Note: Descriptions are shown in the official language in which they were submitted.
~2913~83
ATTORNEY DOCKET NO. 8305
20MB5/8305PAFOR/DR3/111
METHOD AND APPARATUS FOR DETECTION
AND QUANTITATION OF BACTERIA
Field of the Invention
This invention relates generally to testing
procedures for bacteria in fluid samples such as human
body fluids and mora particularly to a method and
apparatus for efficient, less cost, rapid testing
procedures for identifying the presence and concentration
of gram negative and gram positive bacteria strains in
fluid samples.
Background of the Invention
A need exists for a method to rapidly detect
bacteria in urine and various other fluid samples.
Urine specimens are the ma;or work load of the
diagnostic laboratory. The most common urological disease
is urinary-tract infections in children, pregnant women,
diabetics and geriatric patients, (bacteriuria). In
hospitals, bacteriuria ls the predominant form of
nosocomial infection. In view of the frequent
asymptomatic urinary-tract inections bacteriuria tests
must be sufficiently simple and economical to permit
routine testing. A need, therefore, exists for simple,
rapid, inexpensive screening tests to facilitate diagnosis
and ensure prompt treatment of bacteriuric patients.
The conventional method for detection of
urinary-~ract infections ls by inoculation of urine onto
agar culture plates and incubation of these plates at
optimal temperatures (usually 37 degrees C) for 24-48
hours; bacteria are detected by formation of colonies on
'
~2~ 33
the agar surface. Fastidious bacteria may not grow on
conventional culture media, and a variety of agar media
may be required to detect these bacteria. This procedure
is time consuming and expensive.
Although staining techniques, such as the Gram
Stain Method, are known in clinical microbioloyy, light
microscopy is required, and the procedurss are time
consuming and require a skilled microbiologist. Other
prior art processes involve staining of bacteria followed
by concentration by centrifugation or filtration. These
processes require chelating agents and positively-charged
small pore filters, and suffer clogging and interference
from anionic pigments, blood and other substances which
may be present in urine.
It has now been unexpectedly discovared that
both gram negative and gram positive bacteria can be
stained for si.mple, rapid detection by means of the
aomposltlon of the present invention. Concentrated
bacteria stained with the composition described herein are
readily visible and can thus be rapidly detected without
resorting to light microscopic examination by specially
trained personnel, or the use of expensive, perishable
agar cultures. The present invention allows the efficient
detection of bacteria while reducing clogging and pigment
interferance common to prior art methods. Furthermore, it
was discovered through tests that inexpensive, simple, and
rapid quantitative analysis of bacteria is possible
employing the prasent staining composition.
Summary of the Invention
A composition for staining both gram negative
and gram positive bacteria is provided. The composition
comprises an inorganic acid diluent, a dye soluble at a pH
of 8 to 12 which preferentially stains bacteria, a washing
1~9~83
reagent composed of an inorganic acid, and a filter paper
or glass filter of a net negative charge. Bacteria are
electrostatically immobilized and concentrated on the
filter and are stained with the dye solution. The free
dye is then removed with the washing reagent. The
bacteria, which retain the dye, become visible and thus
may be detected and quantitated by comparing the color
intensity of the filter surface (due to the stained
bacteria) tc a nomograph or other calibrated standard
based on color intensities obtained using known amounts of
bacteria. The total test time is usually less than one
minute.
It is therefore a primary feature of the presen-t
invention to provide a novel method and apparatus for
rapid detection and quantitation of bacteria to thus
enable rapid initiation of treatment of patients for
bacterial infections.
It is also a feature of this invention to
provide novel method and apparatus for bacteria detection
wherein a number of tests may be conducted simultaneously
for detection and quantitation of bacteria to thus
minimize the time period for detection of bacteria that
might be present in samples of body fluid.
It is another feature of this invention to
provide novel method and apparatus for identifying the
presence and concentrations of both gram negative and gram
positive bacteria strains in fluid samples.
Among the several features of this invention is
contemplated the provision of a novel method and apparatus
for bacteria detection through employment of a dye for
staining of the bacteria together with a filter composed
of paper, glass, or any other suitable material having a
net negative charge for electrostatically immobilizing and
~sa~s3
concentrating the bacteria on the filter prior to
staining.
It is another feature of this invention to
provide a novel method and apparatus for quantifying the
concentration of bacteria in a fluid sample by means of
visible comparison of the color intensity of stained
bacteria on the filter surface with a nomograph or other
calibrated standard based on color intensities obtained
using known concentrations of bacteria.
Brief Description of the Drawings
So that the manner in which the above recited
features, advantages and ob;ects of the present invention
are attained and can be understood in detail, more
particular description of the invention, briefly
summari~ed above, may be had by reference to the
embodiments thereof which are illustrated in the appended
drawings.
It is to be noted, however, that the appended
drawings illustrate only typical embodiments of this
invention and are therefore not to be considered limiting
of its scopa, for the invention may admit to other equally
effective embodiments.
Preferred embodiments of this invention have
been chosen for purposes of illustration and description
and are shown in the accompanying drawings forming a part
of this speciication wharein:
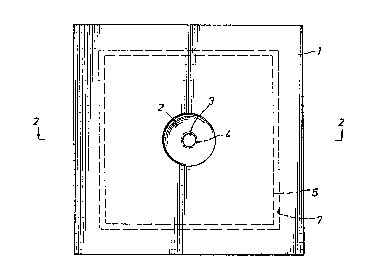
Fig. 1 is a plan view of filter apparatus
constructed in accordance with the present invention.
Fig. 2 is a sectional view taken along line 2-2
; of Fig. 1 and showing the various parts of the filter in
exploded relation.
Fig. 3 is a sectional view showing the assembled
filter apparatus of Figs. 1 and 2.
-
~298:18~
-- 5 --
Fig. 4 is a plan view of filter apparatus
representing an alternative embodiment of this invention
and providing for simultaneous multiple testing of a body
fluid sample.
Fig. 5 is a sectional view taken along line 5-5
of Fig. 4 which illustrates one position of the filter
apparatus of Fig. 4.
Fig. 6 is a sectional view similar to that of
Fig~ 5 showing another position of the filter apparatus.
Detailed Description of the Invention
This invention relates to compositions useful
fvr staining both gram negative and gram positive bacteria
and to various methods of detecting and quantitating
bacteria in fluid samples. Broadly statad, the staining
apparatus and composition of the invention comprises a
filter matrix having a net negative charge, an acid
diluent, a dye capable of staining bacteria at basic pH,
and a washing reagent which removes the free dye from
filter fibers but not from the bacteria. The immobilized
bacteria, electrostatically adsorbed to the filter, are
readily visible and easily distinguished from free dye
; which is diffused away from the stained bacteria by the
washing reagent.
Sinae the color intensity of the filter is
proportional to the number of bacteria in the fluid
sample, quantitative analysis of bacteria may be
accomplished by comparing the color intensity of the
stalned bactaria on the filter sur~ace to a known
standard.
The composition and methods of the invention
have particular application to the detection and
quantitation of bacteria in urine. By means of this
~1 29 !3~33
-- 6 --
invention, rapid and economical detection of urinary tract
I infection is possible.
More particularly, it has been discovered that
dyes soluble at pH 8 to 12 which stain bacteria will do so
in the absence of chelating agents. Bacteria are stained
by contacting these organisms, which are electrostatically
adsorbed to negatively charged filtar sur~aces, with a
protein staining dye at about pH 8 to 12. Any dye capable
of staining bacteria which is soluble at about pH 8 to 12
may be employed. However, preferable dyes are Safranin-O
or Basic Fuchsin.
Bacteria stained in accordance with the present
invention may be readily detected if present at a
concentration of 50,000 or higher per milliliter, or at
lower amounts if concentrated. Stained bacteria which ara
dispersed in solution will, upon concentration, be readily
visible. When concentrated bacteria at sufficient
concentration are stained without dispersion, the presence
of bacteria is immediately manifest.
Sedimentation and entrapment of bacteria by
filtration are examples of effective means for
concentrating bacteria. In this current invention,
baateria in urine ttheir isoelectric charge having been
modified by an acid diluent) are adsorbed to a negatively
charged filter surface. As the urine-diluent solution
diffuses over a wide area, the bacteria therein
electrostatically adsorb to the filter at the site where
the urine is deposited, providing an effective method for
concentrating bacteria. In accordance with the method of
this invention, the pores of the ~ilter are not required
to be smaller than the bacteria under test.
Filter surfaces which have a net negative charge
rather than a net positive charge must be employed. The
diluent indicated in this application will reverse the
~.
~98~L~33
isoelectric charge of negatively charged bacteria,
altering the same to a positive charge. Thus, the
bacteria in the urine-diluent solution will be immobilized
electrostatically by adsorption to the filter matrix
whereas free dye, urine pigments and other urinary
compounds will diffuse away from the restricted area site
of the adsorbed bacteria allowing detection of the stained
organisms.
Dye used to stain bacteria at the site of the
deposit of the urine-diluent mixture is diffused away from
the immobilized stained bacteria adsorbed to the filter.
Addition of the washing reagent at the site at which the
urine-diluent solution was deposited enhances the
diffu~ion of the free dye from this site without removing
the stain from the immobil~zed bacteria.
Referring now to the drawings, a preferred
embodiment of the invention is illustrated in Fig. 1 and
consists of a flat, round or square, rigid or semi-rigid
disk 1. This disk may be machined, molded or thermoformed
2~ from a variety of materials, e.g., polystyrene. The disk
prefarably measures about one to four inches in diameter
or width and features a conical or round depression or
reservoir 2 at or near the center of the disk. Locatsd at
the bottom of the reservoir the disk defines a short bore
forming a small diameter hole or orifice 3 which extends
through the entire thickness of the disk. The orifice
diameter may be in the range of from about 0.06 inch to
about about 0.25 inch.
Though it is not an absolute requirement for
performance of the procedure, the dis~ may feature an
orifice lip 4 protruding from the lower surface around the
orifice. This orifice lip allows for more efficient flow
of fluids into a precise location on the filter paper pad
and serves to inhibit tha spreading of fluid on the upper
~298i83
surface of the filter pad. The filter pad 5 is positioned
ad;acent to the lower surface of the disk so that the
filter pad is in contact with the orifice or orifice lip.
The filter pad may be composed of cellulose, fiberglass or
other suitable filter material and exhibits a net negative
electrostatic charge.
The position of the filter pad is maintained by
"sandwiching" the pad between the disk and a lower filter
retainer plate 6. The lower plate may be machined, molded
or thermoformed, and features a square or round depression
7 slightly larger in size than the filter pad. The lower
plate may be attached to the disk to secure the filter pad
in place. This attachment may be by any suitable means
such as by an adhesive, solvent welding, or sonic welding.
Alternatively, the disk and lower plate may be designed to
hinge, snap or screw together to house the filter pad.
; In another embodiment of the invention, as
evidenced by Figs. 4-6, an adhesive label or tape 8 is
applied to the lower surface 9 of the disk or plate 10 to
secure the filter pad, thus eliminating the need for a
lower ilter retainer plate.
The embodiments described above are useful as a
single use disposable, portable unit for detection of
bacteria and have particular application for detection of
bacteria in fluids, e.g., urine, in situations where
conventional methods for bacteria detection are cumbersome
or unfeasible, e.g., over-the-counter for consumer home
use, physicians' offices, screening clinics, etc.
The disk may be formed to define a reaction well
11 which extends transversely from the tapered reservoir
as illustrated in Fig. 4. When the device is placed in a
vertical position, with its base 12 resting on any flat
horizontal surface, as shown in Fig. 5, the reaction well
- 11 is positioned to accept and retain sample fluid. When
~L~98~33
the device is subsequently placed in a horizontal or near
horizontal position as shown in Fig. 6 by 90 degrees
clockwise rotation from the position shown in Fig. 5, the
fluid flows out of the reaction well and into the
reservoir. The fluid then travels through the orifice and
upon contast with the filter pad, is absorbed into the
pad. This feature is especially usaful in tests requiring
incubation or reaction of a bacterial suspension with a
chemical substance prior to performance of the bacterial
quantitation test. In particular, a test for
determination of antibiotic susceptibility may be
performed by incubating a bacterial suspension with an
appropriate antiblotic for a period of time during which,
if the organism is resistant to the antibiotic, growth of
the organism will occur. After the incubation period, the
device is placed in a horizontal position to allow the
antibiotic-treated bacterial suspension to flow to the
reservoir and filter pad, thus allowing for quantitation
with the staining composition of the current invention.
A disposable multiple test device is illustrated
in Fig. 4 which may be made by molding or thermoforming a
device featuring two or more units of the embodiments
described above in connection with Fig. 4. This m~ltiple
test device has particular application in a test for
determination of antibiotic susceptibility. The
appropriate antibiotics may be pre-loaded into the
reaction well thus allowing for simple test set-up and
multiple determination of antibiotic susceptibility.
Although specific embodiments o~ the invention
have bsen shown and described, it is understood that other
embodiments adaptations and modifications may be utilized
without departing from the true spirit and scope of the
lnvention. The embodiments, composition and methods
described herein may be similarly applied to the staining
-
~:9818;~
-- 10 --
and detection of bacteria in other fluids, such as culture
media, blood, spinal fluids, food washings, milk and
water, as well as to staining bacteria from such fluids
which have been deposited on filter surfaces.
The minimum quantity of bacteria which can be
detected by this staining procadure varies somewhat with
the condition and growth phase of the bacterial culture.
In general, actively growing, viable bacteria are strongly
detected at levels of about 100,000/ml. Non-viable
organisms, cr organisms in lag phase stain less intensely
~nd thus higher levels of bacteria are required for
detection.
The following examples are illustrative of the
invention and are not to be taken in a limiting sense.
Example 1
Two drops of urine (using a conventional
dropping pipette) from a known positive bacteriuric
patient was added to 8 drops of acid diluent, pH 1.5 HCl
in a test tube. Four drops of the mixture was added to
the center of a negatively charged filter. Three drops of
Safranin-0 dye at 1:1000 in pH 10 carbonate-borate-
hydroxyl buffer was added to the filter surface at the
site of inoculation of the urine-diluent mixture. Three
drops of washing reagent, pH 2.5 HCl was added to the same
site to allow diffusion of free dye away from the site of
inoculation. Three additional drops of washing reagent
was used as a second rinse. The results manifested a 3-4
mm diameter intensely stained filter surface at the site
of inoculation with a peripharal ring of free dye about 25
mm from the site of the stained bacteria. Such a result
is indic~tive of a positive bacteriuria test. Comparison
of the intensity of the color of the stained bacteria to a
~298~3
-- 11 --
known amount of bacteria added to urine indicated about
1,000,000 bacteria per ml urine. Plating of the sample on
agar culture verified these results.
Example 2
Two drops of urine (using a conventional
dropping pipette) from a healthy volunteer (free of
urinary-tract infection by conventional assay) was added
to eight drops of acid diluent, pH 1.5 HCl in a test tube.
Four drops of the mixture was added to the center of a
negatively charged filter. The remainder of this
experiment was conducted exactly as described in Example 1
above. Upon observation, the filter surface at the site
at which the urine-diluent solution was deposited was
completely white and the free dye ringed the filter at
about 25 mm from the site of the inoculation. Such a
result is indicative of a negative bacteriuria test. This
result was verified by plating of the sample on agar
culture media.
Example 3
Employing the procedure ~et forth in Examples 1
and 2, and the preferred embodiment illustrat~d in Fig. 1,
experiments were conducted with a variety of organisms
using Safranin~0 as a model dye at pH 10 in carbonate-
3~ borate-hydroxyl buffer. The results were as follows:
1298~L83
- 12 -
Test Organisms Intensity of Stain*
100,000 Bacteria/ml On Filter Surface
Normal urine, no bacteria 0
E. coli +~
S. aureus ++
Proteus vulgaris ~+
Pseudomonas ++
Group A Strep. ++
Group D Strep. ++
Klebsislla pn. +~
*Site of inoculation of sample of urine-diluent onto ths
filter surface. Scoring o color intensity: O = white,
no color;
: += light pink; +~= pink; ++~=red; ++~+= dark red or
magenta.
Example 4
Employing the procedures set forth in Examples 1
and 2, and the preferred ambodiment illustrated in Fig. 1,
experiments were conducted adding different final
concentrations of bacteria/ml to normal urine, and
proceeding to test 4 drops of the urine-diluent mixture
with results as ollows. The scoring of filter color
lntensity is described in Example 3.
E. coli
: CFU/ml Added to Color Intensity
Normal Urine Of Filter Surface
O O
10, 000
50,000 +
100,000
1, 000, 000 f ++
10, 000, ooo +,~,+,~,
~ le 5
A test for determination of antibiotic
susceptibility may be performed as follows: The multiple
test device illustrated in Fig. 4 is placed in a vertical
1298~83
- 13 -
position. Antibiotic solutions (one drop each) are
dispensed into reaction wells #1 through #8. One drop of
formalin solution is dispensed into well #9 and one drop
of distilled water is placed into well ~10. A broth
suspension of bacteria is diluted to yield approximately
100,000 bacteria/ml, and one drop of this dilution is
added to each reaction well #1 through #10. The device is
then incubated for three to four hours at 37 degrees C.
Ater the incubation period, 4 drops of pH 1.5 HCl diluent
are added to each reaction well. The device is placed in
a horizontal position to allow travel of the fluid from
the reaction well into the reservoir. After the fluid is
absorbed into the filter pad, the dye solution and washing
reagent are placed on each filter site as described in
Examples 1 and 2 above. After completion of the staining
process, the filter pad is visually examined for color
development. Formalin treated site #9 manifests a pink
(~+) color and represents the zero-hour control. Site #10
manifests a dark red (~++~) color and represents the
uninhibitad growth control. The color intensity of each
site #l through #8 is compared to the controls for
interpretation of antimicrobial susceptibility. Color
intensity equal to or greater than the growth control
indicates that the organism is resistant to the antibiotic
under test. Color intensity equal to or less than the
zero-hour control indicates that the organism is
susceptible to the antlbiotic.
What is Claimed is: