Note: Descriptions are shown in the official language in which they were submitted.
1297265
- 1 -
The present invention relates to a process of
produciny lithium carbonate from lithium-containing de-
crepitable ores or ore concentrates.
It is known that lithium ores or lithium ore
concentrates, such as spodumene or petalite, can be
subjected to a thermal treatment, which is usually described
as decrepitation, and results in a state in which the
material can be solubilized by an acid. By the
decrepitation, which is usually effected in a rotary kiln at
about 1000 to 1100C, spodumene is transformed from the
alpha modification to the beta modification and petalite is
transformed to beta-spodumene and quartz.
/
/
, / .
~2g~2fiS
-- 2 --
After the decrepitation the beta-spodumene
may be ground further and is treated with concentrated
sulfuric acid used in a surplus with respect to the lithium
content of the ore. The mixture which is ~irtually as moist
as earth is charged into a suitable furnace, such as a
rotary kiln, and is solubilized therein at temperatures
from about 250 to 300 C. When tne lithium content of the
ore is in the form of soluble lithium sulfate, the reaction
mixture is charged into suitable l-achin~ tanks, in which
the lithium sulfate is leached out by means of hot water,
w!lich is usually conducted in a countercurrent to the
re~ction mixture~ Sodium carbon_te is added to tne extract
in a quantity Nhich is sufficient for the adjustment of a
pH value of about 6 to 7 so that impurities such as calcium,
magnesium, iron and aluminum will be precipitated as insoluble
compounds~ When the residue has been separated, a ~ncentrated
solution of lithium sulfate is available, which is treated
with a concentrated sodium carbonate solution at elevated
temperatures so ~at lithium carbonate is precipitated, which
is subsequently recovered.
It is also known that the decrepitation
can be effected in furnaces other than a rotary kiln, e.gO,
in muffle furnaces and in fluidized bed reactors (U.S.
Patent 3~017~243)o The fact that rotary l~ilns, multiple-
hearth furnaces or muLffle furnaces can be used just a~
fluidized bed reactors permits of th~ conclusion that it has
been believed that the nature of the treatment and the manner
1297Z6S
-- 3 --
in which the ground ore is heated to the transformation
temperature are not significant (U.S. Patent 2,516,109).
It is an object of the invention to permit the
production of ]ithium carbonate from decrepitable lithium
ores or ore concentrates in a process which ensures that the
lithium will be virtually completely extractable.
According to the present invention there is
provided a process of producing lithium carbonate from
lithium-containillg decrepitable ores or ore concentrates,
wherein the ore or ore concentrate is transformed at
temperatures from about 800 to 1100C to a form from which
the lithium component can be leached, the lithium in the
decrepitated material is converted to lithium sulfate by a
solubilizing roasting treatment with a surplus of
concentrated sulfuric acid, the roasted product is leached
with water and the extract is filtered from the residue,
precipitated impurities are removed from the filtrate and
the latter is subsequently treated with a concentrated
sodium carbonate solution to effect a precipitation of
lithium carbonate, which is recovered, characterized in that
the disintegrated ore or ore concentrate is transformed to a
leachable form in that it is subjected to a shock heating to
at least 800C as it is introduced into a circulating
fluidized bed.
The ore or ore concentrate is preferably shock-
heated to a temperature which is not in excess of 1300 C.
The term shock heating means that ore or ore concentrate
which has not been thermally treated before is charged into
a circulating fluidized bed and the temperature of said bed
is controlled by a combustion of gaseous, liquid or solid
fuel, such as coal. Any fuel may be used which will not
adversely affect the subsequent treatments.
Within the scope of the invention the decrepiting
thermal treatment is effected in an expanded fluidized bed,
129726S
which is basically known per se. From an "orthodox"
fluidi~ed bed, in which a dense phase is separated by a
distinct stepindensity from the ove~
12972~S
an expanded fluidized bed differs in that it comprises
states of distribution having no defined boundary layers.
There is ~o step in density between a dense phase and an
overlying gas space but the solids concentration in the
reactor decreases gradually from bottom to top.
On principle, the oxygen-containing
gases required for the combustion can be added in one
s~age. But it will be particularly desirable to supply
the oxygen-containing gas required for the combustion
process to the fluidized bed in two partial streams on
different levels. That mode of operation will result in a
SOI`t combustion in two stages so that there will be no
local overheating and the formation of NOx gases will be
suppres 5 ed.
In case of a multista~e combustion, vi~u-
ally any gas which will not adversely affect the nature
of the exhaust gas can be used as a fluidized gas. E~r
instance, the fluidizing gas may consist of a gas which
is non-reactive under the conditions existing in the
circulating fluidized bed, e.g., of a recycled flue gas
(exhaust gas), nitrogen and water vapor. But for an
intensification of the combustion gas it will be desir-
able to use a fluidizlng gas consisting of a partial stream
of the ox~gen-containing gases to be supplied.
~ t is apparent that in a preferred
embodiment the process may be carried out in the following
ways:
~29~265
10 The fluidizing gas consists of a gas which is inert
under -the conditions in the fluidized bed. In that
case the oxygen-containing combustion gas is supplied
as a secondary gas on at least two spaced apart levels.
20 An oxygen-containing gas is used also as a fluidizing
gasO In that case it will be sufficient to suppl~y
secondary gas on one level although in that case the
secondary
gas may also be supplied on a plurality of levels.
It will be desirable to provide a plurality of
inlets for the secondary gas on each level on wnich said
gas is suppliedO The volume ratio of fluidizing gas to second-
ary ~as should be in the range from 1:20 to ~
l'he secondary gas is suitably supplied on
a level ~ihich is spaced above the inlet for the fluidizing
~as at least 1 meter and up to 3~j~ of the overall hei~ht of
~he fluidized bed reactor. If the second~r~ gas is supplied
on a plurality of levels, the reference to 3~/o is appli-
~able to the uppermost inlet level for the secondary gas.
A supply on that level will provide for a sufficiently
l~rge space for the first combustion st~ge for an almost
complete reaction between combustible constituents and
oxy~en-containing gas - whether it is sup~lied on a lower
level as a fluidizing or secondary gas - and -~ill permit
the provision of a sufficiently large zone for a complete
combustion in the upper reaction space above the secondary
129~26S
gas inlet level.
The gas velocities maintained in the fluidized
bed reactor above the secondary gas inlet may amount, as
a rule, to more than 5 m/sec. and up to 15 m/sec..
The ratio of the diameter to the height of
the fluidized-bed reactor should be selected to provide
for gas residence times from 0.5 to 8.0 sec., preferably
1 to 4 seconds.
The mean suspension density to be maintained
in the fluidized bed may vary within wide limits and may
be as high as 100 kg/m3. To minimize the pressure drop,
a mean suspension density in the range from 10 to 40 kg/m3
should be maintained above the secondar~ gas inlet level.
The definition of the operating conditions
by the Froude ~nd ~rchimedes numbers results in the follow-
ing ranges:
001 _ 3/4 x Fr2 x ~g --- ~ 10
~ Pk Pg
or
0.01 ~ Ar ~ 100
wherein
Ar = k ~k
~g x
Fr2 =
and
~97~6S
u = the relative gas velocity in m/sec.
Ar = the Archimedes number
Fr = the Froude number
~g = density of the ga~ in kg/m3
k = the density of the solid particle in kg/m3
dk = the diameter of the spherical particls in m
= the kinematic viscosity in m2/sec.
g = the acceleration due to gravity in m/sec.2
After the thermal treatment in the
fluidized bed the product is usually cooled, suitably
in a fluidized bed cooler, which may comprise a plurality
of stagesc ~he sensible heat of the product may be used to
generate steam and/or to heat the oxygen-containing gas
which is to be supplied to the circulating fluid~zed bed.
~ he exhaust gases from the circulating
fluidized bed are cooled in a waste heat boiler for a
generation of steam, which may be used, e.g., in the
subsequent leaching stages. Alternatively, if the su~se
quent roasting is effected in a directly heated rotary
kiln, the exhaust gases may be used to heat such kiln.
The invention permits of a high through-
put rate and permits those components from which the
lithium component can be leached out to be transformed to
a particulerly highly reactive formO Besides, a material
~29726S
is made a~ailable which has a large surface area and in
which the lithium ions are particularly easily and complete-
ly accessible for chemical reactions. dn additional acti-
~ation can be effected in that the thermal treatment in
the fluidized bed reactor is effected in the presence
of additives wnich are known per see and influence the
modification.
In the process in accordance with the
invention it is not critical to use an ore or ore concen-
trate having a particularly small particle size. It is
generally sufficient to effect a disin~egration to a
particle size from 1 to lO mmO Smaller particle sizes,
e.g., below 10CO!um, .~ill further increase the heating-up
rateO
The decrepited material is subsequently
solubilized in Xnown manner in a homogeneous mixture with
sulfuric acid. Sulfuric acid having a concentration from
90 to 94 ~ is usually employed for that purpose in a surplus
from 30 to lO0 ~ with respect to tne litnium content of the
oreO In the process in accordance with the present invention
t`ne homogeneous mixture of heat-treated ore or ore concen-
trate an~ concentrated sulfuric acid is solubilized by a supp^-
ly of heat at a temperature from 250 to 320 C. ~hat solubi-
lizing roasting treatment is desirably ef ected in a rotary
Xiln, which may be directly or indirectly heatedO An indirect
heatin~ nill afford the special advantage tnat the surplus
free sull'uric acid which has been expelled during the
~297Z6S
roastiug treatment can be directly re-u~ed and can be
directly recycled to the solubilizing stageO Adirect
heating will afford the advantage that the exhaust
gases from the circulating fluidized bed can be used
and that the expelled sulfuric acid can also be re-used.
For a recovery of the soluble lithium sul
fate now contained in the roasted product, the latter is
leached in known manner in a plurality of stirred vessels,
which are connected in series and in which the roasted
product and water flow in countercurrent streamsO After
the leaching t~e residues, which mainly contain silicates,
are separated. In accordance with the invention the process
parameters are so selected that the resulting solution
contains lithium sulfate in a concentration from about
200 to 250/1 Li2S04.
In addition to lithium sulfate and sodium
sulfate, the filtrate may contain other impurities, such
as magnesium, iron, aluminum, calcium. ~aid impurities
are precipitated in known manner by a~ addition of alkali
and/or sodium carbonate and the precipitates are removed.
A concentrated sodium carbonate solution is then added
to tne filtra~e so that the lithium car~onate is precipi-
tated under conditions known per se. The precipitate is
recovered, washed a~d dried. ~n a special embodiment of
the invention only 60 to 85 ~/0 ra~her than all of the
lithium carbonate in the filtrate are precipitated and
the mother liquor is recycled to tne leacning stage.
~29726S
- 10 -
lithium carbonate of high purity is thus recovered~
The process in accordance with the
invention can be carried out to special advantage in
co~junction with a continuous leaching process because
this will permit lithium carbonate to be produced in a
particularly economical manner. The process in accordance
with the invention particularly distinguishes in that it
can be used not only to prooess lithium ore concentrates
in the usual manner but also to process so-called raw
ores consisting of lithium ores which have not been
processed before and this can be effected economically
and with a high yieldD Such ores particularly include
alpha-spodumene, petalite and lepidolite. ~or in~tance,
it is possible to process an alpha-spodumene having the
following approximate composition:
~i2o 3.3 to 401 ~ by -~veight
SiO2 60 to 70 ,o "
r~123 15 t 25 j
Fe203 0-3 tO 0.6 ~G~
CaO 0005 tO 0.2 1
K20 002 tO 0~5 1
Na20 3 to 0.8 ~
The invention will be described by way
of example and more in detail with ~ference to the drawing
and to the ~xample~
~ igure 1 is a simplified flo.v diagra~
illustrating the process in accordance with the invention.
~297265
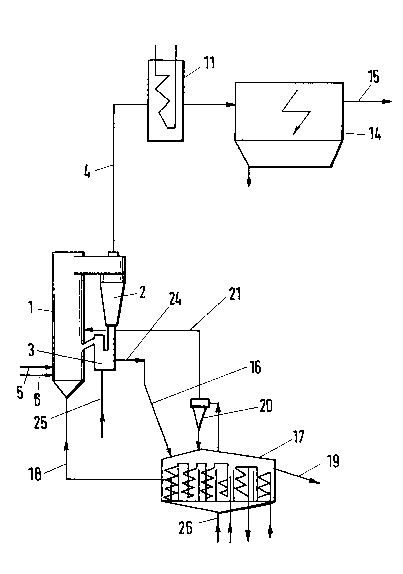
Figure 2 is a diagrammatic represent~-,tion
of a reactor with a circulating ~hidized bed.
~ e feed and fuel are supplied through res-
pective lances 5 and 6 to a circulation system consisting
of a fluidized bed reactor 1, a cyclone separator 2 and
a recycle line 3. A product stream at the same rate as
the feed is withdrawn through line 24 and delivered to
the fluidized bed cooler 17, in which the product stream
first flows through four cooling stages to deliver a sub-
stantial part of its heat by an indirect heat exchange to
oxygen-containing gases which flow countercurrently to
the solids. Said gases are then supplied as a dustfree
fluidizing gas to the fluidized bed reactor 1. The solids
are then cooled further in subsequent cooling chambers,
which are cooled, e.g., with water. The product stream
is subsequently discharged through line 19. ~he fluidized
bed cooler 17 is supplied with an oxygen-containing
fluidizing gas, which takes up a substantial further
quantity of heat from the solids and after a dust-
collecting treatment in the separator 20 is supp]ied in
line 21 as a secondary gas to the fluidized bed reactor 1.
The exhaust gas from the fluidized bed reactor
1 flows throu~b line 4 to the waste heat boiler or heat
exchanæer 11 and subsequently to an electrostatic preci-
pitator 14 for a dust-collecting treatment and is dis-
charged through line 15 into the chimney.
Lines 25 and 26 serve to supply fluidizing
gas or entraining gasO
~,s7~is
- 12 -
Example
A pilot reactor for a single-stage combustion
was supplied through lance 5 at a rate of 5007 kg/h with
alpha-spodumene which had the above-mehtioned composition
and had been ground to a particle size 1 mm (average
particle diameter dp50 = 377 ~m) and was supplied
through lance 6 with light fuel oil at a rate of
22 kg/ho Oxygen-enriched air (215 sm3/h air and 15 sm3/h
oxygen) was supplied only as a fluidizing gas through line
1~ .
A temperature of 1070 C was obtained in the
circulation sys~em consisting of the fluidized bed
reactor 1, the cyclone separator 2 and the recycle line
3. The pressure drop across tbe fluidized bed reactor
amounted to 40 millibars.
Solids (dp50 = 252 ~m) at a rate of 47~1
kg~h were withdr~wn through line 24. Solids (dp50 = 6.0 ~m)
at a rate of 4050 k,~/h were discharged through line 4.
'~he solids were subsequently recovered.
The recovered solids were combined and
subjected to conventional afterprocessing. ~he lithium
introduced with the alpha-spodumene was recovered with a
total yield of 52 to 93 ~ by ~eight.
1297265
~ranslation of the Inscriptions on Figure 1 of the
Drawings
alpha-Spodumen
Reaktor mit zirkulO Wirbelschicht reactor with circulating
fluidized bed
beta-Spodumen beta-spodumene
Mischstufe mixing stage
. . .
concO H2S04 concentrated H2S04
Roststufe roasting stage
H2S04 H2S04
Laugung leaching
Na2S04-Abtrennung separation of Na2S04
Ruckstand (Silikat) residue (silicate)
Filtration filtration
Fallung Verunreinigung precipitation of impurities
Alkali, Soda alkali, sodium carbonate
Verunreinigungen Mg, Ca, Al, Fe impurities Mg, Ca, Al, Fe
Filtration filtration
Li2C03-Fàllung . precipitation of Li2C03
conc. Sodalosung concentrated sodium
carbonate solution
FiltratioD filtration
Li/Na-Sulfat-Losung Li-Na sulfate solution
Li2C3 Li2C3