Note: Descriptions are shown in the official language in which they were submitted.
~z~33359
-- 1 -
71-150381+
Process for removing oxides of nitrogen and sulfur from wa~te ga~es
The present invention relates to a proces~ which makes it po~sibla
to ramQve oxides of nitrogen snd sulfur from wsste gases si~ulta-
neously without the u~e of lime, sodium hydroxide, ammonia or other
chemicals.
The separated n~trogen oxides are used as catalyst for oxidising
sulfur dioxidæ with oxygen-containing gas, preferably atmopsheric
oxygen. Ultimately, ~arketabl~ nitric acid and sulfur~c acid are
obtained from the pollutants.
Tha advantages of the process of this invention will be explained by
~taking;as an example the purif~cation of flua gase~ from a power
station ~uelled by ~alt-containing brown coal having a calorlfic
valoe greater thsn 4500 kcallkg.
lt will be assumed that the power station produces the following
wa-t~ gas, where~x in N0x i8 1 and 2~
amount o~ flue gas `1 mio. Nm3 per hour ~ ;
eoncentration of N0
(ca1cu1at~d~as N02)1.43 g/Nm3 (c. 700 ppm) ~
concantration of S02 ~~12.6 g/Nm3 ~c. 4300 ppm).~ :
Throughout~`thls specification, ppm concentrations will alway~ be~
understood as meaning parto by voIume per m1-l10n units of vo1ume.
: : ~ :
: :: '
:1~9335~
-- 2 --
The process of tbis invention makes it possible to reduce the
pollutant gases to valus~ lower than
S02 0.1 g/Nm3 (c. 34 ppm)
N0 0.2 g/Nm~ (c. lO0 ppm).
Provided cooling water wi~h an average annual temperature of l5C ~ 8
available, the operating requirements for the waste gas purification
are:
electrical energy : le~s than 3 % of the energy produced in the
power station,
heat consumption for concentrating the acid in the course of the
process: corresponds to a consumption of lsss
than 2 % of the coal burned in the power plant.
When the nitric acid (concentration higher than 30 %~ Rnd sulfuric
acid (concentration high~r than 75 %~ produced by ths proce6s of
this invention can be utilised, for example, in a fertiliser
factory, the value of the acids cover~ the operating costs referred
to above. The capital expenditure for a procsss according to ths
pr~sent invention is le6s than a quarter of that required for the
powsr station.:
It is known to remove flue gases by adding ammonla to the still hot
flue ga~es, before complete heat utilisation, and passing them over
a catalyst, thereby reducing the nitrogen oxides to nitrogen. A
drawback of this process i8 the cost of the ammonia and the limited
life of the catalyst. When using salt-containing coal, salt6
vaporise in the combu~tion chamber and form aerosols that contamin-
~ ate the catalyst. A further drawback of the dry and also of the wet
; ~ use of ammonia is tha formation of ammonium sulfate aerosols and
other salt aerosols which cannot bs separated completely despite the
elaborate procedures employed. Ths aerosols released into the
: :
,
: ~
3~Z93359
-- 3 --
atmosphere along with the treated flue gas promote vapour formationin the atmosph0re and prevent natural solar radiation by forming
s~og.
In contradistinction to the known prior art, in the process of this
invention the operating cost~ fall wlth increasing coDcentrations of
N0 in the flue gases. Th~s any combu~tion measures for reducing
the formation of N0x are rendered unnecessary.
The nltrogen oxides are essentially present in flua gase~ in the
form of N0, which i8 Yery 3paringly absorable. It i5 known to
convert N0 into N02 by addition of ozone and to absorb it, for
example in ammoniacal liquids. In addition to the high costs of
producing ozone, a drawback i8 the previously mentioned unavoidable
formation of aerosols.
In recent year~, a~ a result of efforts to control atmospheric
pollution, the nitrogen oxide-sulfuric acid process ha~ been
proposed for separating SOz and N0x from waste gases containing
about 1 % by volume of S02. The work carried out in this field forms
the basis of the present in~ention and, in this connection,
reference i~ made to the followlng publications:
- Fattinger, V., Proc. Brit. Sulphur Corp., 3rd Int. Conf. Fert.,
London, November 1979, Paper XXYI
~ Blanken3tein, K., Neumann, G.E., U.B.A., F and E Rsport
~o. 10403311, June 1980
- Sander, ~., Fattinger, V., Chem. Ing. Techn., 55 (1983) No. 8,
S. 601l607
- Fattinger, V., CIBA-GEIGY CorporatioD US Patent 4 148 868 (1979)
- Fattinger, V., CIBA-GEIGY Corporation US Patent 4 242 321 (1989)
Ullmanns Enzyklopadie der technischen Chemie, 4. Deubearbeitete
und erweiterte Auflage, Verlag Chemie, Weinheim, Vol. 21 (lg82),
p. 148
" :
~93359~
-- 4 --
~lue Kases emitted by power stations contain mostly only 0.02
to 0.5 % by volume of S02; ~nd up to now, an S02 enrichment has been
considered necessary before processing in a nitrogen oxide-sulfuric
acid system becomes po6sible. FurthermorP, expert opinion is that a
nitrogen oxide-sulfuric acid process would cau~e atmospheric
pollution through N0 losses.
A principal reason for the inapplicability of known nitrogen
oxide-sulfuric acid processes for processing waste gases from power
stations is the lengthy ~ojourn time for the oxidation of the N0 set
free in the denitration tower. A prerequisite for nitrogen oxide
absorption is a suitable ratio oE ~O:N02.
It has now been found that nitrogen oxide-sulfuric acid systems with
a reaction requirement of less than 25 m3 per Nm3tsec of flue gas
can be operated if more than half the concentration of nitrogen
oxide present in the waste gas introduced into the process is
absorbed, and the liquid which contains nitrogen oxide iB gubge-
quently sub~ected to desorption, whereupon
a) the nitrogen oxide set free is mixed with an oxygen-containing
gas to give a gas which is relatively enriched with nitrogen oxide
and oxygen, and in which nitrogen monoxide oxidises to nitrogen
dioxide, and the nitrogen dioxide so obtalned and/or nitric acid
produced therefrom, as well as a residual content of nitrogen
monoxide, is introduced into the main stream of waste gas to be
treated, the molar amounts of quadrivalent and pentavalent nitrogen-
oxygen compounds introduced into the system being greater than the
molar amount of nitrogen monoxide also introduced, and
b) the liquid from which nitrogen oxides have been at least parti-
ally removed, or the bulk thereof, is fed ~ack, after passing
through the desorption plant, to one or more of the gas treatment
towers of the main stream of gas. The acid which has been partially
or completely freed from nitrogen compounds can be fed into the
first towers of the sy~tem or else in its entirety or in part into
~ 933~
-- 5 --
one of the N0x absorption towers (or also into a number of N0x
absorption towers) at the end of the system. Both kinds of liquid
feedback can be combined.
Because the acid for the purpo~e of N0x desorption has been heated
andlor diluted by water or by a weaker acid, it is exp~dient to
concentrate or cool the acid before the denitrated or partly
denitrated acid enters an N0 absorption tower.
When treating the stream of waste gas, nitric acid is introduced,
before the S02 absorptioD, into the denitration cycle or into the
S2 absorption cycle in an amount such that an optimum ratio of
NO:N02 for the N0x absorption is established. This step is not novel
and is described e.g. in US patent specification 4 242 32l.
It has further been found that surprisingly good absorption results
are obtained and that relatively high concentrations of nitrose can
be employed in the absorption acid by carrying out the absorption at
temperatures below 30C. It 18 advantageous to use heat pumps
(cooling systems) to produce a cooling brine which, by indirect heat
exchange, lowers the temperature of the acid in the cycle to below
20, 15 or even 10C. This measure has proved much less expensive
than an excess1ve enlargement of the absorption ~one.
.
An important feature that distinguishe~ the process of this inven-
tion from known processes resides in performing the denitration
using Iow concentrations of scid. Sulfuric acid in a concentration
of less than 73 % or even 70 % makes it possible to effect a
sufficient acid denitration even at the low concentration of S02 in
the flue gases emitted by power stations. The denitration tower
employed in the main stream of gas is conveniently operated in
direct~current between gas and acid.
, ~ :
The acid~discharged from the~pre-denitration ln the main current of
gas can be sub~ected to indirect heating and to a fine denltration
in~a tower through which flows a partial stream of sulfur dioxide.
: :
,
:~
`:
~LZ9335~
It is also possible to carry out the entire acid denitration out3ide
the main stream of gas and to pass a current of air and/or a partial
stream of gas which contains S02 through the denitration apparatus.
If necessary, the current of air or t~e stream of gaA which contains
S02 can be heated in order to accelerate the expulsion of the
nitrogen oxides.
It is known that the vapour pressure of the nitrogen oxides dis-
solved in the sulfuric acid a8 nitrosyl-salfuric acid is greatly
increased by addition of nitric acid. Thus, if necessary, the acid
denitration can be considerably accelerated by addition of nitric
acid. The requi3ite nitric acid is formed in the process of this
invention from nitrogen oxides and returns from the denitration
apparatus to the process in the form of nitrogen oxides.
Before the denitrated acid enters the N0x absorption zone, it is
brought, in a concentrating tower, to the concentration required for
a good absorption (73 to 76 % HzS04). The hot acid originating from
the concentrating tower can conveniently be used for heating the
cold exit gas from the N0x absorption zone.
The heated gases of very low relative humidity can be transported
to a bricked flue without the rl.k of tbeir becoming moist.
The advantages of the process of the present invention compared with
known nitrogen oxide-sulfuric acid processes become all the more
signlficant the lower the concentrations of S02 and the higher the
concentrations of N0x in the waste gas to be purified. Preferably
the concentration o~ SOz should be below 0.8 % by volume and the
concentration of N0 should be above 100 ppm.
x
Pigures 1, 2 and 3 and a working Example will serve to illustrate
~ the invention in more detall.
::
~93359
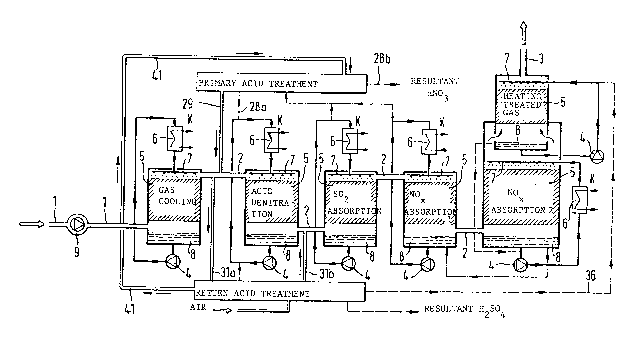
Figure 1 illustrates the treatment of the stream of waste gas in
the gas washiDg towers which are connected in series and have the
following functions: gas cooling, acid denitration, SO2 absorption,
NOx absorption 1, NO absorption 2 and heating treated gas.
The following Table indicates the concentrations of SO2, NOx, H20
and the gas temperature before and after the treatment steps:
Conc0ntrations and tempera- SO2 N3 H20 O
tuFes of the 9tream of ga3 g/Nm3 as 2 glNm3 C
before gas cooling 12.6 1.43 125 130
after gas coollng 12.5 - 1.43 30 26
after denitration 6 22 8 38
after SOz-absorption 1 20 5 25
after NOx-absorption 1 0.5 4.5 0.1 10
after NOx-absorption 2 0.1 0.2 0.1 7
after hssting treated gas 0.1 0.2 3 60
Each treatment ÆOne is provided with an acid cycle which is kept in
circulation by pumps 4. The reference numbers 1 and 9 indicate
respectively the waste gas inlet and the gas feed valve. The
reference number 2 indicates connecting lines between gas treatment
zones and 3 denotes the treated ga~ outlet. All gas treatment tower
contain packing layers 5. As shown in figure 1, individual acid
treatment stages are equipped with acid coollng means 6 for lowering
the temperstures of the acid in order to reach the gas temperatures
indicated in the above Table. The reference number 7 indicates the
acid spray nozÆles in the gss treatment zones. The reference number
8 indicates the acid sumps in the bottom part of the towers. The
packings in the layers 5 hare a surface area of more than 300 m2im3.
The reaction 3pace requirem2nt per 1 Nm3/sec of gas is smaller than
3 m3 for the denltrat1on, s=aller than 4 =3 ior th~ SOz absorpti~n,
: `
~L~933~i9
smaller than 4 m3 for the N0 absorptlon 1, smaller than 12 m3 for
the N0 absorption 2, and ~maller than 1.5 m3 for heating the
treated gas.
As in every nltrogen oxlde-sulfuric acid system, an acid exchange
takes place between the acid denitration and the N0 absorption. The
acid freed from nitrose and which flows into the N0 absorption zone
i8 hereinafter raferred to as "return acid", whilst the acid
containing N0 ia referred to as "primary acid". The amount of
sulfuric acid continuously formed in the S0z absorption zone is
mixed ~ith the primary acid.
High concentrations of nitrose in the acid are achievad as a
consequence of the strong cooling in the N0 sbsorption zone.
Expressed in HN03 equivalents, the concentration of nitrose in the
primary acid is higher than 200 g of HN03 per litre. This high
concentration makes it possible to work with small amounts of
primary and return acid. In the working Example, amounts of less
than 500 g/Nm3 of waste gas to be purified suffice.
The low temperatures in the entire treatment zone of the main stream
of gas simplify the problem of chooaing suitable materials. In the
entire area of the large treatment zones between gas cooling and
heatlng treated gas, there are no temperatures hlgher than 40C.
Figure 2 illustrates the treatment of the primary acid. The primary
acid is heated, in the heatlng means 20, to a temperature of about
60C and fed to the N0x stripper 22. Spent air ls drawn off from the
primary acid treatment zone through this N0x stripper via the
llne 4l~ and the ventilator 21. This spent air is essentially
slightly impure air w~hlch becomes enriched with N0x in the stri,oper
22. The bulk of the N0 ls converted into N02 in the oxidation zone
24a~. Th~ gases inally enter the HN03 absorber 25, which ia equipped
with a circulating pump 26 and an acid cooling means 27. The
line 28a serves to introduce HN03 into the denitration zone.
:: : :
: : : :
:: :
'
~L2933S9
_ 9 _
Excess HN03 produced is drawn off through the line 28. The exit
gases containing N0 from the HN03 absorber pass into the oxidation
zone 24b. The stream of gas containing more NOz than N0 anters the
acid deDitration zone via the line 29.
Figure 3 illustrates the treatment of the return acid.
The return acid leaving the denitration zone snters the heatlng
means 30 in which it i8 heated to 80C and then flows through the
fine denitration tower 32. Lines 31a and 31b serve to charge this
tower with gas which contains S02. It ~uffices to pass about 5 % of
the total amount of waste gas through this tower.
The return acid then enters the S02 stripper 33 and subsequently
paases into the cycle of the concentrating tower 34c The reference
number 39 indicates the circulatlng pump of the concentrating towor,
and 35 denotes the acid heatlng means. The H2S04 brought to a
concentration of 75 % passes through the line 36 into the purified
gas heating cycle. The water vapour containing air from the
concentrating tower 34 pau~es through the line 37 into the condenser
38. Thiu condenser has an acid cycle with the coollng means 40.
Continuou ly forming condensate i9 drawn off through the line 42.
The dried air i~ used as stripping air in the S02 stripper before it
i9 passed through the line 41 to the suction slde of the
~ventilator 21 (see figure 2).
:: : :
::: ~: :
:: :
.:
.