means a straight- or
branched-chain alkyl group, such as methyl, ethyl, propyl,
isopropyl, butyl or t-butyl etc. "Halo-(Cl 4-alkyl)" means
an alkyl group as defined earlier carrying one or more
halogen atoms; e.g. trifluoromethyl or 2-chloroethyl. The
acyloxy group can be derived from an aliphatic, cycloali-
phatic, araliphatic or aromatic carboxylic acid, examples of
such acids being formic acid, acetic acid, propionic acid,
butyric acid, cyclopentylpropionic acid, phenylacetic acid
Mé/18.6.87
lZ9Z~?87
-- 2
and benzoic acid. Preferred acyloxy groups are Cl 4-
-alkanoyloxy group. ~Aryl" means unsubstituted phenyl group
or a phenyl carrying one or more substituents selected from
halogen, hydroxy, Cl 4-alkyl, Cl 4-alkoxy, trifluoro-
methyl, nitro and phenyl. Examples of such substituted-
-phenyl groups are 2-chlorophenyl, 2,4- or 2,6-dichloro-
phenyl, 2-methylphenyl and 2,6-dimethylphenyl. "Aryloxy"
means an aryl group as defined above which is bonded via an
oxygen atom. Examples of aryloxy groups are phenoxy,
2-chlorophenoxy and 2,4-dichlorophenoxy. "Halogen" means
fluorine, chlorine, bromine or iodine.
The compounds of formula I and their tautomers in which
Y represents -CH(OH)-CHz- or in which R and R have
different meanings,-i.e. when R3 represents a hydrogen
atom and R represents a Cl 4-alkyl group or when R
and R represent different Cl 4-alkyl groups, contain an
agymmetric carbon atom and can accordingly exist as
diastereoisomers. The pre8ent invention embraces within its
8cope not only the individual diastereoisomers, but also
mixtures thereof.
In formula I above R preferably is Cl 4-alkyl
especially ethyl. R preferably is hydroxy or Cl 4-
-alkanoyloxy, especially hydroxy or acetoxy. R3 and R4
each preferably are hydrogen. R5 preferably is dihalo-
phenyl, especially 2,6-dichlorophenyl. X preferably is 0. Y
preferably is -CO-CH2-. -CH(OH)-CH2-, -CH2-CH2- or
-S02-, especially -CO-CH2- or -CH(OH)-CH2-.
Especially preferred compounds of the invention are
those in which R is ethyl, R is hydroxy or acetoxy,
R3 and R4 each are hydrogen, R5 is 2,6-dichlorophenyl,
X is 0 and Y is -CO-CH2- or -CH(OH)-CH2-.
Particularly preferred compounds are:
lZ9Z987
3'-O-Acetyl-5'-[3-(2,6-dichlorophenyl)-2-oxopropyl]-
-2~,5~-dideoxy-5-ethyluridine,
5'-[3-(2,6-dichlorophenyl)-2-oxopropyl]-2~,5~-dideoxy-
5-ethyluridine and
55'-~3-(2,6-dichlorophenyl)-2(RS)-hydroxypropyl]-21,5l-
-dideoxy-5-ethyluridine.
Other preferred compounds provided by the present
invention are:
3'-O-Acetyl-2',5'-dideoxy-5-ethyl-5'-[3-(2,6-dimethyl-
phenyl)-2-oxopropyl]uridine,
3'-O-acetyl-2',5'-dideoxy-5-ethyl-5'-[3-(2-methyl-
phenyl)-2-oxopropyl]uridine,
153'-O-acetyl-5'-[3-(2-chlorophenyl)-2-oxopropyl]-2',5'-
-dideoxy-5-ethyluridine,
3'-O-acetyl-Z',5'-dideoxy-5-ethyl-5'-(2-oxo-3-phenyl-
propyl)uridine,
3'-O-acetyl-2',5'-dideoxy-5-ethyl-5'-t2-oxo-3(R5)-
-phenylbutyl]uridine~
2',5'-dideoxy-5-ethyl-5'-(2-oxo-3-phenylpropyl)uridine,
2',5'-dideoxy-5-ethyl-5'-[3-(2-methylphenyl)-2-oxo-
propyl]uridine,
2l,5l-dideoxy-5-ethyl-5l-[3-(2,6-dimethylphenyl)-2-oxo-
propyl]uridine~
5'-[3-(2-chlorophenyl)-2-oxopropyl]-2',5'-dideoxy-5-
-ethyluridine,
2',5'-dideoxy-5-ethyl-5'-t2-oxo-3(RS)-phenylbutyl]-
uridine,
302',5'-dideoxy-5 ethyl-5'-t2(RS)-hydroxy-3-phenylpropyl]-
uridine,.
2',5'-dideoxy-5-ethyl-5'-t2(RS)-hydroxy-3-(2-methyl-
phenyl)propyl]uridine,
5'-[3-(2~chlorophenyl)-2(RS)-hydroxypropyl]-2',5'-
-dideoxy-5-ethyluridine,
2',5'-dideoxy-5-ethyl-5'-t2(RS)-hydroxy-3-(Z,6-dimethyl-
phenyl)propyl]uridine,
~Z9Z987
-- 4
2',5'-dideoxy-5-ethyl-5'-(3-phenylpropyl)uridine and
5'-benzylsulphonyl-2',5l-dideoxy-5-ethyluridine.
Other interestinq compound~ are:
2',5l-Dideoxy-5'-t3-(2,6-dimethylphenyl)-2-oxopropyl]-
uridine,
3'-0-acetyl-5'-t3(RS)-(2,4-dichlorophenoxy)-2-oxobutyl]-
2',5'-dideoxy-5-ethyluridine,
5~-t3(RS)-(2,4-dichlorophenoxy)-2-oxobutyl]-2',5'-
-dideoxy-5-ethyluridine,
5~-[3(RS)-(2,4-dichlorophenoxy)-2(RS)-hydroxybutyl]-
-2',5'-dideoxy-5-ethyluridine,
5l-benzylthio-2l,5'-dedeoxy-5-ethyluridine,
5'-(2-chlorobenzylthio)-2',5'-dideoxy-5-ethyluridine,
5'-(2,4-dichlorobenzylthio)-2',~'-dideoxy-5-ethyluri-
dine,
5'-(2,6-dichlorobenzylthio)-2',5'-dideoxy-5-ethyluri-
dine,
5'-(2-chlorobenzyl~ulphonyl)-2l,5~-dideoxy-5-ethyluri-
dine,
5'-(2,4-dichlorobenzylsulphonyl)-2',5'-dideoxy-5-ethyl-
uridine and
5'-(2,6-dichlorobenzylsulphonyl)-2',5'-dideoxy-5-ethyl-
uridine
According to the process provided by the present
invention, the compounds of formula I above and their tauto-
mers are manufactured by
(a) for the manufacture of a compound of formula I or a
tautomer thereof in which R i~ hydrogen or acyloxy and Y
is -CO-CH2-, catalytically hydrogenating a compound of the
formula
12~Z98~
-- 5
X
-J~,
HN
R4 0 o N
5 1 ll (E)
R - C- C- CH= CH ~ II
~Y
wherein Rl R3 R4 R5 d X h h
significance and R2 is hydrogen or acyloxy,
or a tautomer thereof, or
(b) for the manufacture of a compound of formula I or a
tautomer thereof in which R is hydrogen or acyloxy and Y
i8 -CH(OH)-CH2-, reducing a compound of formula I or a
tautomer thereof in which R2 is hydrogen or acyloxy and Y
is -CO~CH2-, with a complex metal hydride, or
(c) for the manufacture of a compound of formula I or a
tautomer thereof in which R2 is hydrogen or acyloxy and Y
is -CH2-CH2-, replacing the hydroxy group in a compound
of formula I or a tautomer thereof in which R2 i8 hydrogen
or acyloxy and Y is -CH(OH)-CH2- by hydrogen, or
td) for the manufacture of a compound of formula I or a
tautomer thereof in which Y is -S-, reacting a compound of
the formula
~Z9Z987
-- 6
X
J~
HN
O~N ~
R 52 ~ Ill
l 2
wherein R , R and X have the above 8 ignificance and
R is Cl 4-alkyl or aryl,
or a tautomer thereof with an alkali metal derivative of a
compound of the general formula
HSC(R3~R ,R ) IV
3 4 5
wherein R , R and R have the above significance,
at an elevated temperature, or
(e) for the manufacture of a compound of formula I or a
tautomer thereof in which Y is -SO- or -SO2-, oxidizing a
compound of formula I or a tautomer thereof in which Y is
-S-, or
(f) for the manufacture of a com~ound of formula I or a
tautomer thereof in which R i6 hydroxy, deacylating a
compound of formula I or a tautomer thereof in which R2 is
acyloxy.
The catalytic hydrogenation in accordance with embodi-
ment (a) of the proceæs can be carried out in a manner known
per se, e.g. in an inert organic golvent, such as an
alkanol, e.g. methanol or ethanol, using a noble-metal cata-
lyst, such as a palladium or platinum catalyst, which may be
supported on an inert carrier material. Palladium-on-carbon
(Pd/C) is the preferred catalyst. Conveniently, the cataly-
~Z~Z987
-- 7
tic hydrogenation is carried out at about room temperatureand under atmospheric pressure.
The reduction in accordance with embodiment (b) of the
proces~ can be carried out in a manner known per se, e.g. by
treatment with sodium borohydride or potassium borohydride
or, when R is hydrogen, also with lithium borohydride or
an alkali metal aluminium hydride, such as lithium aluminium
hydride. This treatment is conveniently carried out in an
inert organic solvent and at room temperature to the reflux
temperature of the mixture, preferably at about room tempe-
rature. When the reduction is carried out using an alkali
metal borohydride, suitable solvents are alkanols, e.g.
methanol or ethanol, aliphalic ethers, e.g. diethyl ether or
dimethoxyethane, and cyclic ethers, e.g. tetrahydrofuran and
dioxan. Suitable solvent6 which can be used when the reduc-
tion i8 carried out using an alkali metal aluminium hydride
are aliphatic and cyclic ethers such as those mentioned
earlier.
The replacement of hydroxy by hydrogen in accordance
with embodiment (c) of the process can also be carried out
in a manner known per se. For example, the compound of
formula I or a tautomer thereof can firstly be converted
into the corresponding sulphonic acid ester, such as the
mesylate, by treatment with a sulphonic acid halide, such as
methanesulphonyl chloride, conveniently in the presence of
an acid binding agent, especially a tertiary amine, such as
pyridine, and at a low temperature, e.g. about 0C. The
obtained sulphonic acid ester can then be converted into the
corresponding iodide, e.g. by treatment with an alkali metal
iodide, such as sodium iodide, in acetone at an elevated
temperature, preferably at the reflux temperature of the
mixture. The resulting iodide can then be converted into the
desired compound of formula I or a tautomer thereof in which
Y represents -CH2-CH2- by catalytic hydrogenation in a
lZ~Z~87
-- 8
known manner, e.g. using a palladium on barium sulphate
catalyst.
In the reaction of a compound of formula III or a tauto-
mer thereof with an alkali metal derivative, preferably the~odium derivative, of a compound of formula IV in accordance
with embodiment (d) of the process the R -S03- group is
displaced by the -SC(R3,R4,R5) group. The reaction is
conveniently carried out in the presence of an inert organic
solvent such, as dimethylformamide, and at about 100C. The
alkali metal derivative is expediently formed in situ from
the compound of formula IV and an alkali metal hydride, such
as sodium hydride.
15 The oxidation in accordance with embodiment (e) of the
process can also be carried out in a manner known per se.
For example, a compound of formula III or a tautomer thereof
is treated with an organic peracid, such a~ peracetic,
perbenzoic, m-chloroperbenzoic or perphthalic acid, expe-
diently in a suitable solvent, such as a halogenated hydro-
carbon, e.g. chloroform, or an alkanoic acid, e.g. acetic
acid and at a temperature between about 0C and room tempe-
rature. When peracetic acid is used for the oxidation, this
can conveniently be prepared in situ from glacial acetic
acid and hydrogen peroxide. When 1 equivalent of an organic
peracid is used there is obtained a compound of formula I or
a tautomer thereof in which Y represents -S0-, whereas the
use of 2 equivalents of organic peracid leads to a compound
of formula I or a tautomer thereof in which Y represents
30 ~ SO2 ~ .
The deacylation in accordance with embodiment (f) of the
process can be carried out in a manner known per se, e.g. by
treatment with an alkali metal Cl 4-alkoxide, such as
sodium methoxide, in a Cl 4-alkanol, such as methanol.
Conveniently, this treatment is carried out at about room
tempe~ature, although it may be carried out at an elevated
lZ92987
temperature if desired.
The compounds of formula II and tautomers thereof which
are used as starting materials in embodiment (a) of the
present process are novel and also form an object of the
present invention. They can be prepared, for example, by
firstly reacting a compound of the general formula
ClCHzCOC(R3,R ,R ) V
wherein R , R and R have the above significance,
with a triarylphosphine, preferably triphenylphosphine,
conveniently in an inert organic solvent, such as a halo-
genated hydrocarbon, e.g. chloroform, and at an elevated
temperature, suitably at the reflux temperature of the
reaction mixture, to give a phosphonium chloride of the
general formula
Cl ~(R )3PCH2COC(R ,R ,R )~ VI
wherein R , R and R have the above significance,
and R6 is aryl.
The phosphonium chloride of formula VI is then treated
with a strong inorganic base, such as an alkali metal
hydride, e.g. sodium hydride, or an alkali metal hydroxide,
e.g. sodium hydroxide, and the resulting phosphorane of the
general formula
(R )3P-CHCOC(R ,R ,R ) VII
wherein R3, R4, R5 and R6 have the above 8igni-
ficance,
is finally reacted with a compound of the general formula
12~Z~87
-- 10 --
x
,1~,
~ 11
O N ~
OHC 1 VIII
R2 '
wherein R , R and X have the above significance,
or a tautomer thereof under the conditions of a Wittig
reaction to give a compound of formula II.
The compounds of formula V, which are required for the
preparation of the compounds of formula II and their tauto-
mers, are known compounds or analogues of known compounds
which can be prepared in a similar manner to the known
compounds. The compound~ of formula III and their tautomers,
which are used as starting materials in embodiment (d) of
the present process are known compounds or analogues of
known compounds which can be prepared in a similar manner to
the known compounds.
The compounds of formula I and their tautomers possess
antiviral activity and can be u~ed in the control or preven-
tion of viral infections, for example of herpes simplex
viral infections. The in vitro activity of these compounds
in inhibiting herpes simplex virus type 2 (HSV-2) thymidine
kinase can be demonstrated by means of the following test
procedure:
The assay mixture contains 50 mM Tris-HCl, pH 8, 5 mM
magnesium chloride, 5 mM ATP, 0.3 ~M 3H-thymidine
(50 Ci/mmol), suitably diluted thymidine kinase extract and
various concentrations of the test compounds in a total
volume of lOO ~l. Assays are incubated at 37C for 30
l~Z987
-- 11 --
minutes and the reaction i6 terminated by immersion in a
boiling water bath for 2 minutes. ~5 ~1 aliquots from each
assay are then dried on cellulose paper discs and the
unphosphorylated H-thymidine i6 removed by washing in
4 mM ammonium formate. The radioactivity remaining bound to
the di6cs i8 then measured by scintillation 6pectrophoto-
metry. The degree of inhibition at each concentration of the
test compound is expressed as a percentage of a control
reaction. The IC50 value, namely the concentration of the
test compound which inhibit6 enzyme activity by S0%, is then
calculated. The results obtained with representative com-
pound~ of formula I are compiled in the following Table:
Table
Compound of Example No. IC50 (~M)
... . .
3 0.0024
0.016
7 0.17
g 0.6
14c 0.072
The compounds of formula I and their tautomers can be
used as medicaments in the form of pharmaceutical prepara-
tions which contain them in a6sociation with a compatiblepharmaceutical carrier material. This can be an organic or
inorganic carrier 6uitable fo~ entera~, e.g. oral, or
parenteral administration. Examples of such carriers are
water, gelatin, gum arabic, lactose, starch, magnesium
stearate, talc, vegetable oils, polyalkylene glycols and
petroleum jelly. The pharmaceutical preparations can be made
up in a solid form, e.g. as tablets, dragees, suppositories
-- lZ~2987
- 12 -
or capsules, or in a liquid form, e.g. as solutions, sus-
pensions or emulsions; they may be subjected to standard
pharmaceutical operations, e.g. sterilization and/or may
contain adjuvants, e.g. preserving, stabilizing, wetting or
emulsifying agents, salts for varying the osmotic pressure
or buffers. They may also contain other therapeutically
valuable substance~.
The compound~ of formula I and their tautomers can be
administered to adults in a daily dosage of from about 1 to
1000 mg, preferably about 5 to 500 mg. The daily dosage may
be administered as a single dose or in divided doses. The
above do6age range is given by way of example only and can
be varied upwards or downwards depending on factors such as
the particular compound being administered, the route of
administration, the severity of the indication being treated
and the condition of the patient.
~xamDle
A solution of 3.30 g of (E)-3'-0-acetyl-5' -t3-(2,6-
-dichlorophenyl)-2-oxopropylidene]-2l,5l -dideoxy-5-ethyl-
uridine in 1.50 1 of methanol was hydrogenated over 1.10 g
of 10~ Pd/C catalyst at room temperature and under atmos-
pheric pressure for 3 hours. The mixture was filtered andthe filtrate was evaporated. The residue was triturated with
diethyl ether to give 2.45 g of 3'-0-acetyl-5~-[3-(Z,6-
-dichlorophenyl)-2 -oxopropyl]-2',5'-dideoxy-5-ethyluridine,
mp 186.
The starting material was prepared as follows:
(A) 50 ml of oxalyl chloride and 0.5 ml of dimethylformamide
were added to a stirred suspension of 5.12 g of (2,6-di-
chlorophenyl)acetic acid ln 120 ml of toluene. The mixturewas stirred at room temperature for 2.5 hourc and then
evaporated to dryness. The residue was suspended in 40 ml of
l~Z987
diethyl ether and the suspension was added gradually to
250 ml of a 0.25M solution of diazomethane in diethyl ether.
The mixture was stirred at room temperature for 2 hours and
then cooled to oC. Hydrogen chloride was then bubbled
through the mixture for 10 minutes. 300 ml of water were
added to the mixture and the phases were separated. The
organic phase was washed with 200 ml of saturated sodium
hydrogen carbonate solution and 300 ml of water, dried over
anhydrous sodium sulphate and evaporated to give 6.04 g of
1-chloro-3-(2,6-dichlorophenyl)-2-propanone in form of a
white solid. This solid was taken up in 21 ml of chloroform,
7.19 g of triphenylphosphine were added and the solution was
stirred and heated under reflux for 6 hours. The mixture was
cooled and poured into 200 ml of diethyl ether. The resul-
ting precipitate was collected, washed with diethyl ether
and dried to give 8.605 g of t3-(2.6-dichlorophenyl)-2-oxo-
propyl]triphenylphosphonium chloride in the form of a white
solid. Thi~ solid was taken up in 1,5 1 of warm water and
the mixture wa~ filtered. The filtrate was stirred while
12.5 ml of 5% sodium hydroxide solution were added. The
mixture was extracted twice with 600 ml of diethyl ether
each time and the combined extracts were washed with 1 1 of
water, dried over anhydrous sodium sulphate and evaporated.
The residue was recrystallized from 150 ml of diethyl ether
and yielded 4.286 g of t3-(2,6-dichlorophenyl)-2 -oxopropy-
lidene]triphenylphorphorane of melting point 98-100C.
(B) A solution of 2.759 g of 3'-O-acetyl-2'-deoxy-5-ethyl-
uridine, 5.75 g of dicyclohexylcarbodiimide and 0.375 ml of
dichloroacetic acid in 24 ml of dimethyl sulphoxide was
s~irred at room temperature for 27 hours. 0.375 ml of
pyridine and 4.286 g of [3-~2,6 -dichlorophenyl)-Z-oxo-
propylidene]triphenylphosphorane were added and the mixture
was stirred for a further 23 hours. The mixture was filtered
and the filtrate was evaporated. The residue was dissolved
in 100 ml of ethyl acetate and the solution was washed twice
with 100 ml of water each time, dried over anhydrous sodium
~Z~Z987
- 14 -
sulphate and evaporated. The resulting gum was subjected to
flash chromatography on a column of silica gel using ethyl
acetate/hexane (2:13 for the elution. There were obtained
3.84 g of (E)-3'-O-acetyl-5'-[3-(2,6-dichlorophenyl)-2
-oxopropylidene~-2',5'-dideoxy-5-ethyluridine in form of a
white solid of melting point 165C.
ExamDle 2
Analogously to Example 1, there were obtained:
a) from (E)-3'-O-acetyl-2',5'-dideoxy-5-ethyl-5~-[3-(2,6
-dimethylphenyl)-2-oxopropylidene]uridine, mp 126-130C,
which was prepared in a manner analogous to that described
in Example lA) and B) starting from (2,6-dimethylphenyl)-
acetic acid:
3l-O-acetyl-2',5'-dideoxy-5 -ethyl-5'-t3-(2,6-dimethyl-
phenyl)-2-oxopropyl~uridine, mp 150-150.5C
b) from (E)-3'-0-acetyl-2',5'-dideoxy-S-ethyl-5~-[3-(2-
-methylphenyl)-2-oxopropylidene]uridine, mp 124-126C, which
was prepared from (2-methylphenyl)acetic acid:
3l-O-acetyl-2',5'-dideoxy-5-ethyl-5' -t3-(2-methyl-
phenyl)-2-oxopropyl]uridine, mp 156.5C
c) from (E)-3'-O-acetyl-5' -[3-(2-chlorophenyl)-2-oxo-
propylidene]-2~,5~-dideoxy-5-ethyluridine, mp 144-145C,
which was prepared from (2-chlorophenyl)acetic acid:
3'-O-acetyl-5'-[3-(2-chlorophenyl)-2-oxopropyl]-2',5'
-dideoxy-5-ethyluridine, mp 168.5C
d) from (E)-3'-O-acetyl-Z',5'-dideoxy-5-ethyl-5' -(2-oxo-3-
-phenylpropylidene)uridine, which was prepared from phenyl-
acetic acid:
3l-O-acetyl-2',5'-dideoxy-5 -ethyl-5'-(2-oxo-3-phenyl-
propyl)uridine, nmr (CDC13); ~1.10 (t,3), 1.85-2,03
(m,2), 2.10 (s,3), 2.10-2.18 (m,l), 2.30-2.40 (m,3),
~Z9Z987
2.57-2.75 (m,2), 3.72 (s,2), 3.93 (m,l), 4.97 (m,l), 6.27
(dd,l), 7.03 (s,l), 7.17-7.35 (m,5), 8.83 (s,l)
e) from (2)-3'-0-acetyl-2',5l-dideoxy-5-ethyl-5l -(2-oxo-3-
-phenylbutylidene)uridine, which was prepared from 2-phenyl-
propanoic acid:
3'-0-acetyl-2',5'-dideoxy-5-ethyl-5'-[2-oxo-3(RS)
-phenylbutyl]uridine, nmr (CDC13): ~1.07-1.17 (dt,3),
1.35-1.42 (dd,3), 1.72-2.02 (m,2), 2.05-2.13 (m,l),
2.07(s,3), 2.30-2.43 (m,3), 2.50-2.60 (m,2), 3.72-3.82
(m,l), 3.89 (m,l), 4.94 (m,l), 6.20 (m,l), 7.01 (d,l),
7.19-7.35 (m,5), 8.52 (d,l).
ExamDle 3
A solution of 2 g of 3'-0-acetyl-51-[3-(2,6-dichloro-
phenyl)-2-oxopropyl] -2',5'-dideoxy-5l-ethyluridine in 45 ml
of O.lM methanolic sodium methoxide solution was stirred at
room temperature for 1.5 hours, The solution was diluted
with 500 ml of methanol, a polystyrene divinyl benzene
cation exchange resin containing sulphonic acid groups (Hl
form) was added, the mixture was stirred for 10 minutes and
then filtered. The filtrate was evaporated and the residue
was triturated with diethyl ether to give 1.72 g of
5'-[3-(2,6-dichlorophenyl)-2-oxopropyl]-2l,5~_dideoxy 5
-ethyluridine, mp 229-230C.
ExamPle 4
Analogously to Example 3, there were obtained:
a) from 3'-0-acetyl-2',5'-dideoxy-5 -ethyl-5l-(2-oxo-3-
-phenylpropyl)uridine:
2',5'-dideoxy-5-ethyl-5'-(2-oxo-3-phenylpropyl)uridine,
mp 148-151C
125~Z987
- 16 -
b) from 3'-0-acetyl-2',5'-dideoxy-5 -ethyl-5'-[3-(2-methyl-
phenyl)-2-oxopropyl]uridine:
2',5'-dideoxy-5-ethyl-5' -[3-(2-methylphenyl)-2-oxo-
propyl]uridine, mp 165C
c) from 3'-0-acetyl-2',5'-dideoxy-5 -ethyl-5l-[3-(2,6-di-
methylphenyl)-2-oxopropyl]uridine:
2~,5~-dideoxy-5-ethyl-5~ -[3-(2,6-dimethylphenyl)-2-oxo-
propyl]uridine, mp 223-224C
d) from 3'-0-acetyl-5l-[3-(2-chlorophenyl)-2 -oxopropyl]-
-2',5'-dideoxy-5-ethyluridine:
5~-[3-(2-chlorophenyl)-2 -oxopropyl]-21,5~-dideoxy-5-
-ethyluridine, mp 180-181C
e) from 3l-0-acetyl-2l,5'-dideoxy-5 -ethyl-5'-[2-oxo-3(RS)-
-phenylbutyl]uridine:
2 ', 5 ' -dideoxy-5-ethyl-5' -~2-oxo-3 (RS)-phenylbutyl]-
uridine, mp 145C.
ExamDle 5
A golution of 149 mg of 3'-0-acetyl-5'-[3-(2,6-dichloro-
phenyl)-2 -oxopropyl]-2',5'-dideoxy-5-ethyluridine and 26 mg
of sodium borohydride in 7 ml of dimethoxyethane was stirred
at room temperature for 2.5 hours. The solvent was removed
by evaporation and the residue was taken up in 22 ml of 5%
ammonium chloride solution and extracted twice with 20 ml of
ethyl acetate each time. The extracts were washed with 20 ml
of water, dried over anhydrous sodium sulphate and evapora-
ted to give 3'-0-acetyl-5'-~3-(2,6 -dichlorophenyl)-2(RS)-
-hydroxypropyl]-2',5' -dideoxy-5-ethyluridine in the form of
a colourless gum. This was dissolved in 3 ml of O.lM sodium
methoxide solution and gtirred at room temperature for 1
hour. The solution was then diluted with 150 ml of methanol,
stirred with a cros~-linked polystrene/divinyl benzene
cation exchange resin containing sulphonic acid groups (H~
lZ~Z987
- 17 _
form) and then filtered. The filtrate was evaporated and the
residue was crystallized from ethanol to give 45 mg of
5'-[3-(2,6-dichlorophenyl)-2(RS)-hydroxypropyl]-2',5'
-dideoxy-5-ethyluridine, mp 185-186C.
ExamDle 6
Analogously to Example 5, there were obtained:
a) from 3'-O-acetyl-2',5'-dideoxy-5 -ethyl-5'-(3-phenyl-2-
-oxopropyl)uridine:
2l,5l-dideoxy-5-ethyl-5' -[2(RS)-hydroxy-3-phenyl-
propyl]uridine, mp 142C
b~ from 3'-O-acetyl-2',5'-dideoxy-5 -ethyl-5l-[3-(2-methyl-
phenyl)-2-oxopropyl]uridine:
2',5'-dideoxy-5~ethyl-5' -~2~RS)-hydroxy-3-(2-methyl-
phenyl)- propyl~uridine, mp 161.5-163C
c) from 3'-O-acetyl-5'-t3-(2-chlorophenyl)-2 -oxopropyl]-
-2',5'-dideoxy-5-ethyluridine:
5'-t3-(2-chlorophenyl)-2(RS) -hydroxypropyl]-2',5'-
-dideoxy-5-ethyluridine, mp 215-217C
d) from 3'-O-acetyl-2',5'-dideoxy-5 -ethyl-5'-[3-(2,6-
-dimethylphenyl) 2-oxopropyl]uridine:
2',5'-dideoxy-5-ethyl-5' -[2(RS)-hydroxy-3-(2,6-
-dimethylphenyl)propyl]uridine, mp 174-178C.
Exam~le 7
A ~olution of 300 mg of 3'-0-acetyl-5' -[2(RS)-hydroxy-
-3-phenylpropyl]-2~,5' -dideoxy-5-ethyluridine and O.Z ml of
methanesulphonyl chloride in 5 ml of pyridine wa~ left to
stand at 0C overnight. The mixture was poured on to 40 ml
of ice/water, stirred and extracted with 40 ml of ethyl
acetate. The extract wa~ dried over anhydrou~ sodium
Z987
- 18 -
sulphate and evaporated to yield 330 mg of 3~-~-acetyl-
-2',5'-dideoxy-5-ethyl-5' -t2(RS)-methanesulphonyloxy-3-
-phenylpropyl]uridine.
A mixture of 240 mg of the latter and 190 mg of sodium
iodide in 5 ml of acetone was stirred and heated under
reflux for 5.5 hours. The mixture was allowed to cool and
was then filtered. The filtrate was evaporated. The residue
was taken up in 50 ml of dichloromethane and washed with
10 50 ml of water, twice with 50 ml of 5% sodium thiosulphate
solution each time and 50 ml of water, dried over anhydrous
sodium sulphate and evaporated to yield 250 mg of
3'-0-acetyl-2',5'-dideoxy-5 -ethyl-5'-[2(RS)-iodo-3-phenyl-
propyl~uridine.
A solution of 80 mg of the latter in 5 ml of ethanol was
saturated with ammonia. 50 mg of palladium on barium
sulphate catalyst were added and the mixture was hydrogena-
ted at room temperature and under atmo~tpheric pres~ure for
3 days. The mixture was filtered and the filtrate was
evaporated. The re~idue was extracted with several portions
of ethyl acetate and the combined extracts were evaporated
to give 70 mg of 3'-0-acetyl-2',5'-dideoxy-5 -ethyl-5~-(3-
-phenylpropyl)uridine.
A solution of 70 mg of the latter in 2 ml of O.lM sodium
methoxide solution was stirred at room temperature for 1
hour. A polystrene divinyl benzene cation exchange resin
containing sulphonic acid groups (H+ form) was added and
~ 30 the mixture was stirred and filtered. The filtrate was
; evaporated and the residue was triturated with diethyl ether
to give 22 mg of 2',5'-dideoxy-5-ethyl-5'-(3-phenylpropyl)-
uridine, mp 160-162C.
Example 8
A solution of 0.5 g of benzyl mercaptan in 10 ml of dry
129Z987
-- 19 -- . .
dimethylformamide was treated with 60 mg of a 80% dispersion
of sodium hydride in mineral oil. After the effervescence
had ceased a solution of 1.64 g of 2'-deoxy-5-ethyl-5'-0-
-(p-toluenesulphonyl)uridine in 20 ml of dry dimethyl-
formamide was added. The mixture was stirred and heated at100C under a nitrogen gas atmosphere. The course of the
reaction was followed by thin layer chromatography. After 4
hours the mixture was evaporated to give an oily residue.
This was purified by flash column chromatography on silica
gel using methanol/dichloromethane (1:9) for the elution.
The fractions containing the product were combined and
evaporated to yield an oil which solidified and was
recrystallized from diethyl ether to give 1.46 g of
5'-benzylthio-2',5'-dideoxy-5-ethyluridine, mp 149-151C.
Exam~le 9
1 g of 5'-benzylthio-2',5'-dideoxy-5-ethyluridine was
dissolved in 10 ml of glacial acetic acid and the solution
was cooled to 0C, whereupon 0,9 ml of 30% hydrogen peroxide
was added. The mixture was ~tirred at 0C for 1 hour and
then at room temperature for 17 hours. The acetic acid was
removed by evaporation and the resulting solid was crystal-
lized from approximately 100 ml of methanol to give 0.43 g
of 5'-benzylsulphonyl-2~,5l-dideoxy-5-ethyluridine of
melting point 232-233C.
ExamD l e 10
In a manner analogous to that described in Example 1,
from (E)-3'-0-acetyl-5'-[3(RS)-(2,4 -dichlorophenoxy)-2-oxo-
butylidene]-2',5'-dideoxy-5 -ethyluridine, prepared in a
manner analogous to that described in Example l(A) and (B)
starting f~om 2(RS)-(2,4-dichlorophenoxy)propionic acid,
there was obtained:
3l-0-acetyl-5'-[3(RS)-(2,4 -dichlorophenoxy)-2-oxo-
lZ9Z987
- 20 -
butyl]-2~,5l-dideoxy-5-ethyluridine of melting point
122-142C.
ExamPle 11
In a manner analogous to that described in Example 3,
from 3'-0-acetyl-5'-[3(RS)-(2,4 -dichlorophenoxy)-2-oxo-
butyl]-2',5'-dideoxy-5-ethyluridine there was obtained
5'-[3(RS)-(2,4-dichlorophenoxy)-2 -oxobutyl]-2',5'-dideoxy-
-5-ethyluridine, mp 157-159C.
ExamDle 12
In a manner analogous to that described in Example 5,
from 3'-0-acetyl-5'-[3(RS)-(2,4 -dichlorophenoxy)-2-oxo-
butyl]-2',5'-dideoxy-5-ethyluridine there was obtained
5l-t3(RS)-~2,4-dichloroPhenoxy)-2(Rs) -hydroxybutyl]-21,5'-
-dideoxy-5-ethyluridine of mslting point 108-111C.
Example 13
In a manner analogou6 to that described in Example 7,
from 3',0-acetyl-2',5'-dideoxy-5-ethyl-5'-f2~RS)-hydroxy-3-
-(2,6-dimethylphenyl)propyl]uridine there was obtained
2',5'-dideoxy-5-ethyl-5'-t3-(2,6 -dimethylphenyl)propyl]-
uridine of melting point 213.5-214C.
ExamDle 14
Analogously to Example 8, there were obtained:
a) from 2'-deoxy-5-ethyl-5'-0-(p-toluenesulphonyl)uridine
and 2-chlorobenzyl mercaptan:
5'-(2-chlorobenzylthio)-2',5'-dideoxy-5-ethyluridine, mp
147-148C
lZ~987
- 21 -
b) from 2'-deoxy-5-ethyl-5'-0-(p-toluenesulphonyl)uridine
and 2,4-dichlorobenzyl mercaptan:
5'-(2,4-dichlorobenzylthio)-2',5'-dideoxy-5-ethyluridine,
mp 169-170C
c) from 2'-deoxy-5-ethyl-5'-0-(p-toluenesulphonyl)uridine
and 2,6-dichlorobenzyl mercaptan:
5'-(2,6-dichlorobenzylthio)-2',5'-dideoxy-5-ethyluridine,
mp 206-207C.
Exam~le 15
Analogously to Example 9, there were obtained:
a) from 5l-(2-chlorobenzylthio)-2l,5l-dideoxy-5-ethyl-
uridine:
5'-(2-chlorobenzyl~ulphonyl)-2',5'-dideoxy-5-ethyluridine,
mp 189-lgOC
b) from 5l-(2~4-dichlorobenzylthio)-2l~5l-dideoxy-5-eth
uridine:
5'-(2,4-dichlorobenzylsulphonyl)-2',5'-dideoxy-5
-ethyluridine, mp 217-218C
c) from 5'-(Z,6-dichlorobenzylthio)-2',5'-dideoxy-5-ethyl-
uridine:
5'-(2,6-dichlorobenzyl~ulphonyl)-2',5'-dideoxy-5
ethyluridine, mp 236-237C.
The following Example illustrates a pharmaceutical
preparation containing the compounds of formula I:
lZ9Z987
- 22 -
Tablets may contain the following ingredients:
Inaredient Per tablet
5 Compound of formula I lO0 mg
Lactose 70 mg
Maize starch 70 mg
Polyvinylpyrrolidone 5-mg
Magnesium stearate 5 mn
Tablet weight250 mg