Note: Descriptions are shown in the official language in which they were submitted.
5~
- PEPTID~ I~MUNOSTIMUIANT~
. .,
~ his invention relat~s to novel acyl glutamic acid
containing peptides useful as immunost~lant and
antiinfective agents; to phar~aceutical compo~itions
thereof and to the use thereof in treating inections-
~
~ he relatively new field o~ immu~opha~macology, andparticularly that segment thereo~ which qeal8 with
immunomodulation, continues to develop at a rapid pace.
A variety of ~aturally occurring co~po~nd~ has been
investigated, including the tetrapeptide tuftsin, known
chemically as N2-tl-(N~-L-thr~onyl~L-ly~yl)-L-prolyll-
L-arginine. ~uch attention has been directed to
1~ synthetic peptidoglycan derivati~es, ~specially those
known as muramyl dipeptides. For summaries of the wide
range of com~ounds investigated as Lm~uno~odulators, and
especially as immunostimula~ts, attention is dir~cted to
Duker et al., Annu. Rep. ~ed. ChemO, 1~ 6~167 (1979),
Lederer, J~ ~ed. Chem., 23, 819-825 ~19~0) and tc
J. ~salovec, ~ of the ~uture, 8, 615-638 ~1983).
Immunostimulant peptides have been described in a
number of patent specifications:
L Alanyl-alpha-glutaric acid N acyl dipeptides in
German 3,024,355, published January 15, 1981,
tetra- and penta-peptides containing D~alanyl-L-
glutamyl moieties or L-ala~yl-D-gluta~yl moieties in
British 2,053,231, published Fe~ruary ~, 1981 and Germa~
3,024,281, published January 8, 1981~ respectively; and
acyl-alanyl-gamma-D-glutamyl tripeptide
derivatives in which the C-terminal a~ino acid is lysine
or diaminopimelic acid in German 3,024,369, published
January 150 1981; a~d
-2- 72222-~
- lactoyl tetrapeptides composed of N-lactylalanyl,
glutamyl, diaminopimelyl an~ carboxymethylamino
components in EP-11283, publi,shed ~ay 23, 1980.
Furthex immunosti~ulant ~polypeptides having the
5 formula ~A)
-(~NCHCO)n-EN-C~ R
12
R ~ 1~2)m
co~ CH_R4 ~A)
(1~2)3
R6~ R5
wherein R1 is hydrogen or acyl; R2 is nter alia
hydrogen, lower alkyl, hydroxymethyl, benzyl; R3 and R4
are each hydrogen~ carboxy, -CoNR7R8 wherein R7 is
hydrogen, lower alkyl optionally substituted with
hydroxy; and R8 is mono- ~icarboxy lower alkyl; R5 is
~yarogen or carboxy with the proviso tha~ when o~e of R4
and RS is hydrogen, the other is carboxy or CoNR7R8;
is hydrogen; m is 1 to 3 a~d ~ is 0 to 2, and
derivatives thereof in ~hich the carboxy and amino
groups are protected are di~closed in U.S. Patents
4,311,640 and 4,322,341; EP applications 25,4~2; 50,856;
51,812; 53,388; 55,~46 and 57,419.
i7~3~
-3- 72222-8
gitaura et al., J. ~ed. Chem., 25, 335-337 (1982)
report N2-~gamma-D-glutamyl~-m~so-ZtL),2~D)-di~mino-
pimelic acid ~ the ~inimal ~tructure cap~ble o~
ellciting.a biological r~sponne characteri~tic of the
compound of formula ~A) wherein n i5 1; Rl ~ 5
CH3C~O~)-CO-; R2 ~s CF13: each of R3 and R5 ~8 -COO~, R~
i8 -CONEICl~2COO~l and R6 i3 ~. 5aid compound oP fonnula
(A) i~ Icnown as FR-156.
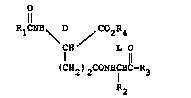
l ~he novel i~munost~mulant~ of the pr~ent invention
are of the formula
O
Rl ~ H \ D / CO~R4
I n
~CR2)2CON~IHC R3
R2
'
and a pharmaceutically acceptable base salt thereof,
wherein R1 is cycloalkyl of four to seven carbon atoms, alkyl
of two to ten carbon atoms or cycloalkylmethyl of six to eight
carbon atoms; R2 is hydrogen; and R3 is an amino acid residue
of the formula D
where X is hydrogen, alkyl of one to two carbon atoms or
hydroxymethyl and n i8 an integes o O to 4; and R~ and
RS ar~ each hydrogen, alkyl of one to ~ix carbon atoms,
cycloalkylmethyl of ~ix to eight carbon atoms or benzyl.
'' ,~",
- ,-.: .: ::..:
. .,, ., :;
. ., ,~ , . -: .:
~ 7~
; ,
-4- :
:
A preferred group of compounds are those where Rl ~
is alkyl of five to eight carbon atoms, R2 is hydrogen,
R3 is said amino a~id residue where X, n and R5 are as
defi~ed, and R4 is hydrogen. Particularly pref~rr~d are
S those compounds within the group whexe n i3 0 and R5 is
hydrogen, said alkyl or ~yclohexylmethyl. Especially
pre~erred compounds are those wherein Rl i~ (R~S)
2-ethyl-1-butyl, R~ is hydrcgen and ~ i~ methyl, Rl i8
~R,S~ 3-heptyl, R5 is hydrogen and X is methyl, Rl is
~R,S) 2-methyl-1-pentyl, R5 is hyarogen and X ~g methyl,
Rl is (R,S) 2-heptyl, R5 is hydrogen and X is methyl, R~
is ~R,S) 2-ethyl-1-pentyl, R5 is hydrogen and X is
methyl, Rl is (R,S) l-hexyl, R5 is hydrogen and X is
IS methyl, Rl is ~R,S) 2-ethyl-1-he~yl, R5 i8 hydrogen and
X is methyl, Rl is (S) or ~R,S) 2-methyl-1-hexyl, R5 is
hydrogen and X is methyl, Rl is IS) or (~,S) 2-ethyl-
l-hexyl, X is methyl and R5 is hydrogen and Rl is
l-hexyl, X is methyl and R5 is ~ydrogen. Also espe-
cially preferred is the compound wherein Rl is l-hPxyl,
X is hydrogen and n i~ 3. Especially pr~ferred esters
are those wherein Rl is (R,S) 2-ethyl-1-pe~yl, X is
methyl and R5 is n-butyl, i_butyl or cycloh~xylmethyl,
R1 is (S) or (R~S) 2-methyl-1-hexyl, X i5 methyl and R5
is n-butyl, i-butyl or cyclohexy}methyl and Rl is ~S3 or
(R~S3 2-ethyl-1-he~yl, X is ~ethyl and R5 is n-butyl,
i-butyl or cyclohe~ylmethyl.
R ~econd preferred group of compounds are tho~e
wherein R1 is cycloalkyl of four to ~even carbon atoms,
R2 is hydrogen and R3 is said amino acid residue where n ~-
: is 0, X is alkyl o~ one to two c~r~on atoms and R~ and
R5 are each hydrogen. E~pe~ially preferred within ~his
group is the compo~nd ~here R~ is ~yclohexyl and X is
methyl.
.
~2~3~7t~4
-5 72222-8
A thixd preferred ~roup of compound$-are those
wherein Rl is alkyl of five to eight car~on atoms, R2 is
hydrogen, R3 is ~ai~ ~mino ~cid residue where X is
hydrogen or alkyl o~ one ~o two carbon atoms, n i8 an
integer of O to 4 and R5 is hydrogen and R4 is ~lkyl o~
one to 5iX carbon atoms, c~cloalkylmethyl of six to
eight carbon atoms or benzyl.
The present invention is al~o directed to ~ pharma~
ceutical composition in unit do~age form comprising a
phar~aceutically acceptable carrier and a~ antiin~ect~ve
or i~muno~timulant effective amount of a compound of
formula 1 and a use for trea-ting an inEection in a
human ~uffering therefrom of a comound of formula 1.
1~
~ y pharmaceutically acceptable base salts o~ said
compounds of formula 1 is meant salts ~ith inorganic or
organic bases such as alkali metal and al~aline earth
2D metal hydroxides, ammonia, triethylamine, ethanolamine
and ~i~yclohexylamine.
The configuration of the amino acid moieties which
make up the compounds of formula 1 is ~ignificant as
regards the pharmcological activity of said compounds.
The mo~t potent act~vity is observed in the compounds o~
for~ula 1 having the ~ter~ochemi~try indicated ~n ~aid
for~ula. In those compounds of formula 1 where R2 a~d X
are other than hydrogen, the preferred stereochemi~try
at said carbon is indicated as L and ~, respectively.
Also considered within the scope o~ the pre~ent
invention are compounds of formula 1 where R3 is al~oxy,
cycloalkoxy, aralkoxy or alkoxy substituted by one or
more of the substituents ~elected from amino, dial~yl-
ami~o, hydroxy, alkoxy and halo.
57~
- 5a - 72222-8
The present invention is further directed to a process
or preparing a compound of formula (1) or a pharmaceutically
acceptable base salt thereof. This process comprises:
[A] acylating the amino group in a peptide of the
formula:
H2N ~
CH
I (L) o
11
(CH2)2CONHCH~C-R3
R2
(wherein R2, R3 and R4 are as defined above except that if they
interfere the reaction, they may be blocked by a blocking group),
with a carboxylic acid of the formula:
RlCOOH (4)
(wherein Rl is as defined above)
or an activated derivative thereof, followed by, if necessary,
the removal of the blocking group, or
[B] amidating the carboxyl group or the activated
form thereof in a compound o the formula:
~; ~
7~
- Sb - 72222-8
RlCONH (D) / C2R4
fH ~L) (5)
(CH2)2CONHCH-CO~OH
R2
(wherein Rl, R2 and R4 are as de~fined above except that if they
interere the reaction they may be blocked by a blocking group,
and the carboxyl group attached to the group -CHR2- may be in
an activated form),
with an amino compound of the formula:
(D)
2 1 ( 2)nC~R5 (6)
X
(wherein X, n and R5 are as defined above except that if they
interfere the reaction, they may be blocked by a blocking group),
followed by, if necessary, the removal of the blocking agent,
thereby producing a compound, or
[C] amidating a D-glutamic acid derivative of the
ormula:
- 5c - 72222-8
l
RlCNH (D) / C2R4
CH (7)
I
( 2)2
(wherein Rl and R4 are as defined above except that if they
interfere the reaction, they may be blocked by a blocking group),
or an activated derivative thereof, with an amino compound of
the formula:
(L)
H2N-CHCOR3 (8)
R2
(wherein R2 and R3 are as defined above, except that if they
interfere the reaction, they may be blocked by a blocking group),
followed by, if nece~sary, the removal of the blocking group,
and
[D] if required, converting a compound (1) produced
by any one of processes [A] through [C] into a pharmaceutically
: acceptable base salt thereof.
, . . .
~,~f~
.
T~e compounds of for~ula 1 ~re prepared by a~y of
several methods known to tho~e skilled in th~ art. ~he
methodology involves the fo~tion of peptid~ linkages
be~ween amino acid~ which, b~cause of their ami~o and
carboxy groups, a~d ~requently the prese~ce o~ other
reactive groups, necessitate the protection of said
groups and/or the activation o~ such groups,
particularly the carboxy group, in order ~o achieve a
certain reaction or to optimize ~uch a reaction.
P~efef~b/~
n-ge~ two routes are employed in the
synthesis of the compounds o~ fonmula 1. The first
procedure ut lizes the coupling of the fragment
RlCN~ ~ D / C02R~
7~ L :~
(C~2~2co~ aco2~
R~ :
with the a~ino acid fragment
D
~2NCH (~ H2) nC02R5
2S The seco~d procedure co~rises acylation of the
p~ptide
~2N \ D C02~4
C~ L O
- 30 1 ~
~2~2CON~ R3 ;'
R2
with ~he appropriate acid RlCO2~,
In the exa~ples presented herein, cert~in
protecting and activating groups are speci~iGally
illustrated. ~owever, o~e skilled in the ~rt will
S recognize that other protecti:ng or activ~ting groups
could have been used. The choic~ of a particular
protecting group is dependent to a gre~t extent UpOD the
availability of the necessary reagent, i~s e~fect upon
solubility of the ~protected~ compound, its ease of
removal and the presence of other groups which might be
effected by its use; i.e., its selectivi~y, ox its
removal.
For example, it will be necessa~y, or at least
desirable, in many reactions to protect the amino groups
and/or the carboxy groups. The synthe~ic route chosen
for the peptide synthesis may require removal of one or
the other or both of said protecting groups in order to
permit further reaction at t~e regenerated ami~o or
carboxy group: i.e., the protecting groups used are
reversible and, i~ ~ost instances, ar~ removable
independently of each otherO Additio~ally, the choic~
of protecting group for a giv n amino group depends ~pon
the xole of said amino group in the overall reaction
schemen Amino protecting groups having varying levels
of lability, iOe.; eas~ of removal~ will be used. The
sam~ is true as regards car~oxy protecting yroups. Such
groups are k~own in the art and atte~tion is dlrected to
the re~iews by ~odansky et al., ~Peptide Synthesis~, 2nd
: 30 Ed., John Wiley ~ Sons, ~.Y. (197~ reene, "Protective
Groups in Organic Synthesis~ John ~iley ~ Sons,
(l98l~; ~cCmie, WProtective ~roups in Organic
Chemistry~, Plenum Press, N.Y. (1973~; and to Sheppard
in ~Comprehensive Organic Chemistry, The Synthesis and
j....
j:
- 8 ~ ~ 78~ 72222-8
R~actions of Organic Compounds", Pergaman Press, N.Y. (1979),
edited by E. Haslam, Part 23.6, pages 321-339.
Conventional amino and carboxy protecting groups are
known to those skilled in the art. Representative amino protect-
ing groups, but by no means limiting thereof, are the following:
such as benzyloxycarbonyl, substituted or unsubstituted aralkyl
such as benzyl, trityl, benzhydryl and 4-nitrobenzyl; benzylidene;
arylthio such as phenylthio, niotrophenylthio and trichlorophenyl-
thio; phosphoryl derivatives such as dimethylphosphoryl and O,O-
dibenzylphosphoryl; trialkylsilyl derivative~ such as trimethyl-
silyl; and others as are described in U.S. Patent 4,322,341. The
preferred amino protecting group is benzyloxycarbonyl. Procedures
for substituting said group on a given amino group are well known,
In general they comprise acyla-ting the appropriate amino compound
with benzyloxycarbonyl chloride (benzylchloroformate) in a
reaction-inert solvent, e.g., water, methylene chloride, tetra-
hydrofuran, in the presence of a base (acid acceptor) e.g., sodium
or potassium hydroxide when water is solvent; and, when an organic
solvent is used, in the presence of a tertiary amine such as Cl_4
trialkylamines and pyridine. When an aqueous solvent system is
used the pH of the reaction is held at about pH 8-10, and prefer-
ably at pH 9. Alternatively, when the reactant; i.e., the com-
pound, an amino group of which is to be protected, contains 'basic
groups, it can serve as acid acceptor.
The acyl group, RlCO is introduced into the peptide by
standard acylation procedures as by reacting said peptide with the
~r
9 - ~2~57~ 72222-8
appropriate acicl chloride or bromide in a reaction inert solvent.
Favored conditions are non-aqueous conditions including addition
of a suitable base such as an organic base, preferably a tertiary
amine such as triethylamine, N-methylmorpholine or pyridine. The
preferred solvent is methylene chloride.
Representative carboxy protecting groups are various
esters such as silyl esters, including trialkyl silyl esters,
trihalosilyl es-ters and haloalkylsilyl esters; certain hydrocarbyl
esters such as Cl_4 alkyl, espeeially t-butyl groups, benzyl and
substituted benzyl esters, benzhydryl and trityl; phenacyl and
phthalimidomethyl esters; certain substituted hydroearbyl esters
sueh as chloromethyl, 2,2,2-trichloroethyl, cyanomethyl; tetra-
hydropyranyl; methoxymethyl; methylthiomethyl; protected earbazoyl
sueh as -CONH-NHR wherein R is an amino protecting group as
disclosed above, espeeially benzyloxy earbonyl; and others as are
described in U.S. Patent 4,322,341. A highly favored carboxy
protecting group is the t-butoxycarbonyl group.
The protected amino and carboxy groups are converted to
the unprotected amino and earboxy groups by proeedures known to
those skilled in the`art. The benzyl group, the preferred pro-
teeting groups for earboxy (as part of the proteeted carbazoyl
group) groups are removed by eatalytie hydrogenation over palla-
dium, especially palladium-on-earbon. Alternatively, said pro-
teeting groups are removed by means o trifluoromethanesulfonie
acid in trifluoroaeetie acid and in the presenee of anisole to
suppress alkylation. The t-butoxyearbonyl group is readily remov-
ed by treatment with dioxane saturated with hydrogen chloride.
-
:~2~
-ln- '
Activation of carboxy groups as a menas of expe~ -
diti~g a given reaction is methodology known to those
skilled in the art~ Esp~cially use~ul in the ~erein
described reaction ~equence are the ~5~ of anhydrid~s,
S particularly cyclic anhydride~; and activated esters,
such as those derived from N-hydroxyphthalimide and
N-hydroxysuccinimide, ~oth of which are used in peptide
syntheses.
The activated N-hydroxysuccinimide ester~ expedi~e
subsequent reactions at said activated ester groups. As
the skilled artisan will recognize other activating
groups could be used. A group of particular i~terest i5
the N-hydroxyphthalimido group, which group is u~ed in
the same manner as is the N-hydkoxysuccinimido group.
In both instances, a dehydrative ~oupling agent is used
to form the activated ester. Representative of such
coupling agents a~e l-cyclohexyl-3-(2-morpholinoethyl)-
carbodiimide ~bod-p-toluene sulfonate, dicyclohexyl
car~odiimide, N,~'-carbonyldiLmida~ole, N-~3-dimethyl-
aminopropyll-N'-ethylcar~odiimide hydrochloride, ethoxy-
acetylene, diphenyl~etene and ~-ethyl-S-phenylisoxa-
zolene-3'-sulfonate. The reaction conditions for using
such coupling agents are well described in the litera
2s ture. In general they comprise the use of a react on-
inert solvent and te~pera~ures ranging ~rom ~mbient to
100C. The above-mentioned carbodiimide reagents are
favored since they penmit use of ambient reactiGn
temperature and afford satisfac~ory yields of the
3n desired esters.
Upon completion of the coupling reactions leading
to the final products, the variou~ protecting groups can
be r~moved by t~e appropriate t~ch~iques previously
discussed, and the compo~lnds of fonmula 1 isolated.
~ 5~1~34 :'
::,
~ he pharmaceutically acceptable base-salts of
formula 1 compounds, where R3 is hydroxy or R4 or R5 is
hydrogen, are obtained by treating a solution,
preferably aqueous ~olution, thereof with ~ b ~e such a~
are enumerated above, generally in ~toichiom~tric
proportions. The ~alt~ are isolated by evaporation or
by precipitation.
The products of this invention are useful ~8 agents
in mammals, including humans, for the cli~ical and
therapeutic treatment of diseases caused by various
patho~enic microorga~isms, especially gram-negative
~acteria. They are also use~ul as immu~o~tLnulants in
mammals, including humans, having an inc~ease risk of
infection due to existing or i~creasad risk of infection
due to existinq or clinical~y-induced Lmmunosuppression.
The test procedure, which used C3H/HeN ~le mice
from the Charles River Breeding Laboratory, is presented
below. The mice wer~ acclimatized for 5 days before use
and then treated either subcutaneou~ly ~SC) or orally
~P0) with various dilution~ ~100, 10, 1 and 0.1 mg/kg)
of the test compound or place~o (pyrogen ~ree saline)
usin~ a volume of 0.2 ml. The treatment re~iment was
dependent on the infec~ious organism utilized: -24 and :
O hours before challenge for Rlebsiella ~ne~moniae in
normal ~ice; and -3, -2 and -1 day before challenge for
Escherichia coli or Sta~. aureus in immunocompromised
mice. Challenge ~as administere~ intramuscul~rly ~IMl
in the hip in the case of R. pneumoniae or intr~-
peritoneally (IP) in t~e case of S. coli and ~Q~.
aureus. A volume of 0.2 ml. was u~ed for the challenge.
~ortality was recorded after 7 days in the case of R.
pneumoniae and after 3 days i~ ~he case of the other two
microorganism challenges.
12-
~lture Preparation: -
K. pneumoniae, E. coli, or ~ . 2ureu~: theculture was ~treaked for purity fro~ fro~n blood st~ck
on brain heart infusion (~I) agar. ~hree colonies w~re
picked from the 18 hour plate culture and placed into 9
ml. of BHI broth~ The broth culture was grown for 2
hours at 37~Co on a rotary shaker after which 0.2 ml.
was streaked on the surface of several B%I agar slants.
Pollo~ing an 18 hour incubation at 37C., the slants
lo were washed with B8I broth, the cultur~ density adjusted
using a spectronic 20 and the appropriate dilution made
to achie~ an LD90 challenge level in nor~al mice.
When used as antiinfective or immunos~imulan~
agents in humans, the compounds of thi~ invention are ,,
conveniently admini~tered via the oral, subcutaneous,
intramuscular, intravenous or intraperitoneal routes,
generally in composition form. Such compositions
include a pharmaceutical practice. For axample, they
can be administered in the form of tablets, pills,
powders or granules co~tai~ing such excipie~ts as
starch, ~ilk su~ar, certain types of clay, etc. They
can be administered in capsules, in admixtures with the
same or equivalent excipients. They can also be
administered in the form of oral suspensions, solutions,
~mulsions, syrups and elixirs whicb ~ay contain
flavoring and colo~ing agents. For oral administration
of the therapeutic agents of this invention, tablets or
capsules containing from about 50 to about 500 mg. are
suitable for most applica~ions.
The physician will determina the do~age which will
be most suitable for an individual pa~ient and it will
Yary ~ith the age, weight ~nd response of the particular
patient and the ro~tP of a~ministratio~. The favore~
~2 ~ ~'7~ -
-13- ..
oral ~osage range, in single or divided do~es, is from
about 1~0 to about 300 ~g/kg/clay. The favored
parenteral dose is from a~out 1~0 to about 100
mg/kg/day: the preferred range from about 1.0 tc ~bout
2û n~g/kg/day. :
This invention also provided phar~aceutical
compositions, including u~it dosage for~s, valuable for
the use of the herein descri~ed co~pounds for the
utilities disclosed herein~ The dosage form can be
given in single or multiple doses, as previously not~d,
to achieve the ~aily dosage effective or a particular
utility.
The following examples are provided &olely ~or the
purpose of further illustration. In the intere~t of
brevity, the following abbreviations for pea~ shapes in
the NMR spectra are used: s, ~inglet; d, doublet; t,
triplet; q, quartet; m, multiplet. The terms mole and
millimole are abbreviated as m and mm, respectively.
'
''
"
;7~
' ;;:'
. .
- EXAMPLE 1
N-~eptanoyl-D-gæ~a-glutamyl-glycyl-D-alani~e
(Rl S C~3(C~2)5; R~ DC~C~IcD
lA. N-heptansyl-D-gamma~glUt~myl(alpha benzyl
ester)-~lyci~e ':
.,
To a solution o~ B97 mg. (13.0 ~m~ o~ glycine and
1.3 g. (13.0 mm) of triethyla~ine in 10 ~l. of water was
added 5.0 g. ~11.2 mm1 of N-heptanoyl-Dogamma glutamyl
0 (alpha benzyl ester)-hydroxysuccinimi~e e~ter in lon ~1.
of dioxane, and the resulti~g reaction mixture allowed
to stir at roo~ temperature for 80 ho~rsO ~h~ solution
was poured into 300 ml. of ethyl acetate and the -~
separated organic pha~e washed wi~h 10~ hydrochlorac
acid, water and a brine solution. The organic phase was
separated, dried over magnesium sulfate and concentrated
in vacuo to dryness. The residue ~a~ trituxated with
_ ~ .
diethyl ether and filtered under x~itrogen, 3.43 g. q74~ -
yield).
lB. N-heptanoYl-D~ qlutamy
To a solution o~ 2 . O g. ~4 . 78 ~i of N -heptanoyl-
D-gan~na-glutamyl (alpha benzyl ester)-~yci~e, 1.75 g. l5 ;
mm) of D-alanine benzyl ester ~-toluenesulfonic acid
salt, 506 mg. I5 mm) of triethylamine and 675 mg. ~5 mmJ
of l--hydroxybenzotriazole in 100 ml. sf ~etrahydrofuran -~
was added 3.03 ~. (7.17 m~) of 1-cy~lohexyl-3~(2- :
morpholinoethyl)~arbodiimide metho-~-toluenesulfonate ::
and the reaction mixture stirred at room temperature ~or
18 hours. The reaction mixture was poured i~to 300 ml.
of ethyl acetate and the organic phase separated and
was~ed wi~h 10% hydrochloric aeid, water, a saturated
sodium bicarbonate 601ution and a brine ~olutio~. The
organic phase was separated, dried ov~r magnesium
,
- :~L2
1 5
sulfate and concentrated in vacuo. The residue was
triturated with diethyl ether and filtered under nitro-
gen, 2.7 g. Two grams of the solid i~ 75 ml. of
methanol with 403 mg. of lO~ palladium hydroxide on
5 charcoal was sha~en in a hydrogen atmo~phere at an
initial pressure o 50 p~i for 4 hours. The catalyst
was filtered and the filtrate evaporated u~der reduced
pressure and the residue was clissolved in water and
lyophilizad to give 1.23 g. (5~0% yield) o~ the deæired
product as a white ~olid.
The NMR spectrum ~DMSO-d6) 3howed absorption at
4.35-4.2 (m, 2~)~ 3.83 (~, 2H), 2.35 (t, J~7Hz, 2B),
2.17 ~t, J-7~, 2H), 2.1-1.8 (m, 2~), 1.55-1.45 (m, 2H),
1.3 (d, J=6~z, 3~1, 1.17 (bs, 6H) and 0.75 (bs, 3~) ppm.
XAMPLE 2
N-Hep~a~oyl-D-gamma-glutamyl-glycine (Rl = CR3~CY2)5;
R~ - ~; and R3 ~ O~)
A solution containing 1~0 g. of ~-heptanoyl-D- :~
gamma-glutamyl (alpha benzyl ester)-glycine in 50 ml. of
methanol was treated with 100 mg. of lO~ palladium
hydroxide on charcoal and shaken i~ a hydro~en
atmosphere at 50 psi for 3 hoursO The catalyst was
filtered and the filtrate concentrated in vacuo, The
residue was dissolved in hot water and ~vaporated i~
vacuo. The residue was redi~solved in water and
lyophilized to give 630 mg. (83~ yield3 of the desired
product as a white solid.
The NMR spectrum (D~SO-d6) showed absorption at
4.37-4.25 (~, 1~)~ 3.9 (s, 2~), 2.35 (t, J=7~z, ~
2.18 (t, J=6~z, 2~), 2.~-1.8 (m9 2~), 1.5-1.4 ~, 2H3,
1. 8 (~s, 6~) and O. 7 (bt, 3~) ppm. . .
-16-
EX~PLE 3 - .
N-~eptanoyl-D-ga~ma-glutamyl-qlycyl-glycin~
(~1 = CH3(ch23s; R2 ~ ~; and R3 2 2
3A. ~-heptanoyl-D-gamma-gluta~yl (alpha benzyl ester)-
glYci~e hydroxysuccinamide ester
To a cold 501UtiOII (0C.1 o~E 13rO g~ (31 mm) c~f
N~heptanoyl-D-gamma-glutamyl (alpha ~enzyl est~r~ :
glycine and 3.91 g. (34 mm) o~ N-hydroxy~uc~in~mide in
400 ml. of ~e~rahydrofuran was added 7.0 g. (34 mm) oP
dicyclohexylcarbodii~ide, and the mi~ture ~llowed to
stir at 0C. ~or on~ hour and at roo~ t~p~rature ~or 18
hours. The solids were filtered and the filtxate
concentrated under reduced pre~sure. The residue was
triturated with diethyl ether and filtered ~nd~r nitro-
gen to give 15.4 g. (98~) of the desired intermediate.
3~. ~
To 2.0 g. (3.97 mm) of N-heptanoyl-D-gamma-glutamyl .
(alpha benzyl es~er)-glycine hydroxysuccinamide ester in
~o 100 ml. of dioxane was added ~46 ~g. 15.95 mm) of
glycine and 0.55 ~1. l3~9 mm) of tr~ethylamine in 10 ~1. ',
of water, and the resulting reactisn mixture allowed to
stir at room temperatur~ for 13 hours. The 6~1ution was
poured into 100 ml. of ethyl acetate and the organic ~.
layer washed with 2.5~ hydrochloric acid, water and a
: brine solution. The organic layer was separa~ed, dried ~::
over magne~ium sulfate and concentrated to drynessO The '.
residue was triturated with diethyl ether and filtered
under ni rogen to give 1.7 g. of white ~olid. One and
30 iive-t~nths grams of the solid in 75 ml.. of methanol
containing ~00 mg. o~ lOS palladium hydroxide on carbon ! ~:
was shaken in a hydrogen atmosphere at 50 psi or 3 ~.
hours. The catalyst wa~ filtered and the filtrate '~
!
' .~.
' j`'.''''.
I':
i~
',:.
~57~3~
concentrated ~n vacuo. The residue w~s dissolved in
water and lyophilize~ to give 1.12 g. ~90~ yie~d) of the
desire~ product.
The NMR spectr~m ID~SO-d6) $howed ab~orption
8.2-8.0 (m, 3~), 4.19 ~m, 1~ .8-4.6 tm, ~), 2.25 ~t,
J-7~z~ 2H), 2.1 (t, J-6Hz, 2~), 2.05-1.7 (~, 2~), 1.5
(m, 2~), 1.25 5bs, 6~) and o.as ~t, JY68Z~ 3H3 ppm.
EXAMPI~ 4
N-HeptanoyloD gamma-gluta~yl-glycyl-D~serine
D
1 3(C~2)5; R2 ~ ~; and R3 ~ -N~CHtC~2O~)CO2~)
~ .
Starting with 2.0 g. (3.98 mm) of h-hep anoyl-D-
g~mma-gl~tamyl (alpha benzyl ester)-glycine hydroxy~
succinamide e~ter, 780 mg. ~4.02 ~m) of O-benzyl-D-
serine and .556 ml. (4.02 mm3 of triethylamine and
following the procedure of Example 3B, 902 ~g. ~76%
yi~ld1 of the desired product i~ i~olated, m.p. 130- :~
132C.
The NMR spect~um (DMSO-d6) ~howed a~sorption at
8.36-7.94 ~, 3~t~ 4.~6-4.28 l~, lR)~ ~.28-4.08 (~, lH),
3.94-3.50 ~m, 4~I); 2.25 ~t, J~91~z, 2EI1. 2.17 It, J=g}~z,
lH)~ 2.10-1.04 (~, 14~) and 0.9 (t, J=6~z, 3~ ppm.
EXA~IPL.E 5
~-Heptanoyl-D-gamma-glutamyl-glycyl-D alpha-
æminobutyric acid ~Rl ~ C~3(CH2)5 ; 2
R3- -N~C~(C~2C~33C02H
The procedure of ~xa~ple 3B was repeated, ~tar~ing
with 2.0 g. (3.98 ~m) of N-heptanoyl-D-gamma-glutamyl
(alpha benzyl ester)-glycyl hydroxysuccinamide ester, :~
400 mgO S4.02 mm) of D alpha-~minobutyric acia and .55S
ml. i4~02 m~ of triethylamine, to give 632 mg. (57%
yield) of the desired proauct, m.p. 140-141C.
. .
-lB-
.
The NMR spectrum ~DMSO d6) showed absorption at
8.16-8.04 (m, 3~), 4.22-4.0B ~m, 2~, 3.8~-3.58 (m, 2~),
2.2 It, J~9~z, 2H~, 2.12 (t, J-g~z, 2~ .D4~1.0 ~m,
15~) and 0.35 ~, J~6~z, 6~1 ppm.
E ~ ]~ 6
N-~eptanoyl-D-gamma-glutamyl-glycyl-3-am~nopropionic
1 3~ 2~; R2 ~; and R3 - -N~(CH2)2C02H)
Following the procedur~ of Example 3B and ~tarting
with 1.5 g. (3.0 mm) of N-heptanoyl-D-gam~a-glutamyl
~alpha benzyl ester)-glycine hydroxysuccinia~ide ester,
350 mg. ~3.9 mm) of 3-aminopropionic acid and 055 ml.
(3.9 m~) of triethylamine, 500 mg. ~43~ yield) of the
desired product was obtained, m.p. 135-138C.
The MMR spectrum ~DMS0-d6~ sh~wed absorption at
8.19-3.02 lm, 2~), 7.98-7.87 It, JG5Hz, 1~), 4.25-~.1
(m, 2~), 3.8-3049 (m, 2~), 3.44-3.1 (m, 2~), 2.4 (t,
J36Hz, 2~), 2.22 (t, J=7~z, 2~), 2.14 ~t~ J-7~z, 2H),
2.1-1.67 tm, 2~), 1.6-1.17 (m, 8~) and 0.88 (t, J=6Hz,
3~ ppm.
~X~MPLE 7
~-Heptanoyl-D-gamma-glutamyl-glycyl-~-aminobutyric
~1 C~35~H2~5 ; R2 s ~; and R3 ~ -N~CH~) C0 ~)
.
The procedure of Example 6 wa~ r~peated ~ubsti-
tuting 410 mg. (4.0 mm3 of 4-aminobutyric acid for the
3-aminopropionic acid to giYe 600 mg. l~o~ yield) of the
desired produc~, m.p. 140-1~2C.
The N~R spect~um ~DMSOod6J ~howed ab~orption at
8.18-8.03 Im, 2~), 7.88 (bt, J=4~z, lB), 4.17-4.09 (m,
3~ 2B~, 3.81-3.48 (m, 2~, 2.32-2.0~ ~m, 6~)~ 2.0B-1908 Im,
12~) and 0.88 It~ J=6~z, 3~) ppm.
,
--19~
~ ~ LE 8
N-~eptanoyl-D-gamma-glutamyi-gly~yl-5-amino-
pentanoic acid (Rl ~CH (C~ ~ -; R2 ~ ~; and
Sub5tituting 470 mg. (4.0 mm~ ~ 5-~inopentanoic
acid ~or 3-aminobu~yric acid ,and ~oll~wing the proc~du~e
of Example 6, 520 mg. ~423 yileld~ of the desir~d product
was obtained, m.p. 122-124~C.
The NMR spectrum (DMSO-d6~ ~howed absorption at
I0 8.25-7.9~ ~m, 2~), 7.85 It, J~5Hzt 1~), 4.25~.1 (m,
2~), 3.8~-3.46 (m, 2H), 3~24-2.9 (m, 2~), 2.21-2.08 (m,
6~), 2.08-1.2 (m, 14H1 and 0.88 ~t, J86~Z, 3~) ppm.
EXAMPLE 9
N-Heptanoyl-D-~amma-glutamylglycyl-6-aminohexanoic
IS ~Rl ~3(C~2)5 ; ~2 H; and R3 ~ -N~(CH2~5C0
~ .
The procedure of Example 6 wa~ again repea~ed,
substituting s3n mg. ~4.0 mm) of 6~ ohexanoic acid
for the 3-æminobutyric acid to give 520 mg~ S40~ yi~ld)
of the desired produ~t as ~ whit~ foamO
The NMR spectrum (DMSO-d6) showed absorption at
8.28-7.9 (m, 2~), 7.82 (bt, J=4~z, 1~, 4.27-~.1 tm,
2~), 3.81-3.47 (mt 2~), 3.15-~.9~ ~m~ 2R), 2.3-2.08 (m~
6~), 2.08-1.18 (m, 16H) and 0.88 St, J~6~z, 3~) ppm.
N-Iso~aleryl-D-gamma-glutamyl-glycyl-D-alanine
(~1 (C~332c~2-; ~2 ~ ~; and ~3 ~ CHIC~3lco~
lOA. ~
To a cold (0C.) solution of 100 ml. methylene
chloride contai~ing 10 gO (57 ~m~ of ~-t-butyloxy-
carbonylglycine, 20 g. ~57 mm) of D-alanine benzyl ester
~-toluenesulfonic acid salt and 5.77 g. ~57 ~) of
~5;7~3~
-20-
: ~ triethylamine was added 12.3 g. t60 ~) of dicyclohexyl-
carbodiLmide and the resulting ~eaetion ~ixture allowed
to warm to room temperature. After 18 hours the ~ixture
was filtered and the filtrate ¢oncentrat~d in ~acuo.
The residue was disFolved in 200 ml. of e~hyl acetate
and the organic layer washed with 2.5~ hydrochloric
acid, water, a saturated sodium bicarbonate solution and
a brine ~olution. The organic layer ~a~ ~eparated, '
dried over magnesium sul~ate and evaporated under
r~duced pressure. To the resulti~g oil ZOO ~1. of
dioxane saturated with hydrogen chloride was added.
A~ter 30 minutes 400 ml. of diethyl ether was added and
the product filtered under nitrogen, 10.9 g. (70% .
lS yield).
lOB. N-t-butoxycarbonyl-D-gamma-glutamyl ~alpha
benzyl ester)-kr~ ~n~m~ ester
h yd ~ ~ lsu cc In C h~ t ~
~o 1500 ml~ of methylene chloride containing 50 g. .
(143 mmJ of N-t-butoxycarbonyl-D-gam~a glutamic acid
alpha-benzyl e~ter and 17.3 g. ~150 ~ml of ~-hydroxy-
succinamide was added 30.9 g. (15 mm) of aicyclohexyl-
carbodii~ide and the resulting reaction ~ixtura allowed
to stir at room temperature for 18 hours. The ~olids
were filtered and the filtrate concentrat~d in vacuo.
The residue was triturated with diethyl ether and the :
~slids filtered l-nder nitrogen, 43.7 g. ~68% yield~
lOC. D-qamma-glutamyl (alpha benzyl esterl-glycyl-
C~
:.'
A solution containing 4~3 gO ~9.45 mmj of N-t-
butoxycar~onyl-D-gamma-ylutamyl (~lpha benzyl ester)
hydroxysuccinamide e~iter, 2471 g. (9.92 ~) of glycyl~
~-alanine ben~yl ester hydrochloride and 1.0 g. i9.92
mM) of triethyl~il~e in 100 ml. of ~ethylene chlor~de
-
~g~7~3~
-21-
was allowed to stir at room temperature for 18 hours,
and was then concentrated in ~vacuo. Th~ residue was
dissolved in 200 ml. of ethyl ~cetate and the ~olution
washed with 2.5~ hydrochloric acid, ~ater, 10~ pota~siuM
carbonate and a brine ~olution. The organic pha~e was
separated, dried over ~aqnesi~m sulfate and e~apor~ted
under reduced pressure. The :residue was treated with
200 ml. of dioxane ~aturated with hydroge~ chloride and
allowed to stir for 2 ho~rs. ~he soluti~n was concen- -
trated to dryness in vacuo and the r~idue triturated
~ . ~
with diethyl e~her. The solids were ~iltered under
nitrogen, 3.41 g. (73~ yield~.
lOD. -isovaleryl-D-gamma-gluta~y~ ycyl-D---alanine
15To a solution of l.0 g. (2~03 ~m) o~ D-gamma-
glutamyl (alpha benzyl ester~-glycyl-D alanine benzyl
ester hydrochloride and 616 mg. (6.09 mm) of triethyl-
amine in 50 ml. of methylene chloride ~as added 490 mg.
14.06 mm~ of i~o-valeryl chloride ~nd the reaction
2D mixture stirred at room temperatur~ for 80 hours. ThQ
methylene chloride ~as evapoxated in vacuo and the
residue dis~olved in ethyl acetate. The re~ulting
solution was washed ~ith 2.5% hydrochloric acid, water
10~ potassium carbonate, water, and a brine solution. ..
The organic phase was separated, dried over magnesium
sulfate and concentrated under vacuu~. ~he residue was
triturated with die~hyl ether, iltered under nitrogen
(910 mg.) a~d 700 ~g. dissolved in 50 ml. of mstha~ol.
Palladium hydroxide 200 mg. was added o the solution
and the mixture shaken in a hydroge~ atmosphere at 50
psi ~or 3 hoursO The cataly~t was filtered and the
sol~ent re~oved in vacuo. The re~id~e ~as dissolved in
water and lyophilized to give 36~ ~g. t~5~ yield~ of the
de~ired pr~duct~
7~3~
-22-
The ~MR spectrum (DMSO-d6) showed ab~orption at
8.25-8.05 (m, 3~), 4.33-4.12 (m, 2~), 3.72 ~d, Js6~z,
2~), 2.21 (t, J-8~z, 2~), 1.88--1.68 (m, 1~), 2~0801.9
(m, 4~1, 1.28 (d, J~9~z, 3~) alld 0.~ ld, J~7~z, 6~) ppm.
S EXAMPLE 11
Starti~g with the appropr:iate acid chloride and
D-gamma-glutamyl ~alpha benzyl ester)-glycyl-D-alanine
benzyl ester hydroehloride and employi~g the procedure
of Exæmple lOD, the following compounds were pr~pared:
O .
RlCN~ \ / 2~
I D
~C~12) ;2CON~CH2CON~IIHS:0
~3
'-:
R
~0
CH3(Ca2)8- >l~S I~SO-d~ 8.22
~, 2h), 4.35-~13
tm, 2H), 3.83 (d, J-
6~z, 2~3~ 2,22 ~t,
2~ 3~6~z, 2~), 2.1~ (t,
J~6~z~ 2~) D 2.08-
l.~S ~ 5
1~18 (~, 17~) and
O.gO ~t~ J~7~z, 3H)
-
3.57~
--23--
:
Rl m. p . ~ ~ ~
(CH3) 2C}~- ~110 (dec) (D~5O~a6) 8 . 2-8 . 08
(~, 21~J, 8.02 (d, J- .
S g~æ, 1~ . 3-4 .1 (m,
2~), 3.72 Id, J~7~z,
2~), 2.53-2.40 lm,
1~), 2.~2 ~t, J~g~z, :
2~), 2.,10-1.7 (m,
2~) " 1.~8 ~d, J~9~z,
3~) ~Ad 1.08-0.9
.. (In, 6~)
'~
~ ~110ld0c~ d6) B.3-7.9
(mt 3~), 4 . 33-4 ~ 17
(m, 2~), 3 . 72 (d ,
J~5~z, 2~1, 2 . 3-? .1 ,:~
(~ 2~) and 2 .10-1. 9
. Im, lS~) ;.
CH3(C~2)2- a~110(d2c) (~O-d6) 8.25-8.05
(~, 3~ . 35-4 . 22 :~
(~, 2~), 3.72 ~d, J~
~, 21I), 2.22 ~
.
J~ F~zo ~Pl), 2.12 (to
J~ lz, 2~), 2 . 08-
1 . 77 (~, 2~1, 1 . 55 .
lq, J~z , 2EI), ~ . 28 .
~d, J~ , 311~ and
0 u 88 (t ~ J~7i~z D 3E~
-'.
~' '
--24
Rl - m.p. ~C.
._
C~3 ~C~2) 4- >190 Ide~3 ~D~S0-d~ .33~8.0
~, 3E~), 4 . 35~
lla, 2E~), 3.7 5d, J~ -
6~z, 2R~, 2.17 (1:,
J~B~z, 2~1), 2.1 It~
1~8~ 2t 2~ 2.05- :
1 . 63 (~ 2~), 1 . ~8
~t, ~7Elz , 2~), 1. 2-
1.1 (m, 7E~) and 0 . 85
~t, JSG7~Z t 3~)
CE~3 ~H2)6- >180 5dec) tD~01 ~.42-~28
(m, 211) o 3r91 Is,
1~3, 2 0 4 ~ 7~z ,
2131 ~ 2 a ;27 ( 1 ~ J27~1z,
2~), 2.22 1 . 94 Im, .,
2~), 1.65-1,55 (m,
2H) " 1.39 Id, J~8Hz,
3~) t 1.3~-1,17 (m,
8H) and 0 . 73 (m, 3EI)
' ~''~
,.. .
:
~, '
-
-
-25- ;:
,c. 3~E~
~a3
~3(CH2)3C~c~2~ 6) ~.~7~~-03
~ , 3~), 4.32~
(~ 2~ ~ 3.72 (d,
z , 2E~), 2 . 22
Elz , 2~,
2.27-1 . 68 (m, 6}11,
lo 1.42-1~ 0 ~m, 101~1
aad 0 . 94-0 ~ 8 lm. 6EI)
~ 2CH3
15 C~3~C~2)3C~ S0--d~;) 8.22-8~0
~,S~ ~, 3E~), 4.32~
(~, 2H), 3 . 8-3 . 6
(~, 2~[), 2 . 28-1 ~ 68
SB) " 1.6-1.0
~, 12~ and 0 . 94O
~.7 tls, 6~.
(C~3C~2)2C~ SO-~6) 8.29~ 7.97
3~), 4 . 33-4 . 1
~, 2E~3, 3.8~ 3.~9
(~, 2EI~, 2 . 32-1 . 65
~, 6~) ,, 1.65-1.1?
(~D ~ ~d 1 . 02 -
30 0.68 (m, 6~)
:: `
'-'.
.
,
~2~7
-25
- R
~C~2-- ~ 50-db ) 8 . 3-B 0 0
(~s 3~), 4 . 32-4 . 1
S ~, 2~), 3.85-3.62
~3a, 2H), 2.21 ~t,
J~8~x ~ 211), 2. 02
8Elz , 2~),
2 . 01-1. 9 (m, lE~
0 1.115 l.S ~r~, 8~,
1,Z8 ~d, J~8Hz, 3~1
a~ 1. 28-0 . 8 (m, SH
(CH3CH2CEI2 ) 2C}ICH~ ~5SO~d6 ) 8 .18-8 . O
n, 3~ . 31~4 . 1
~ 2~) " 3. 84-3 .
(~ 2E~), 2 0 2~
J~6EI~, 2H) ~ 2.07
~a, J~BlE~æ , 2H), :~
2,.03~1.7 ~m, 3~),
1 o 15 ~, llE~)
~d O . 87 ~t, J-6Hx,
(CH3C~2 ) 2CE~CE12 (D~3S0--d6 ) 8 . 27~7 " 95
~, 3H), 4 . 3 -~ . 1 (m,
2~3 ~ 307~-3.~ (~,
2~ . 3 1 . 57 (~,
8~), 1..46-1.13
8E~ a~d 0.84 ~t,, J~
8~x, 6
7~4~
:
--27--
Rl m. p . ~,~,
~ H3
C~13 (CEI2~ 3C~C~2 5D~SO-d6) 8 .18-8 . O ,:
S ~R, S) ~,, 3~, 4 . 2~--4 . 05
~m, 2~), 3074-3.S6
~m, 2H), 2017 5t, J~
9Elz , 2EI), 2 . 12-2 . 0
~m, lEl), 2 . 0-1 i, 64
lo 5DI~ ~H~, 1 . 2~ (d,
J-6~z , 7EI), 1 . 14-
O 0 98 ~m, 2EI) ~nd
36Elz, ~)
lS
I H3
CH3 (CH2) 2CHC~2 (D~IS0 d6~ 8 . 2-8 . 04
(R,S) (mg ~), 4.26-4.08
~m, 21~, 3 . 76-3 .. 6
(m, 21I), 2 . 28-1 . 64
~, 7E~ -O.g~
(~, '7~ ~nd 0 . 96-
0.7~ ~m, 611).
.
'
~D ~
. .
:,:
:
. .
,~
.
j7~
-~8--
Rl al~ ~ ,,
H3 ) 2C~ tCH2 ) 3- ~ - 1~S0-d6~ 8 . 2 4-7 . 95
~s, 3B), ~.3-4.08
l~, 2~1), 3 . ~1-3 0 59
~Dl, 2~ ,. 21 (t,
J-6~z, 2h), 2 0 11
(t, J~z ~ 2~31, ;
2.05 1.38 tm. 7EI) 9 ",
l.,Z7 ~d, J~8~z7 3~) ,,
1.17-1.05 ~m, 2
a~ 0.86 ~d, J-lO~z
6~)
c~3 (D~SO-d6~ 8 . 24Tr8 . 0
C~3 (CE~ CH- ~, 3H), 4 . 33-4 .11
iR95) ~m, 2~, 3 . 79-3 . 6
(~ 2~ 2 a 41--;! ~ 29
(1;~1, llE~), 2 ~ ;~2 ~t ~
J~8~31z~ 2~ 66
(SD., 2EI) ~ 1~59-1~43
t~ 1038-1~11 ;.
(m, 11~), 1.06-0.95
1~, 3~ and 0 . 87 ,~
~t, ~-6~z,, 3
.
,
.
34
--29--
m.p.,~C
C~3C~2
5 CH3(C~23 3CHCH2 ~DI~SO-d6~ 8.24-8.02
(R,S) (m, 3EI), 4.32-4.11
~m, 2~), 3 ~ 3 . 6
~t 2~311, 2.24 ~t,
~gt~z , 2~), 2 . 08 : .
Id, J~lz , 2~, 2 . 03-
1.,~3 ~ ), 1.4~-
1.11 (m, 12~) and
0.97-û.71 ~m, 6H)
15 (CE13)2ClItc~2)~ D~IS0 d~j~ 8.24-8.û~
tm, 3~), 4.28-4.1
i~, 2E~, 3.76-3.6
2E~3, 2 . 18 (~
6~Iz, 2~), 2~1 tt,
6~z , 2~3, ~ O 04- ~ .
1.8~ , 1.84-
1.66 (D~ , 1.56-
1~38 Im, 3~), 1.23
(~1, 3~6Elz, 311) " 1.2-
1, 06 a~, 3~ d
~.82 (~ J~6~{z, 6~) ~
.,
::
--30--
C
C113
C~3 (C~2) 4~ 2~ fiSO-d6) 3.23 7098
IR~S) ib~9 3J~1), 4,.3-4.13
~, 2~), 3.~1-3~61
~Q, 2fl), 2 . 22 (t, -
;J5E~ 2~), 2 . 18 - ;:
1.68 ~, 6H), 1.45-
1007 ~m, 12~l~ and
0.98-0.8 (m, 6~)
.. :
C~3
(C~13)2C~(C~2~2C~c~2 (D~SO-d6) 8.37-8.03 ~
(R,S) l~, 3~I~ O ~.31-4.1
~, 2H) 7 3.78-3.6
~El, a~,, 2.26 (t,
J~ z, ~, 2.~-1.36
l~, 7E~) ~, 1.3 Id, J~.
8~1z , 5~), 1 . 25~1 . 05
1~" 2}13 ~nd 1. 05-
0.73 lm~ 9H)
:
~5 :
,~
~ 3~
:
-31- ::~
';
Rl m.~!C. ~ :~
~E~3 ~~ IDMSO~d6) 8.23-7.94
C~3) 2CHC:H2CHC1~2 (Im, 3~1), 4 . 3-~ .13
1~, 2EI), 3.Bl-3.51
(D~, 2~1), 2.21 ~t,
J-8Elg, 2~ . 15-
2 . 0 t~, 2H), 1 . 9
(t, J~8E~z , 2~1),
l . dS-1 . 52 Im, 3EE),
1~ 4--1~ ;!2 (1~1~ 3EIl r
1 ~ 2~2~0 ~ 94 (~ 3~)
~nd 0.94-0.80 (~, 6~
1~ .
C~3C~2
CH3~C}l2~2~HcE~2 IDMso-d6) 8.25-7.9
IR,S~ (m, 3EI~, 4.3~4.0~
Im, 2~), 3.81-3.62
Im, 23~), 2.22 (t,
Js8~z ~ 2~1, 2 . 06
(d, J~81~2, 2~
. 02-1 . 89 (m, 1~,
1~ 87-1. 65 lm, 2~
1., 41-1. 06 (~, llH)
~d 0.~8-~.7 (D, 6E~) :
. ~
,
~0 , .
~.
7~
--3~-
~1 m ~
O~d2- ~- (DISS0-~16) 8.33-7.9S
1~, 3E~ . 3-~ . ofi
(~ 2~) ~ 3.83-3.59 :
~, 2~), 2.21 It, ~:
J~8H;Go 2H), 2.11 l~
3~), 2008-l.a7 ~
1 . 87-1 . 3~ (~,
~B), 1 . 25 ~d, J~88z , ,~
3~ d 1. 22-0. 98
3~
16 (CH3C~2 ) 2C~ ~CR2 ) 2 (1~S0--d6 ) 8 . 21-8 . U
(~ 3~ . 32~
i~n, 2E~), 3.83-3.6
l~, 2~), 2.21 (t
J~8~z, 2E~1, 2.11
~t~ J~ I8, 2H) ~
2~05-1.89 ~m, 1~3,
1.87-1.67 5m, 1~) 0
l.S7-1.38 Im, 3~[3 g
1 . 38Q1. 08 ~m, 9~1
~d 0.83 It, Jz~6~z~
6Elj , ,
'
',
.
.
.
~2~7~3~
--33--
l ~3
C~3 IC~2) 2CE~ ~C~23 2- ~ ~ DlllSO-d6~ 8 . 24 7 . 97
(R,S) ~3~, 3~), 4033~4.1
(m, 2~), 3 . g6-3 . 59
(~, 2~), 2. 35-2 . 08
~n, 4~13, 2 . 08 1 . 9
(Dl, 1~), 1 . 89-1 . 67
(~ 1}~), 1.~3-1.46
1 0 416-1 . 02
im, llB~ and 0 0 98-
~o73 t~, 6EI)
C~3~2
CH3 (C~2l ~,C~C~2 ~S0Wd6~ 8 .2-7 . 94 ;~.
(R,S~ t~, 3E53 0 ~.26~4.06
~, 2~, 3 . 7~-3 . 56 .
a, 2~[), 2. 16 (t,
J1~6~, 2~), 2,.1-l. B4
(~Q, 31~), 1 . 84-1 ,. 6
(~, 2~1), 1 ~ 24 (d, J~
6Bz, 9H), ï.12 0.~2 :;
26 t~, 3~ 9.92~ :
0.64
.- :
~ . ~
- :~ 2~ ;7~
:
C~3
3 (~ ) 5C~C~2~ MSO-d6 ~ 8 . 23-8 . 0
lR,S) (211" 3~), 4.32--~.,06
(~, 2~) ~ 3.72 ld,
J~ , 2~), 2 ~ ~2
(t , J~lOHz , 2B),
2 . 16~ 611),
1. 42-1. 08 (m, 14~1
~nd 0 . 92~7 . 0 (m, 6~)
C2~5
16 c~3lC~2)3c~c~2 (D~qSO-d6) 8.2-8.0
(S) ~ay 3~ . 2~-4 . 1
l~, ;~1~3[) t 3.7~ 3.60
5m, 21~), 2 . 18 ~t~
J~7, 2~), 2.02 ~d,
J~7, 2H~, 2 . 02-1. 6
- (~, 3~1, 1.26 ~d,
J~6 , 3EI), 1 . 26-~ . 08
5~Q" 8El) and . 92-. 74
(~, 6HI
,
3~ . .
.
- 35 - ~ 5~ 72222-8
EXAMPLE 12
N-(3-(S)-Methylheptanoyl)-D-gamma-glutamyl-L-
alanyl-D-alanine (Rl = (5) CH3(CH~)3CH(CH3)CH2-;
R2 = CH3, R3 = ~NHCH(CEI3)CO2~)
12A. N-t-butoxycarbonyl-L-alanyl-D-alanine benzyl ester
To a solution of 23.0 g. (0.121 m) of N-t-butoxycar-
bonyl-L-alanine, 42.6 g (0.121 m of D-alanine benzyl ester ~-
toluenesulfonic acid salt and 17 ml. (0.121 m) of triethylamine in
400 ml. of cold (0C.) methylene chloride was added dropwise 25.0
g. (0.121 m) of dicyclohexylcarbodiimide in 100 ml. of methylene
chloride. After stirring overnight at room temperature the solids
were filtered and the filtrate concentrated to an oil. The resi-
due was dissolved in 400 ml. of ethyl acetate which was washed
with a 1% hydrochloric acid solution, a 10% potassium carbonate
solution, water and a brine solution. The organic phase was
separated, dried over magnesium sulfate and concentrated to an
oil. The residue was triturated with diethyl ether and the resul-
ting solids filtered under nitrogen, 16.0 g. An additional 12.7
g. of the desired product crystallized from the filtrate.
12B. L-alanyl-D-alanine benzyl ester hydrochloride
To a slurry of 28.7 g. of N-t-butoxycarbonyl-L-alanyl-D-
alanine benæyl ester in 150 ml. of dioxane was added 150 ml. of
dioxane saturated with hydrogen chloride and the mixture stirred
for 4 hours at room temperature. The solven-t was removed ln vacuo
and the residue triturated with diethyl ether. The resulting
solids were filtered, redissolved in methylene chloride and the
solution concentrated to about 150 ml. Ether was added and the
solids filtered under nitrogen, 22.0 g.
- 36 - 72222-8
12C. N-t-butoxycarbonyl-D-gamma-glutamyl (alpha
benzyl ester)-L-alanyl-D-alanine benzyl ester
To a slurry of 5.0 g. (9.64 mm)
N-t-butoxycarbonyl-D-gamma-glutamine alpha benzyl ester
dicyclohexylamine and 2.76 g. (9.64 mm) of L-alanyl-D-alanine
benzyl ester hydrochloride in 100 ml. of methylene chloride cooled
to 0C. was added 2.0 g. (9.64 mm) of dicyclohexylcarbodi-imide in
20 ml. of the same solvent. After stirring overnight at room
temperature, the solids were filtered and the filtrate
concentrated ln vacuo. The residue was treated with 150 ml. of
ethyl acetate, the solids filtered and the filtrate washed with 1%
hydrochloric acid, a 10% potassium carbonate solution, water and a
brine solution. The organic phase was dried over sodium sulfate
and concentrated to give a white solid, which when triturated with
ether and filtered gave 4.1 g. of the desired product.
12D. D-gamma-glutamyl (alpha benzyl ester)-L-
alanyl-D-alanine benz~l ester hydrochloride
To a slurry of 4.1 g. (7.21 mm) of
N-t-butoxycarbonyl-D-gamma-glutamyl (alpha benzyl
ester)-L-alanyl-D-alanine benzyl ester in 50 ml. of dioxane was
added 100 ml. of dioxane saturated with hydrogen chloride, and the
reaction mixture stirred for 3 hours at room temperature. The
solvent was removed under vacuum and the residue triturated with
diethyl ether, 3.5 g.
~r
-37-
.:
12~. N-13 (S)-~ethylheptanoyli-D gamma-gluta~yl (alpha
To 1.0 g. (1.98 ~ml o~ D-qamma-gluta~ lpha
benzyl ester)-L-alanyl-D-alani~e benzyl ~ster and .833
~1. ~5.93 mm) of triethyl amin~ in 50 ~1. of ~ethylene
chloride was added 390 mg. (2.37 mm) of 3-(5)~methyl-
heptanoyl chloride and the reaction ~ixture stirred
under nitrogen for 45 ~inutes. ~he reaction w~s poured
into 150 ml. o~ ethyl acetate ,and the organic phase wa~
washed with 10~ hydrochloric acid, ~ 10% potas~ um
carbonate solution, water and a brine solution. The
organic phase was dried over sodium ulfate and conce~-
trated to dryness. The residue was triturated with
ether and filtered under nitrog~n, 900 mg.
12F. N-~3-(S~-methylheptanoyl-D-gamma-glutamyl- .
L-alanyl-D-alanine
A mi~ture of 200 mg. of palladium hydroxide on
car~on and 900 mg. of N-~3-(S)~methyl~eptanoyl)-D-gamma- ¦
2D glutamyl ~alpha benzyl ester~-L-alanyl-D-alanine ben2yl
ester in 50 ml. of methanol was sh~ke~ in a hydrogen I :
a~mosphere at 5~ psi for one hour, The catalyst was
filtered and the solvent removed in vacuo. Water was
added to the residue and removed under reduced-pre~sure
to give ~92 mg. of the product as a white solid, m.p~ ¦
165-168C.
The N~R spectrum (DMSO-d6) showed a~sorption at
8.21-7.98 tm, 3~), 4~41-4~1 tm, 3~j, 2.3-2006 ~, 4~0
2.06-1.56 lm, 6~), 1.43-1.02 (m, llH) and 1.02 0.73 (m,
3~ 6~) ppm7
-38-
EXAXPLE 13
N-(3-(S,R)-Ethylhexanoyl)-~ ga~ma-glutamyl
(alpha n-butyl estes)-glycyl D-alanine
( 1 C~3~C~z)2C~(c2~s)c~2~; R2 ~ H;
R3 - -N~ ~(CH3)CO~ C~
J -~ C~ y /
13A. N-t-butoxycarbonyl-D-gan~a-gl~t~Din~~~alpha
A solution of 39.5 g. I0.,172 m) of N-t-bu~oxy-
1~ carbonyl-D-glutamic anhydride in 75 ml. of dry tetra-
hydrofùran was added dropwise o~verba~at~ol hour period to
a solution of 4~ ml. (0.516 m~ and 34.3 ~1. (0.172 m) of
dicyclohexylamine in 300 ml~ of ether at 0C. The
reaction was allowed to ~tir at 0C. for 3 hours and ~as
15 . stored in a refriger~tor overnight. ~he solids were
filtered, slurried in ethanol and filtered, 43.3 g~
13B. D-gamma-gluta~yl (alpha n bu~yl ester)-glycyl-
~ h~ product of Example 13A tlO g.~ 0.021 ~ and 6.7g. (.024 ~) of glycyl-D-ala~ine ben~yl este~ hydro-
chloride were slurri~d in 200 ml. of methylene chloride
under nitrogen and cooled to 0C. Dicyclohexylcarbodi-
imide (4.25 g., .021 m) was added and the ~ixture
allowed to warm to room temperature overnight. The urea
byproduct was ~ilte~ed and the solv~nt re~ov~ in vacuo.
The residue was treated with ethyl acetate and filtered~
The filtrate was washed succes~ively with water, 2.5%
hydrochloric acid, water, 10% potas6i~ carbonate and
brine. The organic phase was dried over magnesium
sulfate, the solvent removed under vacu~m and the
~,Z~ j7 '~3 L~
--3 9--
,
residue dissolved in 300 ml. of dioxa~e ~aturated with
hydrogen chloride. After stirrin~ 4 ho~rs at room
temperature, the solvent ~as removed ana the residue
triturated in ethyl ac~tate-h~xane (1:1) and filtered,
7.4 g.
13C. N-(3-(S,R)-ethylhexanoyl-D-glutamyl(alpha n-
but~1 ester)qlycy~-D-alanine _ __
To the product of Example 13B (1.0 g., 2.35 mn) and
.99 ml. (7.05 mm) o~ triethylamine in 50 mlO of
I0 methylene chloride was added 460 mg. l2.83 mm) o~
3-(S,R) ethylhexanoyl chloride and the reaction ~tirred
overnight under nitrogen. The solvent ~ai~ re~oved in
vacuo and the residue dissolv~d in ethyl acetat~. The
16 organic phase was successively wash~d ~ith 10%
hydrochloric acid, water 10% potassium carbonat~ and .
brin~. The organic phase was separated, dried vver
~agnesium sulfate ~nd the solvent remoYed under vacuum. :
Th~ residue was dissolved in ~0 ml. of emtha~ol and
shaken with 170 mg. of 10~ palladium hy~roxide in a
hydrogen at~osphere at an initial pres~r~ o~ 50 psi for
1.5 hours.
The spent ~atalyi~t wa~ filtered and th~ solvent
removed in vacuo, 100 mg.
NMR ~D~SO-d6): 8.18 (d, J - 6, 1~ o 8.10 (d, J=6,
1~), 8.02 (t, J-5, 1~), 4.28-4.10 (m, 2~ .09 it, J-6,
2H), 3.78 3.56 Im, 2~), 2.18 St, J 6, 2~), 2002 (d, J=6,
2~), 2.00-1.60 Im, 3H), 1.58-1042 Im, 2~), 1.28-1.08 (m,
8~, 1.24 ld, J=6, 3~)~ 0.92-0.76 Im, 9~).
: 30
~S~
--4 0--
EX~MPLE 1 4
Employing the general procedure of Example 13, and
starting ~tih the requisite reagen~s, the ollowing
s:ompounds were prepared:
O
RlCN~ ~ I) ~ C2R4
fH D
C O ~CH2) 2CONE~CE~2CON~CEI(C~3~2
~ R4 D~R
3 -methylheptanoyl methyl Nlq~ (DMSO-d6 ): 8 . 23 ~d ,
J~ , 1}1), 8 . 15 (d, J -6 ,
1}1), 8 . 03 (t , J~6 , 11~),
4 . 28-4 . 1~ (m, 2E~); 3 . 72
~d, J-6, 2E~), 3.61 (s,
3~ 3 ~t, J-7 , 2~),
~.16-1.70 Im, 6EI), 1.34
loû4 (~a, 8~1) t 1.25 ld,
J~75 31~) 9 o.s2;-o.?~ Sm, ~:
6~).
.
. .
-
.~2~7~
-4 1 -
.J~ ~/ R,a N~fn
3-ethylheptanoyl methyl NMR (Dl~SO-d6~: 8.19 (d,
J~6 , 1~), 8 . 1 1 ~d , J~6 ,
1~, 8003 ~, J-6, 1~
4 . 2~-4 . 10 ~m, 2~), 3 . 74- : '
3,62 ~, 2~), 3.57 I~.
3~, 2.18 (t, J-9, 2H),
2.01 ld, Js6~ 2~1, 1.97~
1 . 60 ~m, 3~), 1 . 32-1 . 10
~m, 88), 1 . 23 (d, J-7 , ;
3EI), 0.90-~.72 (~, 6~) :
ll . 3-methylheptanoyl ethyl N~R ID~SO-d6) s 8.. 248.n2
(m, 3~ .26-3.56 (m, ~ ~ .
~, 4.04 (q, J'9
3.76-3,56 ~m, 2~) ~ 2~17 ;
(t, J~7, 2~ . 12-1 . 63
(m, 6~); 1 . 74-0 . 9d, ~m,
6~), 1.23 (d, J~5, 3~),
1.13 ~:, J~9, 3~;, 0.8~-
0 . 72 (m, 6~3
~ ;.
'
5~
,
'~ R, c o
.~ 4 ~R
3-ethylhepta~oyl ethyl NMR (D~SO-d6): 8.16 (d,
J#6, 1~), 8.09 ld~ J~6,
~ 8~02 ~t, J~6, 1~),
4.~2-4.08 lm, 2~), 4.D2
(q~ J~7, 2~), 3.7~-3,54
~m, 2~3, 2.1~ (t, J-7, ~:~
2~1, 2,00 (d, J~6, 2~j,
1.96-1O58 (m, 3~), 1.30-
1.08 (~, 3~), 1.21 (d,
~'7~ 3~) t 1.13 (t, J~7,
3~)~ 0O88 0~70 (m, 6~)
3-methylheptanoyl iso- NMR tDMSO-d6): 8.14 (d,
bu~yl J~6, 1~), 8.08-7.98 lm,
2~), 4.20-4.04 (m, 2H)~
3.75 (d, JY6~ ~), 306B- :
3.54 (m, 2~), 2.14 ~,
J~6, 2~ .08~ t
1.28-0.96 ~, 6~),
1~19 Id, J~7, 3~, 0.88
0.70 ~m, 12~)
as
1":, .
~ '
'.
:
: ' ' '',.
'' .'' , .'~
~ ~ R ~
__ .
3-ethylheptanoyl iso- ~MR tDt~lso; dc): 8 .21 (d,
butyl J~6~ 1~) t 8.1~-8.04 (m,
2B), ~ . 24-q . 08 ~, 2El),
3-79 Id, J-6, 2E~1 I 3.72-
3.58 (m, 2~1), 2.19 (t,.
J~7, 2B), 2.03 5d, J-6,
28), 1 . ~9-1 . 6~ (~, 4~
1 . 32-1 . 10 ~, 81~), 1 . 23
(d, J-6, 3EI), û.g~ 0~72
Im. 12~)
16 3-ethylh~xanoyl isc>-- NMR (DNSO~-d6). 8.18 (d,
butyl J=6 , lH~, 8 . 10-8 . 00 (m,
2~) ~ 4.26-~.08 Ir~, 2~I),
3.79 (d, J~6 , 2~33, 3~,72-
3.58 Im, 2H1, 2.18 tt,
J~6~ 2~), 2.02 ld, J~6,
2B), 1.98-1 . 62 ~m, 4E3 ),
1 . 3q-1. 08 (mO 6EI), 1 . ~3
~,d, J~7; 3~), 0.96-0.72
(m, 12
,,~.
, :,
''
. .
,
.
i7~3~
~ i -44~
-''' R,~-C
R4 NMR
3-ethylhexanoylmethyl ~R (D~IS0~63: 8.21 ~d,
J~7, 1E13, 8.10 (d, J~7,
11~), 8 . 05 (t, J~6 , lH~,
4.,26-~ .10 ~m, 2H), 3.76
3.~0 (~, 2EI~, 3.5g (~,
3~), 2.18 (11:, J~6, 2~
2.02 (d, 3~6, 2H), 2~0û
1 . 60 (~, 311), 1. 3~ 1 . 08
(~, 7EI), 9.90-~.72 (~
611) .
3-ethylhexanoyl ethyl pa~qR (D~S0~6) 5 8.22 (d)
J~7, 11~ " 8.l8-a.~6 (m,
~3, 4 . 26-4 . 10 (m, 2~),
4,.06 Sq" Js5, 2Hl, 3.7~
3 0 58 ~m, 2E11~ 2 . 20 (~ ,
J~6 , 21~1, 2 . û4 Id , J-6 ,
21I~, 2.02-1.60 Im,, 3H) 0
1~26-~.20 (~3, 7~I~, 1.18
t , J~5 , 3~1~, 0 . 90-0 0 7
~o ~13
.
:io
`
57~3~
~!
--~5--
O
R4 ~R
__ __
3-~ethylheptanoyl butylNMR ~DI~ d6): 8 . 20 (a,
J~7, lE~ ~ 8.16 B.04 i;o,
2~1), 4 . 24-4 ~, 0S ~m, 2EI),
4.00 (t, J~6, 2H), 3.7~-
3 . 56 ~m, 2EI~, 2 . 17 ~t,
J~6 , 2}1), 2 . 12~ 0 ~, !
5}~ 1.58-1.40 ~m, 2~
1.36-l.00 ~, 8~), 1.21
(d~ J-6, 3~), 0.90-0.7~ :
(m, 9EI)
3-ethylhep~a~oyl butyl N~R ~I)IqSO d6): 8.16 ~d,
J~7, lEI) " 8.11 (d, J57,
1~, 8.03 ~, J~5, 1~,
4.26-4.09 ~m, 20~1), 3.99
(t , ;1~7 , 2~I~, 3 . 79-3 . 58
(m, 2H), 2.17 (t, 3~6y
2}1~ 2.01 ~a, Js6, 2~),
2.00-1,,60 ~m~ 3E[) ~ 1058-
1.42 I~, 2~11, 1.36-1.08
(~, lOEI) ~ 1.24 (d, J-S, . :
3~, 0.92-0,,72 (~, 9El)
!
.
,:
.
.
~ 7~3~
~6-
EXAMPLE 15
__
N-~3-(R,S)-Ethylhexanoyl~-D-gamma-yl~tamyl glycyl-
D-alanine ethyl ester ~Rl ~ C~3(C~212C~C2H5)C~2 ;
R ~ ~; R - -~NC~C~I3)C02C~
S 2 3 ~ 5
15A D-gamma-glutamyl (alpha benzyl ester)glycyl-D-
alanine ethyl ester h~d~ ~
To a ~lurry of 14.8 y. ~.0285 ~) of N-t-butoxy- :
carbonyl-D-gamma-glutamic acicgl alpha benzyl e~ter
dicyclohexylami~e salt acid ~ . V285 ~) o glycyl-
D-alanine ethyl ester hydrochloride in 200 ml~ o
methylene chloride was added 5.6 g. ~0270 m.~ o~
dicyclohexylcarbodiLmide an~ the mixt~re ntirred under a
nitrogen at~osphere overnight. The ~rea i~ filtere~ and
the solvent removed in acuo. The r~sidue was treated
with 300 ml. of ethyl acetate, filtered ~nd the filtrate
washed successively with 2.5% hydrochloric acid, water,
10% potassium carbonate solution and brlne. The organic
phase was separated, dried over magnesium and concen-
trated under vacuum. ~he residual oil ~as dissolved in
450 ml. of dioxane saturated with hydrogen ~hloride.
The ~olution was stirred ~or 2 hours and the solvent
removed in vacuo. The resi~ue was triturated with ether :
an~ filtered, 11,2 g.
15~. ~(3-~R,S)-e~hylhexanoyl)-D-gam~a-glutamyl- :.
~lvc~l-D-alanine ethYl est~r :
. , ~ _ _ , . . .
To the product o Exa~ple l5A 11.0 g., 2.33 ~
and .98 ~ 6..98 mm.,) of triethylam~e in 30 ml, of
methylene chloride, under a nitrogen atmosphere, was
added 378 mg. ~2.33 ~.. 3 of 3-(R,S)-ethylhexanoyl
chloride. Aft~r stirring at room te ~ :rature for 1.5
hours the mixture was poured into 100 ~1. of ethyl
.... . . ..
_ 47 _ ~ ~ S 7~ ~2222-8
acetate and the organic phase was washed successively with 10%
potassium carbonate solution and brine. The organic phase was
separated, dried over magnesium sulfate and concentrated in vacuo.
The white solid residue was dissolved in 30 ml. of methanol and
hydrogenated over 0.1 g. of palladium hydroxide in a hydrogen
atmosphere at an initial pressure of 50 psi. After 2 hours the
catalyst was filtered, the filtrate concen-trated to dryness and
the residue triturated with ether and filtered, 275 mg.
NMR (DMSO-d6) 8.26 (d, J=9, lH), 8.14-8.02 (m, 2H),
4.31-4.00 (m, 2H), 4.06 (q, J=10, 2H), 3.78-3.60 (m, 2E), 2.17 (t,
J=8, 2H), 2.08-1.65 (m, lH), 2.03 (d, J=8, 2H), 1.82-1.53 (m, 3H),
1.40-0.96 (m, 5H), 1.23 (d, J=6, 3H), 1.14 (t, J=10, 3H),
0.90-0.64 (m, 6H).
EXAMPLE 16
Starting with the appropriate reagents and employing the
procedure of Example 15A-15B, the following compounds were pre-
pared:
O
11
RlCNH \ D / C02H
CH D
(CH2)2CONHCH2CONHCH(CH3)CO2Rs
X
~2~ 34
--48-- ;
,O
,~, , , 3R5 ," ,NM ... t. "
3-methylheptanoyl iso-butyl MI~R (Dt4SO-d6): 8.24
~d, J~6 , 1~), 8 . 1û-8 . 00
~m, 21~ . 30 ~ .18 (m,
. O~ ~m, 1
3 . 86-3 . 72 (~n, 2H~,
3.72- 3.58 ~m,, 2~),
Z.16 ~t, J-6 , 2El),
2.12--1~64 ~, 6~I1 D
1.52-1. 00 Im. 6~
1 . 27 ~d o J~7 ~, 3El),
0.90-0.76 (m, 12~)
3-ethylhexanoyl iso-butyl ~R ~I:)MSO-d6): 8 . 23 (d,
J~6, lEI) " ~.0~7.99 ~mg
21~), 4.29-4~17 ~m, lEI), :
4 . 17-~. 07 (~, 1~),
3.83-3.71 ~m, 2~),
3.71-3~5$ (m, 2El) ~ 2.15 .
(t, J~7~ ~), 2.0~-1.60
~gl, 4~), 2.00 ~d, J~6,
2~3, 1.31-l~Og Im, 6~1),
1 . 25 5d, J~6 , 3~),
0.90-0.72 (m, 12~I~
-
.2~3~7~3~g, ;
-49- .:
0 R5 N~
3 ethylheptanoyl iso-butyl NMR ~D~SO~d6): 8.23 (d, ;
~8~ lM), 3.08-7.98 ~m,
2~) ~ 4~29-~ol8 tm, 1
4.18-4.07 (m, 1~),
3.86 3.72 (m, 2~,
3.70-3.57 tm, 2~, 2.15
(~ J-7, 2~), 2.04-1.5g
t~, 4~), 2.00 (d, J~6,
2~), 1.30-1.11 (m, 8~),
1~25 ~d, J36, 3H),
~.89l0.70 (~ 9 12H)
lS c~c/oh~ ne7~
3-methylheptanoyl ~ }- NM~ ~D~SO-d~): 8.25 (d,
~ye~he~t~ J~6~ 1~), 8.13-8.00 ~m,
28~, 4,32-4.20 (m, 1
4~20-4.08 ~m, lH3,
3.91-3.76 (~, 2~),
. 76-3.5~ (m, 2~), 2.1
~tJ J~ ), 2.13-1.48 .
(m, 8~), 1.36-1.01 Im,
12~), 1.27 ~d~ J56,
3~), 1.0~ 0.76 t~
50- .
R~ CO ,,~
3 ~}~ ~5
~_ , . .
~)~Clo~< y/~n ~f~y/
3-ethylhexanoyl -a~L- NMR ~D~SO-d6~: 8.~5 ~d,
_4~4~L~h~ J~6, 1~), 8.13-8.09 (~,
2H), 4~32-4~20 (ffl,
4.20-4.08 ~m, 1
3.9~-3.7~ (~, 2~
3.74-3.59 ~m, 2~), 2.18
tt, J~6, 2H3, 2.09-1.86
(m, 1~), 2.03 (d, J~6,
2~), 1.82-1.43 ~m, 8~
1.36-I~01 (~, 9~), 1.27
(d, ~6, 3H), l~01-0.70
l6 (m, 8
~yc/~e~ y/h~ y/
3-ethylheptanoyl -met~y~ NMR tD~SO-d~): 8.26 ~d,
h~hexrl- J-6, lR), 8.12~8.02 ~m,
2~ .31-4.1~ (m, 1~),
4.19-4.0~ ~m~ lH),
3.93-3.7~ (~, 2~),
3~72-3~58 (~, 2~,
2.18 ( , J~6, 2H),
2.08~1.86 (m, lH), 2.03
2S (d, J~6~ 2~)o 1.82-1.48
(m, 8~), 1.34 1.02 ~m,
11~), 1.27 6~, J36,
3~ 00 0~7~ lm, 8~ :
:
. .
;i7~
-51-
(` 0 ~ ~,
R5 ~MR
.; . , .-
3-methylheptanoyl ethyl N~R (D~S0-d6): 8.25 (d,
J~6, 1~), 8.12-B.00 (m,
2~), 4.28-3.96 ~m, 2H)~
4.03 ~q, J~7, 2R),
3.74-3~56 ~m, 2~), 2.16
(~, J~9, 2~), 2.11-1.62
0 Sa9 6~), 1.32-0.98 ~m,
6EI), 1 . 24 ~d , J~7 , 3H),
1.14 ~t, J-7, 3H),
0.88-0~76 ~, 6~)
3-ethylheptanoyl e~hyl ~MR ~D~S0-d6~: 8.28 ~d,
J~6; l~l, 8016-8.04 ~m,
2~), 4.32-4.04 ~m, 2H)~
4.10 (q, J36 9 2~,
3.~8-3.6~ ~, 2~), 2.22
(t, J-6, 2~), 2.11~1.92
~ 3, 2.07 (d9 J 6,
2~ 1.8601064 S~, 2~
1.~0~ m, 8~), 1030
(d, J~69 3~), 1.21 (t,
~J~6, 3~), 0.94~0.7
2~ (~, 6~
i7~
.
-52-
R/_~D
R5 ~MR.
-- ;
3-ethylhep~ar~oyl l~utyl N~IR tD~IlSO~d6): 8.27 Id,
J~8 , 1~1~, 8 ~ . 02 (al, :
21~ ,.32~4.1û (m, 2El),
~l i lO-3 . g~ ~, 2}I),
3.7a 3.60 ~, 2~I), 2.18
It, 3~6 , 2~, 2 . 0~a ~d,
J-~ , 2E~, 2 0 0~-1 . 62
3~), 1060-1.46 Im, 2
1.38-1 . 10 I~, 12~
1 . 27 ~d , ~-6 , 3}I),
0~,90 0.75 Im, 9~1)
16
3-S-~et~yll~eptanoyl butyl W~ (DMSO-d6): 8.30 (d,
J~8, 111), 8.15-8.04 ~,
2Ba, ~.~S-~.12 ~, 2~), !
~L r l 2 3 ~ 9 ~
3078-3~65 (~ 2~ 2~22
(tJ J~7~ 2EI) ~ 2~18-1~69 .-
~" 7EI), l.G1~1.48 (~,
21~), 1.~0-1.11 (m,
llB), 0 . 97-O . B0 (m, 9
2S
30 - . .,
~5i7~3~
,
` . ~ ,.................................................... ..
~ 5 NMR
3-S-ethylheptanoyl butyl N~ ID~SO d6): 8.22 (d,
J87, ~, 8.12~.0 ~, ,
2H), ~ 6 (m, 2~,
4.08-3.95 ~m, 2
3.75~3.~2 t~
~t, J~6, 2~), 2.02 ~d,
J~6~ 2~), 2.04-1.6
3~), 1.6~-1.46 I~, 2~
1.38-1~1 1~, lS~), and
.9_.75 ~m~
. :
lS 3-ethylhexanoyl butyl NMR tDMSO-d6): 8~28 (d,
J~8, 1~), 8.14-8.04 Im,
2~ .34~4.10 (m, 2H),
4.10-3.95 (~, 2~
3.75-3.62 Im, 2~), 2.19
2~ (t, J~6, 2~), 2.04 (d,
J~6, Z~), 2.04-1.6~
(~, 3~), 1.60-1.45 Im, . .
2~), 1.40-1.10 (m~ :;
13~), 0.90-0.76 (m, g~) ;1
2~ 1 '~,.
i ~
--5~
EXAI~PLL 1 7
The procedure of Example 15 i~ a~ai~ ~epeated,
starting with the appropriate reagents, ~ith the
exception that the hydrogenation i8 not casried out, to
give the follo~in~ compou~ds:
Il . 1'
RlCM~ ~D ~ C02R4 ;:
C~[ D
'1~23 2CONHC:E~2CON;E~ C~3~c2R5 ,;
O ,:
~;;` ~ R R5 NMR
3-ethyl- butylbutyl N~R (DMSO-d6): 8.27 (d, J~7,
hexanoyl 1~), 8.20 ld, ~7, 1~), 8.07
(t, J~70 1~ .37-4.13 (~,
2~3, 4.0~ (t, J~6, 4~), .
3.80-3.62 Im, 2~), 2.20 ::~
~t, J~6, 2~, 2.n5 (d, J-6,
2~), 2.92-1064 (m, 3~1, 1.60- :
1.47 (m, 4~3, 1.40-1.13 (m, ..
13~), 0~95-0O~7 (m, 12~3
3-ethyl- butyl butyl NMR ~DMSO~d63: 8.27 ~d, J-7,
heptanoyl lH); 8.20 ~d, JD7Y 1~3 ~ 8-06
(t, J~6, 1~, 4.36-4.13 (m,
2~), 4.02 (~, J-~, 4~),
3.80-3.6q (m~ 2~), 2.20
- l~, ~=6, 2~, 2.04 (~, J=6, .
2H~, 2.00-1.60 g~, 3~) t 1O60-
1.~3 (m, 4~3, 1.40-1.10 ~,
~ t 0.95-0~72 (~, 12H3
-
3571~
,~$~ o _55_ .
~1 R4 R5 N~gR
3-methyl butyl butyl N~IR (DMSO-d6~: 8 . 26 (d, J~7 ,
hepta~oyl 1~ . 19 (d, J~7 , lEI), 8 . 07
~t, J~6; 1~), 4.32-~.11 lm,
2~ . 02 5~ 0 J~5 t 4EI),
3 . 79-3 . 59 ~m, 2R) O 2 . 20
(t, J-6, 2~) f 2.14-1.68 Im,
5H7, 1 . 61~ .6 (m, 4H~, 1 . 40 -
1 . 06 Im, 13~), 0 . 95-0 . 81 (m,
12~I)
3-S benzyl benzyl NMR (DMS0-d6): 8 . 33 td, J~7 ,
15 methyl~ , 8 . 24 ~d, J~7 , 1~), 8 . 08
heptanoyl (t, J-5, 1~), 7~33 (s, 10~
5.08 ~s, 4H), 4.40~4022 (mt
~}~), 3.~û-3.60 ~ , 2.2
(~, J-5~ 2~), 2.14-1.64 ~m,
20 5~I), 1 . 26 ~ 7, 3~), ;
1.22-0,98 ~m, 6El), 0.88-0.73
(~, 6~1 ..
;
:j,
~,,,
,
':
2~ ~7 ~ ~ ~
-56-
EX~MPLE 18
Crystalline ~-(3-tS)-methylhep~anoyl)-D-gamma-
qlutamyl-qlycyl-D-alanine
N-(3-~S)-Methylheptanoyl)~D-qamma glutamyl (~lpha
benzyl ester)-glycyl-D-alanine benzyl ~ster (30.8 g.)
was slurried in 300 ml. absolut;e eth~nol ~n a 2 liter
autoclave. 5~ Pd/C, 1.54 g., S0~ water wet) was added
and ~he ~ixture hydrogenated at: 4 x atmosph~ric pressure
for 1 hour, by which time uptake of hydrogen was
I complete. The catalyst was rec:o~ered by filtratio~,
fir8t over paper, then over 0.~5 ~icro nylon ~ilipo~e,
employing 100-150 ml. ethanol or tran~fer a~d wa6h.
The com~ined filtrate and wash liquors we~e stripped to
1~ a damp, white solid, which was di~solved ~n a 150 ml.of
a hot, 1:10 mixture of ab~olute ethanol and
acetonitrile, clarified by hot filtration, boiled down
to 35 ml., 810wly cooled to ro~m temperature, granulated
and fil~ered to yield crystallin~, den~e, non-electro-
static tit~le product, 20.1 g. (94~) characterized by it8
ir (~u~o~ mull) ~hich includes major, well-r~olved,
sharp peaks at 3340, 3300, 2900, 2836, 1725, 1650, 1628,
15B0, 1532, 1455, 1~10, 1370, 1280, 1240, 1276 and 1175
Cm~l
This crystalline product (9.4 g) was further
purified by dissolving in 1000 ml. of acetone at reflux
for 1 hour. The solution was cooled to room temperature
and seeded with a trace of the above cryctals. Afte~
stirri~g for S hours, title pro~uct was recovered by
filtration with minLmal acetone wash, and dried ln vacuo
at 35C., 7.25 ~., having identical ir characteri~tics.
p~
,
7~
-~7- :
EXAMPLE 19
N-(3~ Methyl-~-heptenoyl~-D-g~mma-glutamyl
(al~ha benzyl ester~-~lyc~l-D-alani~e be~ l ester
Following the procedure o:E ~xample lOD, 2.77 g. (5
mm) of D-gamma-glutamyl ~alpha benzyl e~ter) ;~lycyl~
D alanine benzyl ester hydrochloride an~ the acid
chl~ride prepared ~ro~ 747 mg. (5 mm) o~ 3-(Rj-
methyl-4-heptenoic acid gave the titled co~pound.
XA~PLE 20
N-(3-(S)-~ethyl-4-heptanoyl)-D-gamma~glutamyl-
cllYc~l-D-al.mine
A mixture o~ 500 ~g. of the product from Example 19
and 26 mg. of 5~ palladium-on-charcoal ~50~ water wet)
in 125 ~1. of ethanol was shaken in a hydrog~n
atmosphere at an initial pressure o~ 4 x atmospheric
pressure for 2.5 hours. The cataly~t was iltered and
the solvent removed i~ vacuo. The produ~t was purified
by the procedure of ~xample 18, and was identical in all
2~ respects to the prod~ct of that exa~ple.
,
.:
2~ ~'''
,::
. ~ ~ .. ~ .. . . . . . . . .
-:
i7~
58-
:'
PREPARATIOR A
Al. ethyl cvcl~ e-e
To 4.9 g. of 60~ sodium hydride in oil wa~ ~dded
sufficient hexane to di~solv~! the oil. To the oil free
sodium hydride under nitrogerl ~as added 100 ml. of dry
tetrahydrofuran followed by ~l solution of 22.2 ml. of
triethyl phosphonoace ate in 80 ml. of dry tetrahydroD
furan. After stirring at room te~perature ~or one hour
10.5 ml. of cyclohexanone wa~ added i~l ~0 ml~ o~ tetra-
hydro~uran and the reaction ~ixtur~ ~tirr~d at room
temperature overnight~ The reaction ~ pourad into
water and extracted with diethyl ether. ~he or~anic
lS phase ~as wa~hed with lN sodium hydroxide ~oluti~n,
water and brine. The organic phase was sep~ra ed, dried
over ~agnesium sulfate and concentrated under reduce~
pre~sure .
~he residue was dissolved in 250 ~1. of ~ethanol,
23 trea~ed with l.S g. of 10~ palladiu~ hydroxide on carbo~ :
and the mixture ~haken in a hy~rogen atmo~phsre at 5 0;i
psi for ~ hours. The ¢atalyst was filtered a~d the
f iltrate conce~ltrated in vacuo . The residue wa~ dis-
tilled at 45-50C. /0 . ~ torr to give 15 . 4 g. (9096 yield)
of the desired intermediateq ~::
"
A2. cyclohexylacetyl chloride
To 100 ml. of metha~ol ~s~taining lS.~ g. of ethyl
cyclohexylacetate was added 15.2 g. of pot~ssium
hydroxide and the 301ution refluxed for 3 hours. The ::~
methanol was removed ~n vacuo and the residue trea~ed
with ~ater. The ~olution ~as extracted with diethyl
ether and then a~idified with 10~ hydrochloric a~id~ ;
The acidified ~olution wa~ e~tracted ~ith fresh ether
and ~he organic phase ~eparated and ~ashed with ~ater
. . . , - :
3~ii7~
59 ~:
and a brine solution. Removal o~ the ~olvent after
drying gave a liquid residue.
The residue was dissolved in 60 ~l. o~ ~ethylene
chloride was treated with 18 ml. o~ oxalyl chloride.
After ~tirring at room temperature ~or 4 ~our~ the
reac~ion mixture was concentrated und~r vacuum and the
residue distilled~ 45/50C.t0.4 torr, 12.5 g~ ~86
yield).
PREPARATION B
Following the general prooedure of Pr~paration A
and starting with triethyl phosphonoacetal ~nd the
appropriate aldehyde or ketone the following ~cid
chlorides w~re prepared~
IS ~lCO~l
l Z.P.-C./torr
~C~13C~2C~2i 2CEICEI2 ..
tC~3CH2)2C~c~2 22-~5/0.5
:
1~3
C~3(C~2)3C~C ~ 23-30~0.5
C~ -
1 3
C~3~C~2)2~C~ - 22-25J0
~R,S~
3a (c~3)2c~(c~2)3 Z4-3110.7
'~.'
. .
,~ . . . ~
.; .
i7~3~
-60-
Rl ~ L~ ;
f~3C~2 .,,
C~13 (~ ~2 ) 3CHCH~ 3~-37/ 0 ~ 5
'
f~3 :
CH3 (CH2) 4C9CH 45~47/O.li
,S~ :
25-30/O.S
~CH3C~2) 2CE~(C~2) 2 32 36/0.~ ~
Cl~ I .:
1 3 ,:
CH3 (C~2~ ;! tHR(S~ 2) 2 30-3~/.06
:.
I 3CH2 .'.
C~3 (C~2) 4 (RCS~ 63-65/ .35
CH3 (C92) 5 ~RCS j~ 89~215
:'
f~3
(C~332C~(C~2),~C~C~2 46~;50/0~5
7~
-61~
Rl ~
CH ' "
1 3
1C~3)2C~C~2C~C~ - 30-34~.5 :
~R,S~ ~
.,
3IH2 ..
(R,S~ 31-35/0.7
:;'i
PREP~RATION ~
5~11~= .
Cl. 3-hydroxy-4-meth~ pentene
To 90 ml. of l.OM vinyl magnesium bro~ide in
tetrahydrofuran cooled to ~C. was added dropwise 6.3 -:
ml. of i~ob~tyraldehyde in 30 ml. of tetrahydrofuran and
the mixture then allo~ed to warm to room temp~ratuse.
After 2 hours the reaction was added ~o a ~aturated
ammonium ~hloride solution and extracted with ether.
The ether extracts were combined, ~ashed ~ith a .
sa~urated ammo~ium chloride solution~ a saturated sodium
bicarbonate solution and a brine solution, and dried
over magnesium sulfate. The solvent was removsd i~
vacuo to giv~ 6.0 g. of the desired product.
;.
:
'
'
',.
--62
i~ ~ h e p ~eh o ~
'3~ C2. 5-~ethyl-4-he~n~e_acid ethyl ester
A mixture of 18.2 g. of 3-h~dro ~ 4- ~thyl~l-
pentene, 200 ml. of triethyl ~.r~e~EE~ and 500 ~1. of
~-toluenesulfonic acid ~as tre,ated with 400 ~l. oP
toluene and heated to reflux over 4A ~olecular ~ieve~
for 24 hours. The solvent was removed in cuo a~d the
residue distilled~ The fractilon di tilling at ~5-64C./ ,~
0.5 torr ga~e 7OS g. of the desired pro~uct.
C3. 6-methylheptanoic acid ethyl ester
To 7.5 g. of 6-~ethyl-4- ~ cld ethyl ester
in 75 ml. of methanol w~s adde~ 700 ~g. of 10~ palladium
hydroxide on carbon and the mixture shak~n in a hydrogen
atmosphere at S0 psi for 1.5 hours. The cataly~t ~as
filtered and the solvent removed under vacuum to glve
5.7 g. of the desired productO
C4. 6-meth~lhe~tanoyl chloride
~ ollowing the procedure of P~epa~atio~ A2, 5~7 g.
of 6-methylheptanoic acid ethyl ester gav~ 2~0 g. of the
desired product, b.p. 30~34C./0.5 torr.
:
' ,',~.
I;
7~
--63--
PP P~TI~ D
~ y~oyl chloride
Dl. 2-methylheetanoic acid
To a cold (0C~l ~olution of 100 ~1~ o~ dry
S tetrahydrofuran containing 11.8 ~1. o~ dry dii~opropyl
a~ine and 55 ~1. of 1.6~ n butyl lithi~m was added 5.4
ml. of n~heptanoic acid and the mixture allow to stir at
room temperature for one hour. The resulting solution
was cooled to O~C. and 7.2 ml. of methyl iodide was
added. The reaction was stirred at roo~ temperature
under nitrogen for 105 hour~ 9 and wa~ then poured into
10% hydrochloric acid and extracted with diethyl ether
(3 x 100 ml.). The extracts were co~bined, wa~hed with
10~ hydrochloric acid, water, 20~ ~odium bi~ulfite and a
brine solution and dried over msgne~ium ~ul~ate. The
solvent was removed in acuo a~d the residue, 5.61 g..
dissolved in ~ethanol containing 5.1- g. of potassium
hydroxide~ After stirri~g overnight the mehtanol was
~0 removed and tbe residue dissolv~d in 150 ml. of water.
The aqueous layer was wa~ed with eth~r (2 x 100 ml~3
and acidified ~ith 10~ hydrochloric acid. ~he proauc
was extracted with ether, washe~ with a 20~ sodium
bisulfite solution and brine and dried over magnesium
23 sulf~te. Removal of th~ ether ga~e 5.0 g. of the
product as a y~llow liquid.
D2. 2~ hvlhe~e-~ovl ~hio~id~
~ mploying 5 g. of 2-methylbeptanoic acid and 7 . 6
ml. of oxalyl chloride and usi~g the procedure of
Preparation A2, 3.3 g. of the d~ired product was
obtained, b.p. 32-34 ~C./0.6 to~xO
'7~
-64-
PREPARATION E
3-tS?~ L!~oe~lee~ a ~e`l
~9~b~olE~z~ ~on~ e ~VI ~e5f ,~ ~ re r
To a 5 lo four ~eck~d fla~sk ~itted with a~ r~
and p~ electrode wa6 added 2.5 1. og ~01~ pota~ium acid
phosphate buffer p~ 7.0 followed by 150 mg. of pig liver
esterase and 150 9. of dimethyl 3-methylgluta~ate. The
p~ of the mixture ~as maintaineaa at about 6.85 by
periodic addition o a 10~ potassium carbonate ~olution.
After 2.5 hours the reaction was acidified ~ith 10%
hydr~chloxic acid to p~ 2.0 and the product e~tracted
with diethyl ether. The extracts were so~bi~ed, dried
over magnesium sulfate and concentrated in vacuo to give
1~ 114 g. of the desired product, lalphalD ~ -1.48 ~C~30
C=0.086 g/ml)-
E2. met~l 3-(R)-m~Y¦C~ bo~
To 114 g. of 3-~R)-~ethylgl~taric acid ~ono methyl
ester in 715 ml. of dry tetrahydrofuran cooled to 0CO
~as added slowly 391 ml~ of 2M solution sf bor~ne
dimethylsulfide in tetrahydrofuran. After the ~ddition
was complete the reaction mixture wa~ ~ti~r~d o~ernigh~
at room temperature. The reaction wa~ cooled and 50 ml.
of water slowly added. The reaction was extracted ~3 x
100 ml.~ with ekher and the extracts co~bined, washed
with water, ~ ~aturated sodium bica~bo~ate 801ution and
a brine solution, and dried over magnesium ~ulfate.
~emoval of the solvent gave 37 g. of the de~ired
product. l,
3~ :
~ ,'~ 7~ ~ :
-65-
:,
E3. methyl 3-~R~-methyl-5-(t-butyldimethyl-
To a solution of 37 q. ~.253 ~ o~ ~ethyl
3-(R)-~ethyl-5-hydroxyp~nta~o~te and 37 g. l.5~3 ~) Of
~idazole in 500 ~1. of dLmethylformamide was added 37
g. ~.249 m) of t-butyldimethyl~ilyl chloride a~d the
reaction stirred at room temperature for 2 hour~. The .:
reaction mixture was poured i.nto water ~nd ~xtracted
~4 x 10~ ml.) ~ith ether. The co~bined extra~ts were
10 washed with 10% hydrochloric a~id, a saturated sodiu~ ;
bicarbonate 801ution, water ~nd a bri~e solutisn, and
dried over magne~ium sulfate. Re~oval of the solvent
gave 12I.88 g. of crude product which, on distillation,
gave 107.12 g. of pure product, b.p. 80 81C./0.4 torr.
E4. 3-(S)-methyl-5-(t-butvldimethyl~ilyloX~ Pentanol
To 8.5 g. (.224 m) of lithiu~ aluminum hydride in
250 ml. of die~hyl ether under nit~oge~ was add~d 53.5
g. (.206 m) o methyl 3- ~R~ -methyl-5- (t-butyl~methyl- :
silyloxy)pen~anoate in 1~5 ml. o~ etherO The reactio~
was stirred for one hour at 0C. and wa~ then treated
dropwis~ with 8.4 g. of water, 8.~ ml. of a 15% 80dium
hydroxida solu~ion and 25.2 ml. of water. The solids
were filtered and -he organic phase separated and washed
2S with water, 2.59~i hydrochloric acid arld a l:rine ~olution.
Th2 organic phase ~as dried over ~a~nesi~m ~ulfate and
concentrated in vacuo to give 46 q. oiE produ~:t.
~:5. =~_~
~o 56.3 g. of oxalyl chloride in 3D0 ml. of dry
methylene chloride cooled to -60C. 2nd under a ~i~r~gen
atmosphere was added dropwi~e 74~81 y. of d~methyl~
sulfoxide in 100 ml~ o~ dry ~ethylene chlorideO After
15 minutes 92.0 g. oP 3-(S)-methyl 5 (t-butyldimethyl-
silyloxy)-1-penta~ol in 250 ~1. of th~ same 301~e~t was
i
I., .
.:
-66- :
added dropwise. After 30 minutes 206.1 g. of triethyl-
amine was added to -60C. followed by the r~moval of the
cooling ~ath. The reaction was stirred at room t~mpera- :
ture for l.S hours, and was then poured into water a~d
extracted with methylene chloride. The extract~ were
washed with 2.5~ hydrochlori~, a 8aturat~d ~oaium
bicarbonate solution, water and a b~ine 801ution, dnd
then dxied over magnesium sulfate. The ~olve~t wa~
removed and the re idue dissol~ed in ether and rewashed
and dried as before. Removal o~ the ether ga~e 90.9 g.
of the desired p~oduct.
E6. 5-(S)-methyl-7-(t-butyldimethylsilyloxy?-2-heptene
To a slurry of 80 g. (.2155 m) of triphenyl-ethyl
phosphonium ~romide in 800 ml. of dry tetrahydrofuran
cooled to 0C. was added 165.7 ml. of 1,3~ ~olution of
n-butyl lithium (.215S ~) in the ~ame ~olvent. After 2
hours, 45 gO ( r 196 m) 3-~R)-methyl-5-(t-butyldimethyl-
silyloxy)-1-pentanal in 200 ml. of d~y t~trahydrofuran
was added dropwise to the rea~tion mi~ture. The
reaction was allowed to stir 2 hours at room temp~ratu~e
and was then poured i~to wa~er and e~tracted with ether.
The combined extracts were washed with water and a brine
solution and dried over magnesium ~ulfate. Removal of
the solvent in vacuo gave a ye}low oil which, on di~til-
lation gaYe 37.4 g. of product, b~p~ 74-79C./.2~
torr. -
~7. 3-~S?-methyl-l-heptanol ~-
To a solution of 74.8 g. of 5-~S)-methyl-7-(t-
butyldimethylsilyloxy)-2-hepte~e in 500 ~1. of methanol
was added 7.5 g. of 10% palladium hydroxide on car~o~ :
and the mixture sha~e~ in a hydrogen atmosphere for 1.5
hours at 50 psi. The catalyst wa~ filtexed a~d the
solvent removsd under vacuum, 30 g.
.
67-
E8. 3-(S)-methylhepta~oic ~ci~
To 10 g. of 3-(S)-methyl-1-heptanol in 175 ~1. of
acetone was added over 45 ~inutes 90 ~1. of Jones
reagent dropwise at 15-20C. Aft~r 15 ~inu~s, 15 ml.
of isopropanol was added ~nd stirri~g conti~ued ~or 30
minutes. The reaction was poured into water and the
product extracted with ether. The extracts were
- combined, washed with water, a sodium bi~ulfit~ ~olution
and a brine solution and dried over mag~e9ium ~ulfate.
Removal of the solve~t gave 10 g. o~ the produ~t as a
liquid, b.p. 84-88C./.~ torr, ~alphal~ 4.46 ~C~3O~
C=0.105 g/ml.).
E9. 3-~S~-methylheetanoyl_chloride
Following the proçedure of Prepar~tion A2, 5.0 g. ,
of 3-~S)~me~hylheptanoic acid and ~.5 ~1. of oxalyl
chloride gave 2.9 g. of the desired acid chloride, b.p.
29-32C./.25 torr~ ::
DD '
:
'''
;'.
:-.