Note: Descriptions are shown in the official language in which they were submitted.
T ITLE: COMPOSITE ANODE
BACKGROUND OF THE INVENTION
The present invention relates to sacrificial anodes
and in particular sacrificial anodes for use in providing
cathodic protection of buried steel products.
Sacrificial anodes for the protection of buried steel
or submerged steel products is well known. The most common
sacrificial anodes are made of zinc or magnesium and an
electrical connector serves to connect the anode with the steel
to be protected. The soil environment and/or water provides an
electrolyte between the product to be protected and the anode,
thus completing the circuit and the anode is sacrificed in
preference to the steel. This will continue until the anode is
depleted and the expected life of the anode will be a function
of at least the size of the product being protected and the
environment in which it is placed. The voltage potential used
to drive the circuit is primarily a function of the material of
the anode and the product being protected.
In other cases, an implied current system can be used
where power from an external source supplies the voltage
2Q potential between an electrode and the product to be protected
and typically implied current systems operate with a voltage of
about 300û mv. The standard zinc anode has an output of about
500 mv negative with respect to steel, whereas magnesium anodes
would produce about 800 mv relative to steel. The zinc anode
WH-7211-87 1 Z ~ ~ Z ~ 0505H/0053F
1 is preferred due to its higher efficiency and longer expectant
life, however in some environments it is beneficial to use the
higher output magnesium anode.
It is common with sacrificial anodes to encase them in
a porous material which tends to retain water such as a gypsum
bentonite mixture and, in other cases, this mixture is placed
between the anode and the steel or iron article to be protected.
It would be desirable to be able to manufacture a
sacrificial anode in accordance with a desired voltage
potential with the anode custom designed to the application and
furthermore it would be desirable to have a sacrificial anode
where the output is substantially higher than the common zinc
and magnesium sacrificial anodes now used.
SUMMARY OF THE INVENTION
A composite sacrificial anode according to the present
invention comprises a core of a metal having a negative
potential relati~e to steel and an adjacent electrode of a
material having a positive potential relative to steel. This
adjacent electrode has a first surface generally facing the
core and a second surface in direct electrical communication of
a material of a negative potential relative to steel
substantially shielded from the core by the material having a
positive potential relative to steel. The core includes means
-- 2 --
WH-7211-87 ~29~239 0505H/0053F
1 for securing an electrical lead thereto. The anode includes a
suitable porous material between the core and the electrode
which tends to retain water. The anode produces a potential
voltage in the presence of an electrolyte between the core and
the electrode which is the collective of the material of the
core and the material of the electrodes. Such an anode has a
higher potential than an anode of one of the materials alone.
According to an aspect of the invention, the composite
sacrificial anode has an electrode formed like a ring about the
core.
According to an aspect of the invention, the electrode
is made up of a series of connected segments spaced about the
core.
The composite sacrificial anode of the present
application uses the water or soil environment to connect the
core with the spaced electrode, and the materials of the core
and the electrodes are selected to achieve a battery-like
effect to increase the overall potential. The first material
of the electrode substantially shields the second material from
the core and additional electrodes can be spaced beyond the
first electrode to further increase the potential of the
anode. The ring-like orientation of the electrode about the
core is desirable as the anode can be placed in close proximity
to the article to be protected, however other arrangements are
-- 3 --
WH-7211~87 ~Z~239 0505H/0053F
1 possible. In a preferred form of the invention, the electrode
or electrode segments are made of two plate-like members placed
in back to back relationship with this back to back
relationship providing the electrical connection therebetween.
~RIEF DESCRIPTION OF THE DRAWINGS
-
Preferred embodiments of the invention are shown in
the drawings wherein:
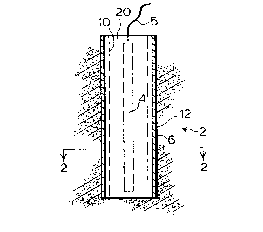
Figure 1 is a cross-section of the composite
sacrificial anodei
Figure 2 is a sectional view taken along line A-A of
Figure l;
Figure 3 is a cross-sectional view of a modified
composite sacrificial anode; and
Figure ~ is a cross-section of a further composite
sacrificial anode having higher output.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
_
The potential voltages referred to below are the
theoretical potentials and actual voltages will vary, perhaps
as much as 300 milvolts, depending upon the configuration and
resistances. Therefore, the potentials are provided as a
guideline and are subject to variation.
-- 4 --
WH-7211-87 ~2~9 0505H/0053F
1 The sacrificial anode generally shown as 2 includes a
core 4 of a suitable material such as magnesium or zinc which
will sacrifice in preference to iron or steel products when
electrically connected thereto. Associated with core 4 is an
adjacent electrode 6 which, as shown in Figure 2, is a
ring-like electrode made of two different materials. The first
surface 10 faces the core, generally surrounds the core 4, and
is preferably of a graphite material or graphite powder. The
second surface 12 of the electrode 6 is shielded from the core
by the first surface 10 and is preferably of a material the
same as the core. Intermediate the adjacent electrode 6 and
the core 4 is a mixture of gypsum and bentonite generally shown
as 2û which is porous and tends to retain water. This retained
water will act as an electrolyte connecting the core to the
adjacent electrode. The core 4 is connected to a article to be
protected by a connector 5.
The sacrificial anode shown as 2 in Figure 2, when
placed in close proximity to a steel tank, ~or example with the
core of the anode directly electrically connected with a steel
tank, will produce a voltage potential greater than that of the
core alone. If the core is made af zinc and the first surface
of the adjacent electrode is made of graphite and the second
surface of the adjacent electrode is a zinc casing, a voltage
is generated which is a result of the potential between the
~ - 5 -
~29~239
WH-7211-87 05~5H/~05~F
zinc core to graphite of being 1500 mv and the relation of the
graphite to the zinc outer surface reversing this to -150~ mv.
The zinc outer casing to the steel pipe or article to be
protected would be approximately ~900 mv. Thus 9 the output of
the anode is approximately 1500 mv when the various materials
are graphite and zinc. If magnesium were used, the potentials
would be different, but the same principles would apply.
Electrode 6 is porous or has means for allowing water to come
into contact with the gypsum bentonite mixture 20 and form an
electrolyte connecting the various surfaces.
The anode of Fiyure 3 again uses a zinc core 4
surrounded by an adjacent electrode 6, however, in this case,
the adjacent electrode is defined by a series of plates having
an inner graphite plate 8 attached to a surface of a zinc plate
14 such that the graphite plate essentially shields the zinc
from the core. Three plates have been placed about the core to
generally surround the core 4 and each of the plates or
segments are electrically connected. Interior to the adjacent
electrode and exterior to this electrode is a gypsum bentonite
mixture 20 and the electrode and bentonite mixture are retaine~
within a porous cardboard container generally shown as 17. The
adjacent electrode 6 need not be a circular riny7 but merely
needs to cooperate with the core and preferably generally
surrounds the core to produce the a~ditional potential.
- 6 -
WH-7211-87 0505H/0053F
1 A higher output zinc anode 40 is shown in Figure 4 and
includes a second ring-like anode 44 about the first ring anode
42, all of which surround core 4. Again, thîs ring-like anode
is made of plates having an interior graphite layer 46 and an
external layer of zinc 48 with a direct electrical connection
between the graphite and zinc plate of each seyment. This
additional ring will result in a -3000 mv voltage potential
relative to the zinc core. The anode is again surrounded by a
cardboard container 50, and a mixture 52 of gypsum and
bentonite is between the electrodes and between the core and
the first electrode and exterior to the the outer ring of
electrodes.
The output of up to -3000 mv is produced by the series
of combined zinc graphite electrodes submerged in water or
soil. The first ring of electrode segments cooperates with the
core to produce ~1500 mv. The polarity is reversed by the zinc
to the exterior of that electrode and the process is repeated
by the second ring oF electrode segments. This results in the
-3000 volt potential between the core and the outer zinc
surface. Note that although the graphite and zinc are the
preferred materials, other materials may be used and different
materials can be mixed. The amount of current generated by the
anode is a ~unction of the outer surface resistance of the zinc
- 7 -
WH-7211-87 ~ Z3~ 0505H/0053F
1 anode, the voltage of the anode and the resistivity of the
environment medium.
A regular zinc solid core anode has an output of -500
mv with respect to steel. The new high potential zinc anode
has a -2400 mv potential to steel; an increase oF about 5.1
over the regular zinc anode.
Aluminum may also be used to replace the zinc to
produce a slightly lower output potential in the range of
1700 mv to 2400 mv with respect to steel. Magnesium would
produce a higher output in the range of up to 3500 mv to
steel. The output potentials of these high output sacrificial
composite anodes are in the range of externally powered
impressed current anodes and can be used to replace them. As
in existing sacriflcial anode applications, special low
resistance back film material may be placed around the anode
and electrodes which further improve the output. Such
materials would include sodium sulphate, gypsum, bentonite
mixtures.
~ Although various preferred embodiments of the present
invention have been described herein in detail, it will be
appreciated by those skilled in the artj that variations may be
made thereto without departing from the spirit of the invention
or the scope of the appended claims.
:
-- 8 --