Note: Descriptions are shown in the official language in which they were submitted.
1297403
Contact-~ens Care Set
The ~nvention relates to a contact lens care set.
Care sets for soft and hard contact lenses serve to clean, to disinfect
and to stabilize the optical properties and to improve the wear comfort
of the contact lenses. ~ydrogen perox;de, as antimicrobial substance,
has become more important recently for disinfecting and cleaning contact
lenses. Compared to chlorhexidine salts or organomercury compounds,
such as th;omersal, which are also used to disinfect contact lenses,
hydrcgen peroxide has a better antimicrobiolog;cal effect, especially
with respect to the retati~ely resistant Aspergillus species. The known
hydrogen peroxide solut;ons, used to clean contact lenses, are 3% per-
oxide solut;ons (German Patent 2,425,714). After treatment of the
contact lenses with these solutions, relatively large res;dual a~ounts
of hydrogen peroxide solution adhere to the lens. When lenses so
treated are inserted, these residual amounts lead to varying degrees of
irritation of the mucous ~embrane. Therefore, after treatment with a
hydrogen peroxide solution, it is necessary to treat the lens with a
neutral~zing solution, by means of which the hydrogen peroxide is split
into water and oxysen. The elimination after the ster;lization treat-
ment of res;dual amounts of hydrogen peroxide by decomposition with the
help especially of heavy metal catalysts is known from the aforemen-
tioned German Patent 2l42~,714. The use of two d~fferent treatment
steps is required here, namely, first of all, the treatment step of
ster;liz1ng the lens and, after discarding the sterilizing solution, the
treatment step of eliminating the residual hydrogen peroxide in an
aqueous system conta;ning the catalyst. ~he handl;ng of the lens durin~
the care and cleaning ~perations therefore is relatively complicated.
The choice of suitable catalysts, which are used for the decomposition
of residual amounts of hydrogen peroxide, also presents d;fflculties.
As catalysts for the decomposition of the hydrogen perox~de, used to
disinfect and clean contact lenses, after the cleaning and dis;nfecting
-
1297403
treatments have been carried out, the use of enzymatic
peroxidases, especially catalase, is known from the EP-A-82
798. In this case also, however, the hydrogen peroxide
solution is poured off after its disinfecting treatment and
replaced by a neutralizing solution which contains the
peroxide-neutralizing catalyst or catalysts (i.e.,
peroxidases). Here also, therefore, two different,
successive treatment steps are employed, so that the care
treatment of the lens remains complicated.
In both cases, a contact lens care treatment
simultaneously using hyd-ogen peroxide and a neutralizing
solution with a decomposition catalyst, for example, has
been impossible because, in such case, the hydrogen peroxide
is decomposed into oxygen and water by the neutralizing
solution via its decomposition catalyst, for example, before
it can have a sufficient sterilizing action to kill the
germs on the lens which requires from about one to about
four hours, especially for a soft lens.
Summary of the Invention
It is, therefore, an object of the invention to
provide a contact lens care set which, in one step, keeps
hydrogen peroxide active sufficiently long at least for
substantially sterilizing the lens and then neutralizes at
least residual hydrogen peroxide on the lens at least
substantially so that the lens can then be worn, at least
generally, without discomfort.
12974~3
To this and other end, the invention provides a
contact lens care set having at least two agents: hydrogen
peroxide and a neutralizing agent. The hydrogen peroxide is
sufficient to be effective at least for substantially
.
sterilizing at least one of a hard or soft contact lens and,
preferably, either. The neutralizing agent is sufficient to
be effective at least for decomposing any residual amount of
the hydrogen peroxide on the lens into oxygen and water
after the hydrogen peroxide has so sterilized the lens at
least substantially, whereby the lens can then be worn, at
least generally, without discomfort. The neutralizing agent
has at least two constituents: a catalyst or enzyme,
preferably for so decomposing the residual hydrogen
peroxide, and a retarding agent. The retarding agent delays
the time of release of at least so much of the neutralizing
agent as decomposes the residual hydrogen peroxide, e.g.,
the catalyst, into the hydrogen peroxide until the hydrogen
peroxide has so sterilized the lens.
The invention therefore substantially facilitates
the care of the contact lens, whether hard or soft, because
only a one-step treatment is required. The lens and the
neutralizing agent with the catalyst and the retarding agent
that controls the time-delayed release of at least part of
the neutralizing agent such as the catalyst, for example,
are placed into a sterilizing hydrogen peroxide solution.
The regarding agent, which comes into contact with the
hydrogen peroxide during the immersion, then releases at
least the neutralizing portion of the catalyst-containing
neutralizing agent after the hydrogen peroxide has acted on
the contact lens that is to be treated for a time sufficient
for the lens sterilization.
12974~13
For this, the neutralizing agent may be coated
with its retarding agent may be coated with its regarding
agent for immersion in the hydrogen peroxide in a water
solution with the lens. The delayed release time then can
be adjusted (especially up to four hours), for example, by
the thickness of the coating which dissolves into the
hydrogen peroxide solution. As a result of the release of
the catalyst-containing neutralizing agent when the coating
dissolves, the neutralizing acts automatically, without
further help from the contact-lens wearer taking care of the
contact lens, on the hydrogen peroxide solution. The
hydrogen peroxide solution is acidic (with a pH of less than
4) in the stable state for effecting both the sterilization
and the dissolving. The catalyst, for example, inside the
coating then decomposes the sterilizing hydrogen peroxide
solution in a treatment container therefore in which the
lens to be treated and the neutralizing agent have also
been placed at the same time.
2 9 7~0 3
~xamples o~ the composition of the hydrogen peroxide solution and of the
catalyst- or enzyme-containing neutral;z;ng agent are gi~en ;n the Ger-
man Offenlegungsschrift 3,410,400.
Moreover, the following composition is s~itable as neutral;zing agent:
0.0~ weight percent catalase concentrate, 260,000 units/mL
0.05 weight percent hydroxypropylmethy kellulose USP XX
O.OS weight percent NaH2P04 x 2~20 DAB B
0.25 weight percent Na2HP04 x 2H~O
0.75 weight percent sodiur chloride EP I
The following is a further suitable composition of the neutrali2ing
agent:
~ - 12 mg suitable buffer substance(s) or mixture, for exa~ple, alkal1
phosphate, borate or citrate, glycine
40 - 70 mg neutral electrolyte (for example, NaCl, KCl)
~ - lO ~9 alkali hydrogen carbonate
- lO mg water~soluble polymer, for example, polyvinylpyrrolitone
0.2 - l mg catalyst (catalase, peroxidase)
per single tose.
this amount is sufficient to deco~pose, to neutralize and to adjust to
an osmolarity of Z70 - 320 mosmol 7 ~L of an hydrogen peroxide solution.
These 7 mL correspond to the volume of a standard contact lens case or
contact lens treatment body.
An exampte of a further special composition of the neutralizing agent-is
the following:
5.6 mg potasslu~ hydrogen phosphate
8 mg disodiu~ hydrogen phosphate
52.4 mg NaCl
7 mg sodium hydrogen carbonate
4.8 mg polyvinylpyrrolidone K25
0.3 mg catalyst
The compositlons of the neutrallzlng agent, qiven above, can be used as
capsule fillings, the capsule being fashloned as the retard~ng agent,
which surrounds the catalyst-containing neutralizing agent. The capsule
co~prises a water-soluble poly~er, espec;ally a polyvinyl alcohol.
The neutralizing agent ~ay also be formed lnto a ta~let, wh kh 1s pro~
vlded with a water-soluble coating for the time-delayed dissoluticn of
the tablet. The water-soluble coating may compr;se a polymer, soluble
ln an ac;d;c mediu~, such as a polymer of dimethylaminomethacrylate and
neutral methacrylate esters. The coating may also compr;se a pH-neu-
tral, sotuble polymer. Polymers, suitable for thls purpose, are, for
example, soluble cellulose ethers such as methylcellulose, methylhy-
droxypropylcellulose, methylhydroxyethylcellulose, hydro~ypropylcellu-
lose, hydroxyethylcellulose, sodium carboxymethylcelluloses; cellulose
acetate phthalate; hydroxypropylmethylcellulose phthalate; polymers of
~ethacrylic acid and ~ethacrylate esters; a coating of an aqueous dls-
persion of a copolymer of methacrylic ac;d and methacrylate esters; a
coating of an aqueous dispersion of cellulose acetate phthalate; copoly-
mers o~ methyl vinyl ether and maleic anhydride and polyvinyl alcohols.
~L2 9 7 ~0 3
Su;table polyalcohols, especially in an amount o~ 0.2 - 1 mg/tablet, can
be added to these polymers to control the time delay, 1,2-propylenegly-
col, polyethylene ~lycols and citrate esters be~ng suitable as polyalco-
hols.
The coating can be produced from the water-soluble polymer by known
processes, for example, by spray-coating a film in the coating pan, by
the fluidlzed bed process (Wurster process) or in closed systems. The
preferred amount of polymer, coating a tablet> is 2.0 to 5.0 ~9.
~he following is an example of a tablet composition, which forms the
catalyst-conta;ning neutrallz~ng agent:
11.2 ~9 disodium hydrogen phosphate
55.8 mg NaCI
4.8 mg polyvinylpyrrolidone K25
0.2 mg catalyst tcatalase, peroxidase)
The following is an example of an acid~soluble polymer, suitable as a
coating:
1.9 mg methacrylate ester
0.4 mg hydroxypropylmethylcellulose
0.2 mg polyethylene glycol (1000)
The following is an example of a coating film of a neutral-soluble
polymer:
2.2 mg hydroxypropylmethylcellulose phthalate
0.3 mg polyethylene slycol ~1000)
It is alsc possible to seal a catatyst-containing neutraliz;ng agent,
which has been formed into a tablet, in a water~soluble ~ilm, for exam-
ple, of polyvinyl alcohol, wh;ch acts as a retarding agent. Plasticiz-
1297403
ers ~f multihydr;c alcohols and water are added in an amount of 3.5X to5Z to the polyvinyl alcohol fi1m, as a result of which a time-delayed
dissotution of the film ~s effected. The fi~ms may be ~s thick ~s 30 -
150 ~m. The gas permeability (D~N 53 380) is 0.9 - 1.4 cc/m2/d/bar.
The f11ms cin be welded at temperatures ranging from 140~C - l90~C w1th
a contact~ng pressure of 4 - 5 bar.
The catalyst-containing neutralizing agent, formed into a tablet, may
also be coated w~th an insoluble, yet semipermeable membrane. This
membrane ~ay also be appl~ed by known coating processes, such as spray-
coating the film, by the flu;d;zed bed process or in closed systems, and
moreover in an amount of 3 to 10 ~g/tablet. The semipermeable membrane
may be formed, for example, as follows from an organic solution of
ethylcellulose, an aqueous dispersion of ethylcellulose, a copolymer of
acrylate/methacrylate esters with tr;methylammoniummethacrylate, an
aqueous dispers~on of mixed ~ethyl methacrylates and ethyl methacry-
lates; to control the diffusion rate, suitable plasticisers can be
added, especially in an amount of 1.0 - 5.0 mg/tablet. As plast;cizer,
1,2-propyleneglycol, polyethylene glycols and citrate esters ~re suit-
able. An example of the co~position of a semiper~eable membrane 1s as
follows;
3.8 mg ethylcellulose N22
1.2 mg polyethylene glycol (6,000)
A further example of a retarding agent, by means of which a time~delayed
release of the catalyst, which decomposes hydrogen perox~de, and of the
neutralizing agent can be attained, ;s a sw~lable, yet sparingly solu~
ble or insoluble ~atrix, especially in tablet form, ;n which the cata-
lyst-contain~ng neutralizing agent is d;stributed. The catalyst-con-
taining neutralizing agent may have one of the aforementioned compos1-
tions, ~hich is incorporated in the matrix that may comprise natural or
partly synthet1c polymers. The matrix, especially in tablet form, can
be produced by conventional processes, for example, by granulating and
molding the starting materials. It is of course also possible to pro-
- ~297403
cess the mixture of start;ng ~a~erlals directly ;nto tablets without
prior granulation. Polymers, suitable for the formation of the ~atrix,
are the soluble cellulose ethers, such as those given by way of exa~ple
above, the alkali salts of alginic acid, ~ethacrylic ac1d der{vatives,
especially acrylate/methacrylate esters, acrylic acid derivatives, tex-
trans (MW 1,000 - 75,000) and polyvinyl alcohols. The following is an
example of the matrix, in which the catalyst-conta;ning neutral~ing
agent i5 1ncorporated:
0.3 mg catalyst (catalase, perox1dase)
10.2 mg disodium hydrogen phosphate
55.8 mg ~aCl
4.7 mg polyv;nylpyrrolidone K25
l~ mg hydroxypropylmethylcellulose
Tablets are molded to have, for example, a weisht of 86 mg and a 6 ~m
round format.
It is also possible to incorporate the components of the catalyst and
the neutralizing agent in a h;ghly-concentrated, aqueous polymer solu-
tion and, after cast1ng and drying, to produce from this sectile films
1.0 - 3.0 mm thick. By cutting the film to s;ze, appropriate dosage
units can be produced in precalculated superficial dimensions. ~he
water~soluble polymers act as retarding asent, through wh1ch the time-
delayed release of catalyst and neutralizing agent ~s attained. Su1t-
able polymers are water-soluble cellulose ethers, 11ke those given above
by way of example, alkali alginates, dextrans and polyvinyl alcohols.
It is known in the medical sector concerned with the admin;stration of
drugs that therapeut k systems may be used in the form of tablets,
sem1permeable membranes and matrices, which are brought into a particu-
lar target area of a biosystem in order to bring about a constant
delivery of drug over a prolonged per;od of tire. In contrast to this,
the invent10n accomplishes that the hydrogen perox;de-decomposlng cata-
lyst, together with the stérilizing hydrogen peroxide, can be used
-
12~7403
simultaneously in the treatment of contact lenses ~ithout adverse effect
by the catalyst, which decomposes the hydrogen peroxide, on the desired
ster~lizing action of the hydrogen peroxide over a period of, for exam-
ple, four hours. The decomposing action of the catalyst on the hydrogen
peroxide sets ~n~only ~t the end of the desired treatment time.
A delayed release of the catalyst can also be achieved by immobilizing
the catalyst on particutate carrier substances, especially acrylic resin
pellets, for example, by bonding over reactive oxiran groups. The
carrier substances, with the catalyst, especially an enzymatic catalyst,
immobllized thereon, are d;sposed in an adeguate amount, for example in
a bottom part or a lid w~th screw thread of a contact lens treatment
container or ~ contact lens case and are separated from the treatment
solut~on, which conta;ns the pH-neutralizing materials and the neutral
salts together with the hydrogen peroxite, by permeable, inert sieve
netting. In this vers;on, the catalyst is disposed in a part of the
treatment container without mixing with the treatment solution. By
these means the danger is avoided, that the catalyst material will
collect or accumulate in the soft lens materlal during the treatm~nt of
soft contact lenses. For example, an amount of carrier substances with
an immob~l~zed enzymatic catalyst, suff~cient for 30-day repeatet use,
can be accommodated in the container part.
The treatment solution, ;n which the release of catalyst-containing
neutralizing agent takes place, may have a pH of about 7 to 7.5 and
espeoially of 7.3 and an osmolarity of about 300 mosmol.
A color change, especially the use ~f a color indicator (pH/redox indi-
cator3, changins color at a p~ of 7.0 to 7.5 and espec;ally at 7.3,
indicates to the user that the hydrogen peroxide, used for the steriliz-
ing treatment, has been decomposed. For this purpose, high molecular
weight dyes, which do no~ penetrate into the 1ens mater;al, are espe-
cially suitable.
So that the catalyst, which preferably is an enzymatic catalyst, exerts
lZ974(~3
its act10n only after the disinfecting or sterilizing treat~ent of the
lens, this catalyst additive, together with auxiliary ~aterials, whtch
are present ;n the neutralizing agent and which serve to neutral;ze the
hydrogen peroxide, may be packaged in a coat;ng, wh kh ensures that the
catalyst for décomposing the hydrogen peroxide is released only after
the necessary treatment time (up to four hours). rhere are a number of
possibilit~es for accomplishing this.
The coating or packaging of the catalyst and the auxiliary materials has
the shape of a capsule, which is ~ater-soluble at the pH, at which the
hydrogen peroxide solution is stable, that is, at a pH of 4 to about 5,
yet does not fl~cculate or precipitate in neutral solution, that is, at
a p~ of about 7.3.
It is, moreover, advantageous if the capsule is provided with one or two
or optionally even more laser perforations, through which the auxtliary
materials are released on contact with the hydrogen peroxide solution,
the capsule dissolving completely when a pH of about 7 is reached in
order to release the catalyst then.
The ca~alyst may also be present as an enzyme pr;ll which, together with
the auxiliary materials, is filled into the capsule, wh;ch has the
aforementioned dissolving properties.
The catalyst-containing neutralizing agent may also be fashioned as a
coated tablet, from which, with delayed dissolution of the coating,
first of all the auxiliary materials are set free to change the p~ of
the hydrogen peroxide solution and then, at a pH of about 7, the cata-
lyst is set free.
Furthermore suitable for the catalyst-containing neutraliz;ng agent is a
two-layer tablet, one of the layers of which, comprising soluble salts,
serves to neutralize the hydrogen peroxide soluti~n and the other layer
of which conta;ns the catalyst, it being possible to release either the
auxil;ary materials or the catalyst first.
.,.
,
~2~4~3
The neutratizing agent may also be present as a basic gel in a coating,
which is soluble at a pH of 7. The catalyst may also be present ss
viscous so1ution ;n a coàting, which surrounds the aux~liary mater1als.
Moreover, the neutral;zing agent may have a sem;permeable membrane as
coating, through which the water to dissolve the auxiliaries penetrates
into the interior and out of which the dissolved salts reach the outside
due to osmotic pressure. For this purpose, the semipermeable membrane
may have a perforation or it may be destroyed by osmotfc pressure,
whereby then the auxiliary materials and the catalyst are set free. The
catalyst may be present here as an enveloped enzyme product.
Moreover, a two-layer syste~ with a soluble and a semipermeable membrane
is suitable for the time-displaced release of the auxil~ary materials
and the catalyst. An insoluble membrane system could be exchanged on
renewed use.
A suitable two-layer system may be constructed so that the catalyst is
enveloped by the semipermeable membrane, at the outside of which the
aux;liary mater;als lie. A coating, water soluble at the pH (less than
4) of the stable hydrogen perox~de solution, moreover envelops the
aux;liary materials and the se~ipermeable ~embrane enclosing the cata-
lyst.
~he decomposition of the hydrogen peroxide can be tetected by a color
ind~cator. ~n this connect;on, it is a question of the addition of a
pH/redox indicator, ~hich does not penetra~e lnto the soft hydrophiltc
contact lenses and which is physiologically safe. ~he color indicator
may also be so designed, that it is colored at pH 7 in the presence of
hydrogen perox;de and colorl~ss at pH 7 in the absence of hydrogen
peroxide.
The container, in which the contact lens care or treatment ~s carr~ed
out, may have facllities for vent;ng, ~or example, in the form of a
12
12974~3
Bunsen valve or the like, through which the oxygen released during the
neutralization and decomposition of the hydrogen perox;de can escape.
The attached Figures serve to explain the invention further.
Figures 1 to 5 show different examples of the systems containing hydro-
gen perox~de and the decompos;tion catalyst.
Figure 6 shows the timewise course of the treat~ent of a contact
lens with an example of the operation of the contact lens
care set.
Figure 7 shows a treatment container ;n side view.
Figure 8 shows a plan view of a container part, which can bescrewed onto the treatment container and which contains
part~culate carrier substances, to wh~ch an enzymatic,
hydrogen peroxide-decomposing catalyst is bound.
In the example shown in Fig. 1, the auxiliary materials 1 ~nd a hydrosen
peroxide-spl;tt;ng catalyst 2. for example in the for~ o~ c~talase, are
randomly distributed in the outer casing ~. The outer casing 3 may be
water soluble or sem~permeable after it has been acted upon for a cer-
tain period of time by the hydrogen peroxide, which has a sterilizing
effect in contac~ lens care on the contact lens to be treated. ~he
thickness of the casing controls the len~th of time (up to four hours)
that the hydrogen peroxide acts on the lens, before sufficient catalase
and auxiliary materials are released by the dissolution of the outer
casing 3 or by the sem~permeability of this cas;ng, so that the steri-
lizing hydrogen peroxide i5 neutralized and e1iminated by catalyt;c
decomposltion. In this example of the operation, the auxiliary mate-
rials and the catalyst may be released simultaneously.
In the example shown ;n Fig. 2, the auxiliary materials 1 are released
first, ~s a result of wh~ch the ster;l;zing hydrogen peroxide solutlon
is neutralized. After this, the hydrogen peroxide-testroying catalyst
2, for example ~n the form of catalase, comes to be used. For this
purpose, the outer caslng 3 is provided whlch, after the hydrogen per-
-. ,
~297~3
oxide has acted for a certain period ~f ti~e, is soluble or wh1ch is
appropriately sem~permeable and which surrounds the auxiliary materials
l. ~he auxiliary materials l surround the catalyst 2, which, for ~ts
part, 1s surrounded by an internal casing 4, which may also be con-
structed so as to be soluble or semipermeable.
~n the example shown ln Flg. 3, the catalyst 2 and the auxiliary mate-
rials l are next to each other and are surrounded by a common outer
casing 3, which is water soluble or semipermeable. In th~s case, the
auxiliary materials l and the catalyst 2 ~ay be separated by a partition
5.
In the example shown in Fig. 4, tne catalyst 2, which may be present ;n
the form of a viscous solution, is released first, after which the
auxiliary materials l are released. The catalyst 2 is surrounded here
by the outer casing 3 and the auxit;ary materials l by the inner casing
4. In this version, the auxiliary materials l lie on the inside and are
surrounded by the catalyst 2.
In the example shown ;n F;g. 5, the catalyst 2, as catalyst prill, and
the auxiliary mater;als l are randomly d;stributed ~n the outer casing
3, which may be soluble or semipermeable.
Fig. 6 graphically shows the timewise course of the treatment of the
contact lens with the hydrogen peroxide and the subsequent destruction
of the hydrogen peroxide, after it has acted for the desired time tl.
The length of ti~e, that the hydrogen peroxide acts, can be f;xed. for
exa~ple, by the thickness of the coating or by an appropriate selection
of the ~aterial of the coating. The hydrogen peroxide content is plot-
ted on the ordinate, the ;n~tial hytrogen perox~de content essentially
remainin~ constant until the end of the time of action tl. After the
release of the au~ aries and/or the catalyst, the hydrogen peroxide
content 1n the treatment container decreases rapidly.
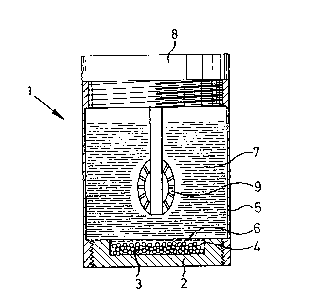
F;gs. 7 and 8 schemat k ally shown an example of the operation of a
14
~ 7~33
treatment conta;ner l, in which in a bottom part 2 particulate carrier
substances 3, on which an enzymatic hydrogen peroxide-deco~posing cata~
lyst ~s immobilized, are d~sposed in a recess 4 of the bottom part 2.
The ~otto~ part 2 may, for example, may be attached by a screw th~ead to
the treatment vessel part 5, as described, for example, in the German
Offenlegungsschrift 3,410,400. The carrier substances 3, coated w~th
the cat~lyst, are separated fro~ the 1nterior 7 of the container by
means of an inert sieve netting 6, which is impermeable to the treatment
solution present in the interior 7 of the container. The color of the
lid 8, which ;s ~lso screwed on and to which the cups 9 for the contact
lenses to be treated ~re attached, as shown ~n the German Offenlegungs-
schrift 3,410,400, may be different from the color of scre~ed-on base
part 2, so that the danger of co~fusion ~s precluded.
It ~ill be understood that the specification and
examples are illustrative but not limitative of the present
invention and that other embodiments within the spirit and
scope of the invention will suggest themselves to those
skilled in the art.
. :- - . .