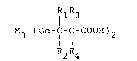Note: Descriptions are shown in the official language in which they were submitted.
i?~77
S~ECIFICATION
Title of the Invention
Salt of Organogermanium Compound and Medicine
Containing the Same
Detailed Description of the Invention
(Field of the Invention)
The present invention relates to novel salts of
organogermanium compounds and medicines containing those
salts.
(Prior Art and Problems)
As shown in many literatures such as "Pharmaceutical
Activity of Organogermanium Compound Ge-132 (Introduction)"
by Hiroshi Satoh and K ohei Miyao and "Inhibition of Tumor
Growth and Metastasis in Association with Modification of
Immune Response by Novel Organic Ge*manium Compounds", J. of
Biological Response Modifiers, 4, 159-168 (1985) by Nobuo
Tanaka, et al., organic germanium compounds expressed by
: Formula (1) exhibit excellent characteristics as biological
response modifiers, for example, they are active in inducing
interferon, can activate macrophage:or NK cells, and have
antitumor activities based thereon. In addition, since
these compounds exhiblt desirable pharmaceutical activities
such as an ability to control enkephalin-degrading enzymes
and improve Ca metabolism, and also have a low level of
.` .
~Y~ $~ 77
toxicity, it is thought that they can be used as useful
pharmaceuticals.
However, since the compounds expressed by Formula (1),
such as carboxyethylgermanium sesquioxide (i.e.
O3(GeCH2CH2COOH)2 in which M in Formula (1) is an oxygen
atom and R1 to R4 are each a hydrog~n atom, and which is
referred to as Ge(O,H) hereinaf-ter) generally have a low
solubility in water and thus do not easily dissolve in usual
organic solvents, they can not be directly prepared as
liquid medicines such as solutions for injection. In
addition, when orally administered, they have disadvantages
with respect to their low levels of bioavailability and
their short half-life in blood due to their extremely low
absorption e~ficiency.
However, since the above-described compounds have
carboxyl groups, it should be possible to change them into
water-soluble compounds by neutralizing them. For example,
the neutrali~ation of the above-described Ge(O,H) with
sodium bicarbonate (NaHCO3) or sodium hydroxide (NaOH)
enables the preparation of an aqueous solution containing
10% by weight of Ge(O,H).
Since an inorganic salt of the above-described Ge(O,H),
such as sodium (Na), potassium (K), or calcium (Ca) salt,
has very poor crystallinity and is hygroscopic, it can not
be obtained as crystals. In addition, since the Ge(O,H) is
~ 77 72057-5
a weak acid, the agueous solution obtained by its complete
neutralization with an inorganic ba6e i8 alkaline.
(Means for Solving the Problem~)
The presen~ invention has been achieved as a result of
energetic research conducted by the inventoxs o~ the present
invention with a view to solving the above-described
drawbacks of the known compound~.
The inventor~ have made ef fort~ to en~ure that ~he
above-described compounds are sae, in view of the fact that
they will be used as pharmaceutical~.
The present invention relateg to pharmaceutically acceptable
s~dlts oE organogermanium comp.ounds expressed by the following
Formula (1):
1113
M3 (~ 7 f-CH ~ 2 ~ ( 1 )
R2R4
(wherein M denotes an oxygen or sulfur atom~ and Rl, R2, R3,
and R4 may b~ the same or di~ferent ~om each other and each
denotes a hydrogen atom, a lower alkyl group, or an aryl
group) with compounds having basic grou~s.
A description will now be made of the above-described
organogermanium compounds.
These compounds have basic skeletons in which propionic
acid derivative~ having sub~tituent~ Rl, R2, R3, and R4 are
bonded to germanium atom~, the ~ermanium atom~ in the basic
27~
skeletons being bonded to oxygen atoms (when M = O) or
sulfur atoms ~when M = S) in the ratio of 2 : 3.
The substituents R1, R2, R3, and R4 may be the same or
different and each denotes a hydrogen atom, a lower alkyl
group such as a methyl, ethyl, propyl, or butyl group, or an
aryl group such as a phenyl group.
The substituents Rl and R2 are bonded to the carbon
atom at the ~-position with respect to the germanium atom
and the substituents R3 and R4 are bonded to the carbon atom
at the ~-position with respect to the germanium atom.
Lysozymes can be first exemplified as compounds having
basic groups used for forming salts with the above-described
organogermanium compounds.
Lysozymes, which are pure basic zymoproteins having a
high level of stability, are generally present in various
tissues of many kinds of plants and animals, including
humans, function as natural protective substances, and are
used as pharmaceuticals~ A lysozyme has the structure of a
simple polypeptide comprising 20 different kinds of 129
amino acid subunits which are brige bonded by 4 disulfide
bridges, and has a molecular weight of about 14,400 + 100
and shows an isoelectric point at a pH of 10.5 to 11Ø
In add,ition, in the present invention, the COOH groups
of each of the organogermanium compounds are bonded to free
amino acids of the lysozyme molecules, or all or part of the
basic groups thereof, such as guanidine groups or imidazole
groups.
The salts of the present invention can be easily
produced by reactions between the compounds expressed by
Formula (1) and lysozymes, in accordance with conventional
reactions between acids and bases. The reaction products
obtained are crystalline and thus can be separated out or
recrystallized from, for example, a system of water and
alcohol.
For example, since the neutral salt produced from the
Ge(O,H) and a lysozyme has good crystallinity and gradually
dissolves in solvents, it has an effect of sustained release
and can be maintained as a long-lasting medicine at a
certain concentration in the blood by a device, such as a
gastric retention type of medicine, so that this salt can be
prepared as medicines which have high levels of
bioavailability.
Basic amino acids can also be used as compounds having
basic groups.
Examples of basic amino acids used in the present
invention include L-lysine, L-arginine, and L-histidine.
In this case, in the present invention, the COOH groups of
the organogermanium compounds are bonded to amino or imino
groups of basic amino acids.
The above-described salts of the present invention can
$~7
be easily produced with a good yield by the reactions
between the compounds expressed by Formula (1) and basic
amino acids, in accordance with conventional reactions
between acids and bases. For example, equivalent amounts
of a compound expressed by Formula (1) and a basic amino
acid are mixed together and the mixture is dissolved in as
little water as possible under heating. Crystals are
separated out by filtering the thus-produced solution then
cooling it, or by concentrating the reaction solution, or
the salt is separated out by adding an organic solvent such
as ethanol to the concentrated aqueous solution, and the
salt is then filtered off and dried. The thus-obtained
salt of the present invention is excellent in crystallinity,
unlike the salts with inorganic bases described above.
When prepared, this salt therefore has desirable physical
properties as a principal ingredient of a solid medicine in
a form such as tables, granules, or capsules. In addition,
this salt is extremely water-soluble, unlike the compounds
expressed by Formula (1), and thus can be used as it is in
the preparation of liquid medicines containing appropriate
concentrations of the salt (for example, 2 to 20% by weight,
preferably about 3 to 7~ by weight, measured as
organogermanium compounds). Unlike salts with inorganic
bases, aqueous solutions of the salts of the present
invention have a pH of about 7, which is within the range of
~ 2~7 72057-5
biological pH values.
The pH of about 7 of aqueous solutions of the salts of
the present invention means that these solutions exhibit strong
buffer actions, and, when the salts are orally administered,
free organogermanium compounds are separated out therefrom at
a much lower rate when they come into contact with acids in
the stomach. On the other hand, when salts with inorganic
bases are orally administered, free organogermanium compounds
have a tendency to be immediately separated out by the acids in
the stomach from the aqueous solutions of the salts, which are
thus made insoluble, resulting in a reduction in the efficiency
with which they are absorbed in the body, and thus a reduction
in their bioavailability. Therefore, since the salts of the
present invention are present as molecules or very fine particles,
without any separation of the organogermanium compounds, they
exhibit excellent levels of absorptivity and thus an increased
efficiency of absorption into the body, resulting in an increase
in their bioavailability.
The present invention also relates to a biological
response modifier comprising a biological response modifying
effective amount of the salt of the organogermanium compound
having Formula (1) and the compound having basic groups, in
admixture with a pharmaceutically acceptable diluent.
Biological response modifiers (referred to as modifiers
~ hereinafter) of the present invention have higher levels of
.~ ~ bioavailability than those of conventional organogermanium
~.
.
~ - 7 -
.
277
72057-5
compounds or salts thereoE. For example, some of these modi-
flers are excellent for activating macrophage or NK cells, or
inducing interferon.
The modifiers of the present invention are much more
active in producing biological response modifications than the
compounds expressed by Formula (1). Namely, in DHT (delayed
type hypersensitive reaction) by a foot-padding method, Ge(O,H)
and a lysozyme salt thereof exhibited increased effects which
were 108% and 11~% relative to a control, respectively, when
administered in doses of 100 mg/kg.
The modifiers of the present invention, therefore,
have excellent levels oE availability compared with conventional
organogermanlum compounds alone.
The present invention also relates to an antitumor
agent comprising an antitumor effective amoun~ of the salt of
the organogermanium compound having Formula (1) and the com-
pound having basic groups, in admixture with a pharmaceutically
acceptable diluent.
As described above, the salts of the present invention
have higher levels of bioavailability than those of conventional
organogermanium compounds or salts thereof, and thus exhibit
excellent antitumor actions.
When animal experiments on rats were performed, salts
which were administered in doses of about half those of the
organogermanium compounds alone exhibited similar antitumor
:,
~ ~ - 8 -
.
~ ~r ~ ~ $~77
effects. Therefore, the medicines of the present invention
can be used in oral or parenteral administration, according
to symptoms and can be prepared into forms which are
conventionally used for oral or parenteral administration,
such as liquids, powders, fine particles, granules, tablets,
coated tablets, capsules, injections, ointments, or creams.
~uch forms can be prepared by mixing the salts produced from
the organogermanium compounds expressed by Formula (1) and
compounds having basic groups with additives conventionally
used in preparations, such as excipients, fillers, binders,
disintegrators, lubricants, perEumes, tinctures, or sterile
water.
The medicines of the present invention can be
administered to adults one to more times per day at a total
dosage of the active component of 20 to 100 mg/kg,
preferably 30 to 70 mg/kg per day, according to the
symptoms.
The medicines of the present invention have virtually
no toxicity. In particular, the lysozymes and amino acids
which are the basic components are natural components and
are used as pharmaceuticals such as oral medicines or
injections. Since the safety and availability of these
substances have thus been fully proven, these substances
will present no problem when used as components of
medicines.
Brief Description of the Drawings
Fig. 1 is an IR spectrum of the compound obtained by
Example l;
Fig. 2 is an IR spectrum of the compound obtained by
Example 2, and
Figs. 3 and 4 are survival graphs of rats obtained by
Example S.
Description of Examples
Examples are described below.
Example 1
14.7 g of lysozyme chloride was dissolved in a small
amount of water and the resultant solution was passed
through a column of liberation-type basic ion exchange
resins. The column was then washed with water to obtain
free lysozymes (pH 10). 1.35 g of carboxyethylgermanium
sesquioxide was added to the thus-obtained aqueous solution
under agitation and was dissolved therein. About ten times
the volume of the obtained solution of ethanol was gradually
added to the solution to separate out the reaction products.
The products were filtered off then dried in a desiccator
to obtain the intended substance as a colorless crystalline
powder (yield, 92%, melting point, 300C or more
~decomposition)). The IR spectrum of the substance is
shown in Fig. 1.
Example 2
27~7
19.6 g of L-lysine and 16.5 g of carboxyethylgermanium
sesquioxide were dissolved in a small amount of hot water
under agitation. Insoluble solids were filtered out and
the filtrate was then gradually added to ten times its
volume of ethanol under agitation to separate out the salt
produced. This salt was allowed to stand in a refrigerator
until it was completely separated out, then it was filtered.
The thus-obtained crystals were dried in a vacuum desiccator
to obtain the intended substance as a colorless fine
crystalline powder (yield, 73%; melting point, 270~C or more
(decomposition)). The IR spectrum of the substance is
shown in Fig. 2.
Example 3
ComponentAmount (mg/tablet)
Carboxyethylgermanium 50
sesquioxide lysozyme salt
Sodium carboxymethyl cellulose 140
Lactose 40
Amounts of carboxyethylgermanium sesquioxide lysozyme
salt and the additives corresponding to 5 tablets were each
weighed then they were uniformly mixed together. The thus-
obtained mixture was weighed to amounts corresponding to
single tablets then was directly formed into tablets by a
tablet machine at a pressure of 200 kg/cm2 to obtain
77
tablets. These tablets contained 50 mg of
carboxyethylgermanium sesquioxide lysozyme salt as an active
component pex tablet.
8-week old ICR mice were used as test animals in groups
of 6 mice. However, a control group comprised 12 mice.
108/0.05 ml of sheep red blood corpuscles (SRBC) was
injected under the plantar skin of each of the mice, and the
same amount of SRBC was injected under the skin 4 days
later. The next day, i.e., after 5 days, the increase in
the thickness of the plantar of each mouse was measured.
The mice were divided into 3 groups comprising group A
to which 100 mg/kg of the lysozyme salt of
carboxyethylgermanium sesquioxide was orally administered
once a day for 4 days after the start of the experiment,
group B to which only carboxyethylgermanium sesquioxide was
administered in a similar manner, and group C as a control
group. The results of treatment are shown in Table 1.
Table 1
Group Increase in thickness of plantar Increase ratio
A 2.9 +1.4 mm 116
~ 2.7 +1.2 mm 108%
C 2.5 tl.8 mm 100%
12
2~7
Example 5
A comparison was made between a lysine salt of
carboxyethylgermanium sesquioxide (A) and
carboxyethylgermanium sesquioxide (B) with respect to their
antitumor effects on ascites hepatoma (AH66) of rats.
Female Donryu rats (each having a body weight of 120 to
lS0 g) were used, divided into three groups comprising a
control group, administration group A, and administration
group B, each containing six rats. A suspension (107/ml)
of ascites hepatoma (AH66) cells of rats was prepared. 72
hours after an infusion graft of 1 ml of the suspension onto
the tail vein of each rat, medicines (A) and (B) were
administered to administration groups A and B, respectively.
100 mg/kg of each medicine was orally administered once a
day for 10 days. Medicine (A) was used in the form of a 10
wt% aqueous solution, and Medicine (B) was used in the form
of a 10 wt% suspension in an aqueous solution of 0.5 wt~
of carboxymethyl cellulose.
The effects of the medicines were judged from survival
graphs (Figs. 3 and 4) obtained from observation of the
control group, administration group A, and administration
group B for 60 days after the grafts of hepatoma cells. If
a case in which the graph of each administration group was
substantially the same as that of the control group, or in
which rats died and the area under the curve was twice or
277
less that of the control group, is denoted by (-); a case in
which 5Q% or more of the rats recovered and the area was
three times or less is denoted by (~); and intermediate
cases are denoted by (~); both the administration groups A
and B show (~
However, the amount of the germanium compound contained
in each of the compounds of the present invention is, for
example, for the lysine salt, 46% of the same amount of the
free germanium compound. Therefore, the salts of the
present invention show activity degrees which are
substantially the same as or higher than that of the same
amount of free germanium compound, which means that the
compounds of the present invention are extremely effective.
14