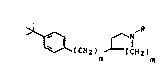Note: Descriptions are shown in the official language in which they were submitted.
~2~7~
- 1 - O.Z. 0050/38413
N-substituted_pyrrolidine and piperidine derivatives
and their salts
The present invention relates to novel N~substi-
tuted pyrrolidine and piperidine derivatives of the general
formula I
~ICH2) ~IH2) ( L)
n m
where R ;s C1-CzO-alkyl, C2-C20-alkoxyalkyl, C2-Czo~
hydroxyalkyl, C3-C12-cycloalkyl, C4-Czo-alkylcycloalkyl,
hydroxyl-substituted C4-CzO-alkylcycloalkyl, C4-CZo-
cycloalkylalkyl, aryl, haloaryl, C7-c20-aralkyl~ C7-C20-
haloaralkyl, Cg-C20-trifluoromethylaralkyl or C7-Czo-
aryloxyalkyl, m is 1 or Z and n is O or 1, and their phy-
tophysiologically tolerated salts.
The present invention furthermore relates to fung-
icides of the formula I which contain these compounds, and
to methods for controlling fungi.
Phenyl- and benzylpyrrolidines and -piperidines,
which may also be substituted in the aromatic radical,
are known to be antidepressants tGerman Laid-Open Appli-
cation DOS 2,801,195 and US-A 3 632 767) and muscle relax-
ants (German Laid-Open Applications DOS 2,017,255 and DOS
2,017,Z56).
Furthermore, the compound N-C3-p-tert-butylphen-
yl-2-methyl-1-propyl]-cis-2,6-dimethylmorpholine (fenpro-
pimorph)I' from German Laid-Open Applicat;on DOS 2,656,747
CH3
~ ~ C~3
is a known commercial fungicide.
It is an object of the present invention to pro-
vide novel effective fungicides.
We have found that this object is achieved by the
novel N-substituted pyrrol;d;ne and piper;d;ne derivat-
;ves I def;ned at the outset and the;r salts. We have
furthermore found that the compounds I and their phyto-
- 2 - O.Z. 0050/38413
physiologically tolerated salts are very useful as fungi-
cides.
The compounds of type I are obtainable by the
following methods.
The compounds I can be prepared, as shown in the
scheme below, by introduction of a tert-butyl group into
the aromatic followed by N-substitution of the secondary
amine, or by subjecting the same starting compound IV to
the reverse reaction sequence.
~ ~H R-X or
r ~ I c H 2 ~lH 2 ¦ cC O m b 8 0 Yn c~
¦ IIV) ¦ 2) H2/ cat-
~(CH2) ~CH2) ~(CH2) ~C~iz)
n m n m
III) IIII)
~-x or
l~carbon L
compouXd ~ N ~ / H3
~H /cat. ~
2 ~;~(CH2) ~-DlCH2)
(I)
a) Introduction of the radical R
a1) with halides R-X
Substitution at the n;trogen of the arylamines
II and IV can be carried out in a conventional manner,
in the presence or absence of a solvent or diluent and/or
of a base and/or of a reaction accelerator. The reaction
is accelerated using, in particular, iodides, eg. potassium
or sod;um iodide. The solvents or diluents used are those
conventionally employed for this purpose, such as chloro-
hydrocarbons, eg. tetrachloroethylene, tetrachlaroethane,methylene chloride, chloroform, carbon tetrachloride, tri-
chloroethane and chlorobenzene, ethers, eg. di-n-butyl
- 3 - O.Z. 0050/38413
ether, diisopropyl ether, tetrahydrofuran, ethylene gly-
col dimethyl ether and dioxane, nitrohydrocarbons, eg.
nitromethane and nitrobenzene, ketones, eg. cyclopenta-
none, cyclohexanone and acetone, and amides, eg. formamide,
dimethylformamide and dimethylacetamide. Suitable bases
are all conventiona~ inorganic and organic acid binders.
These preferably include tertiary amines, alkali metal
and alkaline earth metal compounds and mixtures of these.
a2) With carbonyl compounds
Enamine formation in the reaction of the amines
II and IV with a carbonyl compound
R 1J~R2
R
can be carried out in a conventional manner under acid
catalysis with removal of the water of reaction, or, for
example, by the addition of water binders, such as sodium
sulfate, magnesium sulfate, a molecular sieve or titanium
tetrachloride. Examples of suitable solvents are hydro-
carbons, such as toluene, xylene and naphtha, chlorohydro-
~0 carbons, such as methylene chloride, chloroform and chloro-
benzene, and ethers, such as diethyl ether, diisopropyl
ether, tetrahydrofuran and dioxane. The enamine thus ob-
tained can be hydrogenated to the amine under catalysis,
for example with platinum oxide (Rylander, Catalytic
Hydrogenation over Platinum Metals, page 176, Academic
Press, New York 1967).
b) Introduct;on of the tert-butyl group into the aromatic
Arylamines of type III and IV can be converted
with isobutylene to the p-tert-butyl-substituted phenyl
compounds in a conventional manner in an acid-catalyzed
re~ction. Particularly suitable solvents are halohydro-
carbons, such as methylene chloride, chloroform, carbon
tetrachloride, tetrachloroethane, trichloroethane and
chlorobenzene, as well as the solvents usually employed
for this purpose. Mineral acids such as sulfuric acid
are used, and the reaction is carried out with ice cool-
ing, for example at from -5 to +10C. The product can be
~9~78~3~
- 4 - O.Z. 0050/3~413
obtained either as the salt, for example the bisulfate,
or as the free amine.
c) Preparation of salts of the N-substituted pyrrolidine
and piperidine compounds
The cyclic tertiary amine I can be dissolved in
an inert solvent, and salt formation can be effected in
a conventional manner, by passing the gas into the sol-
ution in the case o~ gaseous acids, eg. HCl gas, by the
drop~ise addition of equimolar amounts in the case of
1Q liquid acids, such as phosphoric acid or sulfuric acid,
and by dissolving an equimolar amount in an inert solvent
and then addin~ the solution dropwise in the case of solid
acids. The reaction can be carried out at room tempera-
ture, ~ith or without ice cooling.
Among the plant-tolerated salts of the cyclic
tertiary amines, it is possible to form, for example, those
with HCl, H~r, HI, H2S04, H3P04, HN03, HC02H, CH~C02H,
C2H5C2H or C12H25-C6H5-so3H~
The novel N-substituted pyrrolidine and piperidine
2Q derivatives of the formula I and their phytophysiologically
tolerated salts may contain chiral centers. They are gen-
erally obtained in the form of racemates bwt may be ob-
tained as diastereomer mixtures. Pure diastereomers can
be isolated in the case of some of the noveL compounds,
for example by distillation or column chromatography or
on the basis of solubility differences. Pure enantiomers
can be obtained, for example, by resolution of the race-
mate uith a chiral auxiliary by a conventional method, for
example via diastereomeric salts. Regarding the use of the
3Q novel N-substituted pyrrolidine and piperidine derivatives
I and their phytophysiologically tolerated salts as fungi-
cides, both the d;astereomers or enantiomers and their
stereoisomer mixtures obtained in the synthesis are suitable.
R in the compounds I is
C1-C20-alkyl, preferably C1-C10-alkyl, particularly pref-
erably C4-Cg-alkyl, such as n-butyl, isobutyl, tert-butyl,
n-pentyl, isopentyl, n-hexyl, n-octyl, n-nonyl, 4,4-dimethyl-
~2~38~
- S - O.Z 0050/38413
pentyl, 3,5,5-tri~ethylhexyl, 3,3-dimethyl-n-butyl, 1,1,2-
trimethyl-n-propyl and 1,1,3,3-tetramethyl-n-butyl;
C2-C20-alkoxyalkyl, preferably C2-C8-alkoxyalkyl, such as
methoxyme~hyl, methoxyethy~, methoxy-n-propyl, methoxybutyl,
methoxyhexyl, ethoxymethyl, ethoxyethyl, ethoxybutyl, pro-
poxymethyl, propoxyethyl, butoxymethyl or butoxypropyl;
C2-C20-hydroxyalky~ preferab~y C2-C8-hydroxyalkyl, such
as hydroxyethy~, hydroxyProPyl, hydroxybutyl, hydroxyhexyl
or hydroxyoctyl;
C3-C12-cycloa~kyl, preferably Cs-cg-cycloalkyl, particu-
larly preferably cyclopentyl or cyclohexyl;
C4-Czo-alkylcycloalkyl, preferably C4-C12-alkylcycloalk-
yl, particularly preferably C8-C10-alkylcycloalkyl, such
as 3-methyl-5-n-propyl-cyclohexyl, 3,5-diethyl-cyclohexyl,
4-(tert-butyl)-cyclohexyl, 1-ethylcyclohexyl, 3,3-dimethyl-
cyclohexyl and 3,3,5-trimethyl-cyclohexyl;
hydroxyl-substituted C4-Czo-alkylcycloalkyl, preferably
hydroxyl-subst;tuted C4-C12-alkylcycloalkyl, particularly
preferably hydroxyl-substituted C6-C1o-alkylcycloalkyl,
such as 3,3-dimethyl-4-hydroxycyclohexyl;
C4-CzO-cycloalkylalkyl, preferably C4-C12-cycloalkylalk-
yl, particularly preferably C6-Cg-cycloalkylalkyl, such
as cyclopentylmethyl, cyclopentylethyl, cyclohexylmethyl,
cyclohexylethyl or 2-cyclohexylethyl;
aryl, preferably phenyl or naphthyl;
haloaryl, preferably fluoro- or chloroaryl, particularly
preferably chloroaryl, such as 4-chlorophenyl, 2,4-di-
chlorophenyl or 3,5-dichlorophenyl;
C7-Czo-aralkyl, preferably C7-C14-aralkyl, such as benz-
yl, phenylethyl, phenylbutyl, phenylhexyl or phenyloctyl;
C7-C20-haloaralkyl, preferably C7-C14-haloaralkyl, particu-
larly preferably C7-C10-fluoro- or chloroaralkyl, such as
4-fluorobenzyl, 4-chlorobenzyl, 4-chlorophenylethyl, 4-
chlorophenylpropyl or 4-chlorophenylbutyl;
Cg-C20-trifluoromethylaralkyl~ preferably C8-C14-tri-
fluoromethylaralkyl, particularly preferably Cg-C10-tri-
fluoromethylaralkyl, such as 4-trifluoromethylbenzyl or 4-
~2~88Cl
- 6 - O Z. 0050/38413
trifluoromethylpheny~ethy~; or
C7-C20-aryloxyalkyl, preferably C7-C14-aryloxyalkyl,
such as phenoxymethyl, phenoxyethyl, phenoxypropy~,
phenoxybutyl, phenoxyhexyl or phenoxyoctyl
In the case of the indices, m is particularly
preferably 2 and n is particularly preferably 0.
Particularly preferred phytophysiologically tol-
erated salts of the compounds I are the hydrochlorides.
Ex~mples of suitable compounds I are:
l~ Compound Compound I
no. _ _ _ _
1 N-Methy!-4-(p-tert-butylphenyl)-piperidine
2 N-Isopropyl-4-(p-tert-butylphenyl)-pipericline
3 N-Isobutyl-4-(p-tert-butylphenyl)-piperidine
15 4 N-Isopentyl-4-(p-tert-butylphenyl)-piPeridine
N-n-Hexyl-4-(p-tert-butylphenyl)-piperidine
6 N-(4,4-Dimethylpentyl)-4-(p-tert-butylphenyl)-
piperidine
7 N-(3,5,5-Trimethylhexyl)-4-(p-tert-butylphenyl)-
piperid;ne
8 N-Cyclopentyl-4-(p-tert-butylphenylj-piperidine
9 N-Cyclohexyl-4-~p-tert-butylphenyl)-piperidine
N-(3-Methyl-5-n-propylcyclohexyl)-4-(p-tert-
butylphenyl) piperidine
11 N-t4-tert-8utylcyclohexyl)-4-(p-tert-butylphen-
yl)-piperidine
12 N-Cyclooctyl-4-(p-tert-butylphenyl)-piperidine
13 N-(Cyclohexylmethyl)-4-(p-tert-butylphenyl)-
piperidine
30 14 N-(2-Cyclohexylethyl)-4-(p-tert-butylphenyl)-
piperidine
N-(3-Cyclohexyl-n-propyl)-4-(p-tert-butylphenyl)-
piperidine
16 N-(2-Cyclohexyl-n-propyl)-4-(p-tert-butylphenyl)-
piperidine
17 N-(2-Hydroxyethyl)-4-(p-tert-butylphenyl)-piper-
idine
- 7 - O.Z. 0050/38413
Compound Compound I
noO
18 N-(2-Methoxyethyl)-4-(p-tert-butylphenyl)-piper-
idine
5 19 N-(2-Methoxy-n-propyl)-4-(p-tert-butylphenyl)-
piperidine
N-(4-Chlorobenzyl)-4-(p-tert-butylphenyl)-piper-
idine
21 N-(4-Trifluoromethylbenzyl)-4-(p-tert-butylphen-
yl)-piperidine
22 N-(2-Phenoxyethyl)-4-(p-tert-butylphenyl)-piper-
idine
23 N-(Cyclohexylmethyl)-4-(p-tert-butylPhenYl)-
pyrrolidine
15 24 N-(2-Cyclohexylethyl)-4-(p tert-butylbenzyl)-
piperidine
N-(4-Chlorobenzyl)-4-(p-tert-butyl-benzyl)-
piperidine
26 N-(Cyclohexylmethyl)-4-(p-tert-butylphenyl)-
Z0 pyrrolidine
27 N-(2-Cyclohexylethyl)-4-(p-tert-butylPhenyl)-
pyrrolidine
28 N-Cyclohexyl-4-(p-tert-butylphenyl)-pyrrolidine
29 N-Cyclopentyl-4-(p-tert-butylphenyl)-pyrrolidine
25 30 N-Cyclohexyl-4-(p-tert-butylbenzyl)-pyrrolidine
31 N-Cyclopentyl-4-(p-tert-butylbenzyl)-pyrrolidine
32 N-Isobutyl-4-(p-tert-butylphenyl)-piperidinium
hydrochloride
33 N-n-Hexyl-4-(p-tert-butylphenyl)-piperidinium
hydrochloride
34 N-Cyclohexyl-4-(p-tert-butylphenyl)-piperid;nium
hydrochloride
N-Cyclopentyl 4-(p-tert-butylphenyl)-piperidinium
hydrochloride
35 36 N-(3,3-Dimethyl-n-butyl)-4-(p-tert-butylphenyl)-
piperidine
37 N-(3,3-Dimethyl-n-butyl) 4-(p-tert-butylbenzyl)-
- 8 - O Z OOSO/38413
Compound Compound I
no.
piperidine
38 N-(3,3-Dimethylcyclohexyl)-4-(p-tert-butylphen-
y~)-piperidine
3cJ N-(3,3-Dimethylcyclohexyl)-4-(p-tert-butylbenzyl)-
piperidine
N-(3,3,5-Trimethylcyclohexyl)-4-(p-tert-butylphen-
yl)-piperidine
10 41 N-(3,3,5-Trimethylcyclohexyl)-4-(p-tert-butyl-
benzyl)-piperidine
42 N-tert-~utyl-4-(e-tert-butylphenyl)-piperidine
43 N-t1-Ethylcyclohexyl-4-(p-tert-butylphenyl)-
piperidine
15 44 N-(1,1,Z-Trimethyl-n-propyl)-4-(p-tert-butyl-
phenyl)-piperidine
N-(1,1,3,3-Tetramethyl-n-butyl)-4-(p~tert-butyl-
phenyl)-piperidine
46 N-(4-Hydroxy-3,3,5-trimethylcyclohexyl)-4-(p
2Q tert-butylphenyl)-piperidine
47 N-(4-Hydroxy-3,3,5-trimethylcyclohexyl)-4-(p-
tert-butylbenzyl)-piper;dine
48 N-(3,3-Dimethyl-4-hydroxycyclohexyl)-4-(p-tert-
butylphenyl)-piper;dine
25 49 N-(3,3-Dimethyl-4-hydroxycyclohexyl)-4~(P-tert-
butylbenzyl)-piperidine
N-(4-Hydroxycyclohexyl)-4-(p-tert-butylphenyl)-
piperldine
51 N-(4-Hydroxycyclohexyl)-4-(p-tert-butylbenzyl)-
p;peridine
52 N-(p-Chlorobenzyl)-4 (p-tert-butylbenzyl)-piperidine
The N-substituted pyrrolidine and piperidine deriva-
tives of the formula I and their phytophysiologically tol-
erated salts or the fungicides which contain them and the
ready-to-use formulations prepared from them can be used,
for example, in the form of directly sprayable solutions,
emulsions, suspensions, dusts, powders, pastes or granules
~ ~ 2~8~
- 9 - o.Z. 0050/38413
in a conventi~nal manner, for example by spraying, atom-
izing, dusting, broadcasting, dressing or watering. The
forms employed depend entirely on the intended uses. They
shou~d at all events ensure fine, uniform distribut;on of
the active substance. The formulations are prepared in a
conventional manner, for example by extending the active
ingredient with solvents and/or carriers, ~ith or ~ithout
the use of emulsifiers or dispersants. Examples of sol-
vents used are aromatics, such as benzene, toluene or the
xylenes, chlorinated aromatics, such as chloro- or bromo-
benzene and their derivatives, paraffins, such as various
mineral oil fra~tions, alcohols, such as methanol, etha-
nol, n- and ;sopropanol or n-, iso- or tert-butanol,
ketones, such as acetone, cyclopentanone or cyclohexanone,
am;nes, such as ethanolamine, dimethylamine, triethylamine
or dimethylformamide, and water. Where water is used as a
diluent, solubilizers are employed. Suitable solubilizers
are organic solvents which are partially or completely
m;sc;ble with water, such as acetone, methanol, ethanol,
ethanolam;ne, dimethylamine or tr;ethylamine. Suitable
carriers are powdered natural rocks, such as kaolin,
chalk, talc or alumina, and powdered synthet;c minerals,
such as finely d;vided silica or silicates. The emulsi-
fiers used are nonionic or anion;c ones, eg. polyoxy-
ethylene fatty alcohol ethers or alkyl- or arylsulfonates,
and the d;spersants employed are, for example, l;gnin-
sulfite waste liquors or methylcellulose.
The N-subst;tuted pyrrolidine and piperidine
derivat;ves of the formula I are distinguished by excel-
lent act;vity aga;nst a broad spectrum of phytopathogenicfungi, ;n part;cular from the class cons;sting of the Asco-
mycetes and 9as;d;omycetes. Some of them have a systemic
act;on and can be used as fol;age and/or soil fungicides~
The compounds I are particularly important for
controll;ng a large number of fungi in var;ous crops and/
or the;r seed, for example for wheat, rye, barley, oats,
rice, corn, cotton, soybeans,coffee, sugar cane, peanuts,
~97~
- 10 - O.Z. 0050/38413
fruit or ornamentals in horticulture or viticulture, and
tor vegetables, such as cucumbers, beans or cucurbits.
The compounds I are particularly useful for con-
trolling the stated plant diseases in or on the corres-
5 ponding crops.
Plant diseases Crops
Erysiphe graminis, Puccinia species cereals
Septoria nodorum wheat
Helminthosporium teres barley
10 Pseudocercosporella herpotrichoides wheat, barley
Ustilago species cereals, sugar cane
Pyricular;a oryzae rice
Cercospora arachidicola peanuts
Podosphaera Leucotricha, Venturia apples
15 inaequalis
aotrytis cinerea strawberries, grape vines
Plasmopara viticola, Uncinula necator grape vines
Alternaria solani, Phytophthora
infestans potatoes, tomatoes
20 Erysiphe cichoracearum, Sphaerotheca
fuliginea cucurbits
Fusarium species, Verticillium
species various crops
The fungicides contain in general from 0.1 to 95,
preferably from 0.5 to 90, æ by we;ght of active ingredient.
The appl;cation rates of the active ingredientare from 0.0~ to 3 kg of active ingredient per ha, or
higher, depend;ng on the type of effect desired. The N-
substituted pyrrolidine and piperidine derivatives I are
applied before or after infection of the plants or seeds,
by either spraying or dusting.
The compounds I can also be used in material pro-
tectionr for example for controll;ng ~ood-destroying fungi,
such as Coniophora puteanea or Polystictus versicolor.
These compounds may furthermore be used as fungi-
cidal components of oily ~ood preservatives for controlling
~ 2 ~ ~ 8~ ~
- 11 - O.Z. 0050/38~13
~ood-discoloring fungi, for examp~e by coating or impreg-
nating the wood.
Some of the compounds I are useful for controll-
ing skin-pathogenic fungi, such as Trichophyton mentagro-
phytes or Cand;da albicans.
The N-substituted pyrrolidine and piperidine de-
rivatives I can also be mixed with other active ingredi-
ents, eg. herbicides, insecticides, growth regulators or
fertilizers, and applied together with these.
Preparation Examples
EXAMPLES 1 T0 3
N-substitution with halides R-X
A solution of 434 mmol of a cyclic amine II, 430
mmol of a halide R-X and 1.3 mol of sodium carbonate in
400 ml of dimethylformamide was heated for 8 hours at
150C, after which the reaction mixture was worked up in
a conventional manner.
Details and results are given in Table 1.
~0 TA~LE 1
Example Compound Compound Halide Compound I
no. no. II X bp.CC]/ Yield
m n R mbar C%]
1 5 2 0 n-hexyl Br 160-170/1.0 82
2 14 2 0 2-cyclo- ar 185-186/0.2 75
hexylethyl
3 36 2 0 3,3-dimeth- Cl 177/2 65
yl-n-butyl
EXAMPLES 4 T0 15
a) Preparat1on of the enamines
A solution of 2 mol of a cyclic amine II~ 1 mol
of a carbonyl compound and 1 g of toluenesulfonic acid in
400 ml of toluene was heated under a water separator and
worked up in a conventional manner.
b) Hydrogenation of the enamines
.
A solution of 460 ~mol of the enamine in 1 l of
~7~
- 1Z - O.Z. 0050/38413
ethyl acetate was hydrogenated in the presence of 10 9 of
10~ strength Pd/C at 60-70C and under 100 bar, and the
mixture was ~orked up in a conventional manner.
Details and results are summarized in Table 2.
S TABLE Z
Exam- Compound Compound Carbonyl Compound I
ple no. no. II compound R bp.[C]/ Yield
n m mbar [%]
4 3 0 2 isobutyr- isobutyl 155-160/0.8 55
aldehyde
4 0 2 isovaler- isopent- 165-168/1.0 40
aldehyde yl
6 6 û 2 y,y-dimeth- 4,4-di- 160/0.3 60
ylvaler- methyl-
aldehyde pentyl
7 7 0 2 2,4,4-tri- 3,5,5- 182-184/0.3 65
methylhex- trimeth-
anal ylhexyl
8 8 0 2 cyclopenta- cyclo- 172-180/1.0 35
none pentyl
9 9 0 2 cyclohexa- cyclo- 188-194/2.0 66
none hexyl
0 Z 3-methyl- 3-meth- 190-192/0.4 70
5-n-prop- yl-5-n-
yl-cyclo- propyl-
hexanone cyclohexyl
11 13 0 2 cyclohex- cyclohex- 165-170/0.3 65
ane carb- ylmethyl
aldehyde
12 23 0 1 cyclohex- cyclohex- 15Z-156/OoZ 70
ane carb- ylmethyl
aldehyde
13 24 1 2 cyclohex- cyclohex- 174-178/0.3 48
ane carb- ylmethyl
aldehyde
- 13 -O.Z. 0050/38413
Exam- Compound Compound Carbonyl Compound I
ple no. no. II compound R bp.[C]/ Yield
n m mbar ~] _
14 38 0 2 3,3-di- 3,3-di- 36
methyl- methyl-
cyclohex- cyclohexyl
anone
0 2 3,3,5-tri- 3,3,5-tri- 130-138/1 34
methylcy- methylcy-
clohexa- clohexyl
none
) mp. ~C~: 119-121
EXAMPLES 16 T0 22
Introduction of the tert-butyl group into the aromatic
1.4 mol of concentrated sulfuric acid were added
dropwise to an ice-cooled solution of 275 mmol of a cyclic
amine in 500 ml of methylene chloride at from 5 to 10C,
and the solution was then gassed with 375 mmol of isobut-
ylene at from 10 to 15C. The mixture was stirred for one
hour at room temperature and then ~orked up in a conven-
tional manner.
Details and results are given in ~able 3.
TA88E 3
Exam- Compound Compound III Compound I
25 ple no. no. bp.~C]/ mp. Yie~d
n m R mbar tC] [%]
16 5 0 2 n-hexyl 160-170/1.0 45
17 8 0 2 cyclopentyl 172-180/1.0 52
18 9 0 2 cyclohexyl 188-194/2.0 62
19 42 0 2 tert-butyl 74 65
20 43 0 2 1-ethyl- 86-88 52
cyclohexyl
21 44 0 2 1,1,2-tri- 108 64
methyl-n-
propyl
22 45 0 2 1,1,3,3-tetra- 86-88 36
methyl-n-butyl
~7~
BAS~ Aktiengesellschaft - 14 - O.Z. 0050/38413
EXAMPLE 23
Preparation of N-isobutyl-4-(p-tert-butylphenyl)-piperidinium
hydrochloride (compound 32)
o5
At room temperature, HCl gas was passed into a solution of
73 mmol of N-isobutyl-4-(p-tert-butylphenyl)-piperidine.
Conventional working up gave 67% o~ compound 32; m.p.:
246C (with decomposition).
Use Examples
EXAMPLE A
Action on cucumber mildew
The leaves of pot-grown cucumber seedlings of the
"Chinesische Schlange" variety were sprayed at the 2-leaf
stage with a spore suspension of cucumber mildew (Erysiphe
cichoracearum and Sphaerotheca fuliginea). After about
20 hours, the plants were sprayed to runoff with aqueous
emulsions consisting (dry basis) of 80 wt.~ of active
ingredient and 20 wt.% of emulsifier. After the sprayed-on
layer had dried, the plants were set up in the greenhouse
at from 20 to 22C and a relative humidity of 70 to 80%.
To assess the action of the novel compounds, the extent of
fungus spread was determined after 21 days.
The results show that compounds 4, 5, 6, 7, 8, 9, 13, 14,
23, 24 and 32 of Examples 1, 2, 4, 5, 6, 7, 8, 10, 11, 12,
13, 14, 15 and 16, when applied as 0.025% spray liquors,
had a better fungicidal action (97 to 100%) than the
comparative agent fenpropimorph (70%).
8~30
BASF Aktiengesel]schaft 15 O.Z. 0050/38413
EXAMPLE B
Action on Plasmopara viticola
05 Leaves of potted vines of the MUller-Thurgau variety
were sprayed with aqueous suspensions containing (dry
basis) 80 wt.% of active ingredient and 20 wt.% of
emulsifier. To assess the duration of action, the plants
were set up, a~ter the sprayed-on layer had dried, for
8 days in the greenhouse. Then the leaves were infected
with a æoospore suspension of Plasmopara viticola. The
plants were first placed for 48 hours in a water
vapor-saturated chamber at 24C and then in a greenhouse
~or 5 days at from 20 to 30C. To accelerate and
intenslfy the sporangiophore discharge, the plants were
then again placed in the moist chamber for 16 hours. The
extent of fungus attack was then assessed on the
undersides of the leaves.
The results of the experiment show that compounds
4, 5, 6, 7, 8, 9, 10, 13, 14, 23, 24 and 32 of Examples
1, 2, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14 and 16, when
applied as 0.05% spray liquours, had a better fungicidal
act~on (90 to 100%) than the comparative agent fenpropimorph
(5%)-
~5
EXAMPLE C
Actlon on Septoria nodorum
.
Leaves of pot-grown wheat seedlings of the "Jubilar"
variety were sprayed to runoff with aqueous spray liquors
consisting (dry basis) of 80 wt.% of active ingredient and
20 wt.% of emulsifier. On the following day, the plants
(with the dried-on layer) were infected with an aqueous
spore suspension of Septoria nodorum, and then cultivated
for a further 7 days at 17 to 19C and a relative
~291~
BASF Aktiengesellschaft 16 O.Z. 0050/38413
humidity of 95%. The extent of fungus attack was then
assessed visually.
The results of this experiment show that compounds
3, 4, 8, 13 and 14 of Examples 2, 4, 7, 10 and 14 had, when
O5 applied as 0.05% spray liquors, a better fungicidal action
(97 to 100%) than the comparative compound fenpropimorph
t90~-